Abstract
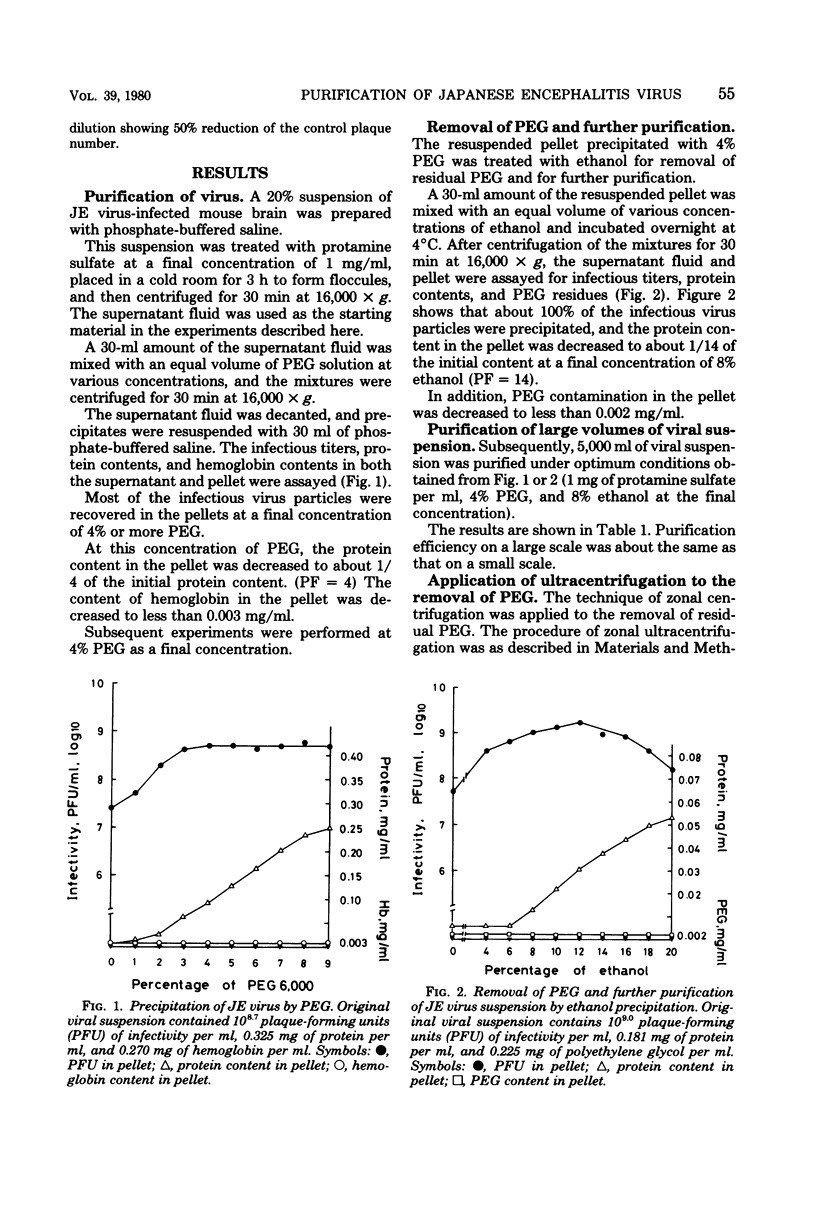
Large volumes of Japanese encephalitis (JE) virus propagated in mouse brain can be easily purified by polyethylene glycol 6,000. By using the polyethylene glycol precipitation method, mouse hemoglobin was almost all separated from the viral suspension, and consequently the total amount of nonviral protein in the viral suspension decreased. The recovery of infectivity was about 100%. The removal of residual polyethylene glycol in the viral suspension was possible without difficulty by means of ethanol precipitation. This method is recommended as an initial step in large-scale purification of Japanese encephalitis virus propagated in mouse brain because it is simple, rapid, and inexpensive.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., ANDERSON S. G., ABBOT A. Purification of Murray Valley encephalitis virus. J Gen Microbiol. 1961 Feb;24:177–186. doi: 10.1099/00221287-24-2-177. [DOI] [PubMed] [Google Scholar]
- CHENG P. Y. Purification, size, and morphology of a mosquitoborne animal virus, Semliki Forest virus. Virology. 1961 May;14:124–131. doi: 10.1016/0042-6822(61)90139-8. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Heinz F., Kunz C. Concentration and purification of tick-borne encephalitis virus grown in suspensions of chick embryo cells. Acta Virol. 1977 Jul;21(4):301–307. [PubMed] [Google Scholar]
- Heyward J. T., Klimas R. A., Stapp M. D., Obijeski J. F. The rapid concentration and purification of influenza virus from allantoic fluid. Arch Virol. 1977;55(1-2):107–119. doi: 10.1007/BF01314484. [DOI] [PubMed] [Google Scholar]
- Horzinek M. A simple method for concentration of arboviruses propagated in tissue culture. Am J Trop Med Hyg. 1969 Jul;18(4):588–591. doi: 10.4269/ajtmh.1969.18.588. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Fukuoka T., Sasao F., Surimarut S., Fukai K. Purification of Japanese encephalitis virus grown in BHK21 and Singh's Aedes albopictus cells by polyethylene glycol precipitation. Biken J. 1973 Jun;16(2):67–73. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Okuda K., Ito K., Miyake K., Morita M., Ogonuki M. Purification of Japanese encephalitis virus vaccine by zonal centrifugation. J Clin Microbiol. 1975 Jan;1(1):96–101. doi: 10.1128/jcm.1.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- PORTERFIELD J. S. Plaque production with yellow fever and related arthropodborne viruses. Nature. 1959 Apr 11;183(4667):1069–1070. doi: 10.1038/1831069b0. [DOI] [PubMed] [Google Scholar]
- Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. I. Correlation of particle density, infectivity, and RNA content of type 2 virus. Virology. 1965 Sep;27(1):103–112. doi: 10.1016/0042-6822(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Zettner A., Sylvia L. C., Capacho-Delgado L. The determination of serum iron and iron-binding capacity by atomic absorption spectroscopy. Am J Clin Pathol. 1966 May;45(5):533–540. doi: 10.1093/ajcp/45.5.533. [DOI] [PubMed] [Google Scholar]
- van KAMPEN E., ZIJLSTRA W. G. Standardization of hemoglobinometry. II. The hemiglobincyanide method. Clin Chim Acta. 1961 Jul;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]



