Introduction
When characterizing the pharmacokinetics of a therapeutic agent, a key goal is to maximize efficacy while minimizing risk. Because pharmacokinetics studies are difficult to do in populations with severe end organ damage, such as end-stage renal disease, dosing recommendations in these populations may be extrapolated from other data or may be missing. In patients receiving dialysis, the added complexity of clearance by the dialysis process, which depends on the degree to which medications are protein bound, must be taken into account. Nevirapine (NVP) is a commonly used non-nucleoside antiretroviral agent that has only minimal renal elimination. However, hemodialysis may represent a significant route of NVP elimination in patients with end stage renal disease due to its relatively low volume of distribution (1.2 L/kg), low molecular weight, and being only 60% bound to plasma proteins.1 In the one previously published case report of NVP use in a patient receiving hemodialysis,2 the area under the concentration time curve was substantially reduced during dialysis with a 46.5% extraction ratio. However, NVP was dosed at only 200 mg once daily in this patient. Therefore, we examined the potential effects of hemodialysis on the steady-state pharmacokinetics of NVP, in HIV-1-infected subjects with end-stage renal disease requiring hemodialysis, when given at the usual dosing strategy of 200 mg twice daily.
ACTG A5177 was a prospective observational study to estimate the steady-state pharmacokinetic (PK) parameters of efavirenz, lopinavir/ritonavir and NVP in HIV-infected subjects requiring hemodialysis. The efavirenz and lopinavir/ritonavir results have been reported elsewhere.3 This letter summarizes the pharmacokinetic results observed in the 3 subjects accrued to the NVP arm.
Methods
HIV-1-infected subjects requiring hemodialysis between the ages of 18 and 65 were enrolled into the NVP arm if they had received as part of their antiretroviral regimen one tablet of NVP 200 mg twice daily for at least 30 days immediately prior to study entry. Use of any other NNRTI or PI was not allowed. The accrual target for the NVP arm was 12 subjects; however due to slow enrollment the study was closed to accrual after 3 NVP subjects enrolled. PK sampling took place on two separate days, a non-hemodialysis day and a hemodialysis day. Observed doses of NVP were administered at exactly 8am on each study day; blood samples were taken at −0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, and 12h. Dialysis was administered from about 12pm to 4pm.
Samples were assayed for NVP at the University of California at San Francisco Pharmacology laboratory, using an LC/MS/MS method approved by the ACTG Pharmacology QA/QC subcommittee. Statistical methods for calculating pharmacokinetic parameters have been previously described3.
To estimate the NVP hemodialysis clearance rate, additional blood samples were obtained on the dialysis day at the inflow and outflow ports at dialysis initiation, and at approximately 2 and 4 hours thereafter. The filtration fraction, represented by FF, was calculated as where Cp,IN and Cp,OUT were the plasma concentrations of NVP flowing into and out of the dialyzer, respectively. The NVP hemodialysis clearance rate (based on blood) was calculated as ClHD = Qdial * FF, where Qdial was the blood flow into and out of the dialyzer, ml/min. The amount of NVP cleared by dialysis was calculated as Qdial*( AUCHD,in – AUCHD,out), where AUCHD,in and AUCHD,out represent the NVP area under the concentration time curve during dialysis of blood entering and leaving the dialyzer, respectively.
Results
All three subjects were black non-Hispanic males, ages 39, 40 and 51. For Subject 1, the dialyzer model and manufacturer was the AM-BIO 100D, Asahi Biomembrane. For Subjects 2 and 3, the dialyzer model and manufacturer were the PSN 210, Gambro Renal Products (by Baxter). Dialysis flow rates ranged from 300 to 450 mL/min, and dialyzed volumes ranged from 72.00 to 128.25 L.
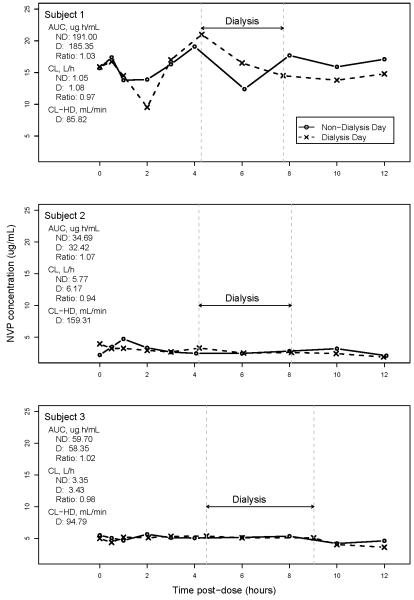
Figure 1 shows the NVP plasma concentration time plots on the nondialysis and dialysis days for each of the three subjects. For all 3 subjects NVP concentrations on nondialysis and dialysis days tracked closely. For Subject 1, NVP concentrations over the dosing interval were higher on both study days than for Subjects 2 and 3. These results do not suggest differences in NVP PK between nondialysis and dialysis days. Across subjects, the geometric means of NVP AUCs were 73.40 and 70.51 μg.h/mL on nondialysis and dialysis days, respectively. The geometric means of minimum observed plasma NVP concentrations were 4.77 and 4.01 μg/mL; and of systemic NVP clearance were 2.72 and 2.84 on nondialysis and dialysis days, respectively.
Figure 1.
Plots of plasma NVP concentration (μg/mL) by time (hr) on nondialysis (solid line, circles) and dialysis (dashed line, x-marks) days. Dashed vertical lines show start and stop times of hemodialysis. AUC, area under the NVP concentration-time curve, plasma, 0-12 hr; ND, nondialysis day; D, dialysis day; CL, systemic NVP clearance; CL-HD, hemodialysis clearance.
Calculated filtration fractions obtained on the dialysis days were negative in several instances indicating a more rapid loss of fluid than drug across the dialyzer. Hemodialysis clearance rates were not calculated in these. Positive filtration fractions ranged from 17% to 37%, and CLHD ranged from 68.40 mL/min (at end of dialysis) for Subject 1 (this subject’s CLHD at start of dialysis was 103.23 mL/min) to 159.31 mL/min (at start of dialysis) for Subject 2 (CLHD could be calculated at a single time only for Subject 2; for Subject 3 CLHD was 77.90 and 111.67 mL/min 2h after start of and at end of dialysis, respectively). Calculated amounts of NVP cleared by hemodialysis were −145, 10 and 107 mg (negative value likely due to assay variability) for Subjects 1, 2 and 3, respectively. Thus, on average less than 5% of the dose was lost, indicating that, an insignificant quantity of NVP was removed from the systemic circulation by hemodialysis. NVP concentrations in both blood and plasma (sampled simultaneously prior to the PK dose) were available for Subjects 2 and 3, yielding similar blood-to-plasma ratios of 1.03 and 1.05, respectively.
All 3 NVP subjects were negative for hepatitis B surface antigen and hepatitis B core antibody. NVP plasma AUCs were higher for Subjects 1 and 3 who were hepatitis C antibody positive compared to Subject 2 who was hepatitis C negative.
In summary, we measured plasma NVP concentrations at steady-state in dialysis patients following their oral doses on both dialysis and nondialysis days. We found that the steady-state pharmacokinetics of NVP given 200 mg twice daily were similar to those in patients without renal failure,4 and we saw only minimal differences in PK parameters between dialysis and non-dialysis days. While some proportion of the NVP is removed by the hemodialysis process, our data suggest that this loss is clinically insignificant. To the extent that plasma levels reflect stability of efficacy in the intracellular compartment, our results do not suggest that the dosing of NVP needs to be coordinated around the timing of dialysis. While limited by small sample size, we conclude that the dose of NVP 200 mg twice daily provides consistent plasma levels that are not clinically changed by the dialysis process.
Acknowledgments
The present work was supported in part by National Institute of Allergy and Infectious Diseases grants to the AIDS Clinical Trials Group (A168636), SDAC/Harvard School of Public Health (1U01AI068634-01) and by Grant Number UL1RR024134 from the National Center for Research Resources as well as by the University of Pennsylvania AIDS Clinical Trial Unit (AI69467).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boehringer Ingelheim Pharmaceuticals, Inc. Nevirapine Package Insert. Nov, 2008. [Google Scholar]
- 2.Izzedine H, Launay-Vacher V, Aymard G, et al. Pharmacokinetic of nevirapine in haemodialysis. Nephrol Dial Transplant. 2001;1:192–193. doi: 10.1093/ndt/16.1.192. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SK, Rosenkranz SL, Cramer YS, et al. The pharmacokinetics and pharmacogenomics of efavirenz and lopinavir/ritonavir in HIV-infected persons requiring hemodialysis. AIDS. 2008;15:1919–1927. doi: 10.1097/QAD.0b013e32830e011f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy RL, Sommadossi JP, Lamson M, et al. Antiviral effect and pharmacokinetic interaction between nevirapine and indinavir in persons infected with human immunodeficiency virus type 1. J Infect Dis. 1999;5:1116–1123. doi: 10.1086/314703. [DOI] [PubMed] [Google Scholar]