Abstract
Background
Coenzyme Q10 (CoQ10) and its analogs are used therapeutically by virtue of their functions as electron carriers, antioxidant compounds, or both. However, published studies suggest that different ubiquinone analogs may produce divergent effects on oxidative phosphorylation and oxidative stress.
Methodology/Principal Findings
To test these concepts, we have evaluated the effects of CoQ10, coenzyme Q2 (CoQ2), idebenone, and vitamin C on bioenergetics and oxidative stress in human skin fibroblasts with primary CoQ10 deficiency. A final concentration of 5 µM of each compound was chosen to approximate the plasma concentration of CoQ10 of patients treated with oral ubiquinone. CoQ10 supplementation for one week but not for 24 hours doubled ATP levels and ATP/ADP ratio in CoQ10 deficient fibroblasts therein normalizing the bioenergetics status of the cells. Other compounds did not affect cellular bioenergetics. In COQ2 mutant fibroblasts, increased superoxide anion production and oxidative stress-induced cell death were normalized by all supplements.
Conclusions/Significance
These results indicate that: 1) pharmacokinetics of CoQ10 in reaching the mitochondrial respiratory chain is delayed; 2) short-tail ubiquinone analogs cannot replace CoQ10 in the mitochondrial respiratory chain under conditions of CoQ10 deficiency; and 3) oxidative stress and cell death can be counteracted by administration of lipophilic or hydrophilic antioxidants. The results of our in vitro experiments suggest that primary CoQ10 deficiencies should be treated with CoQ10 supplementation but not with short-tail ubiquinone analogs, such as idebenone or CoQ2. Complementary administration of antioxidants with high bioavailability should be considered if oxidative stress is present.
Introduction
Coenzyme Q10 (CoQ10; ubiquinone) and its analogs have been evaluated as antioxidant agents and enhancers of mitochondrial functions in patients with mitochondrial disorders and clinical trials of neurodegenerative diseases including Parkinson disease, amyotrophic lateral sclerosis, Huntington disease, Friedreich ataxia, and Alzheimer's disease with modest or no objective benefits [1]–[6]. The use of CoQ10 therapy and its analogs in primary CoQ10 deficiency, an autosomal recessive syndrome due to defects of ubiquinone biosynthesis, could provide valuable data to evaluate the effectiveness of these compounds in restoring respiratory chain activities and preventing oxidative stress. The disorder manifests clinically with four major phenotypes: 1) an encephalomyopathy with brain involvement and recurrent myoglobinuria [7]; 2) an infantile multisystem disorder with encephalopathy usually associated with nephropathy and variable involvement of other organs [8], [9]; 3) ataxic syndrome with cerebellar atrophy [10], [11]; and 4) an isolated myopathy [12], [13].
Molecular defects in genes encoding CoQ10 biosynthetic proteins have been reported in 18 patients. Four patients improved with CoQ10 supplementation [9], [14]–[17], five died before or during the treatment, and 9 had no definite response [14], [15], [18]–[22]; it is therefore difficult to reach definitive conclusions about the effectiveness of CoQ10 supplementation in primary CoQ10 deficiencies. To better understand the pathogenesis of CoQ10 deficiency, we have characterized the bioenergetics and oxidative stress in PDSS2 and COQ2 mutant fibroblasts, and have demonstrated that severe CoQ10 deficiency caused marked defects of ATP synthesis without oxidative stress whereas milder CoQ10 deficiency produced reactive oxygen species (ROS) and oxidation of proteins and lipids [23]. Here, we evaluate the in vitro effects of CoQ10 supplementation on the bioenergetics and oxidative stress status of CoQ10 deficient fibroblasts with mutations in PDSS2, COQ2, and COQ9 (Fig. 1). In addition, because CoQ10 analogs and vitamin C are being used in clinical trials based on their antioxidant properties, we concurrently evaluated the effect of CoQ2, idebenone, and vitamin C.
Figure 1. CoQ10 biosynthesis pathway.
CoQ10 is composed of a benzoquinone and a decaprenyl side chain. PDSS2 is the second subunit of decaprenyl diphosphate synthase, a heterotretameric enzyme that catalyzes the formation of the decaprenyl side chain. COQ2 or para-hydroxybenzoate (PHB)-polyprenyl transferase catalyzes the condensation reaction of PHB and decaprenyl diphosphate. The function of COQ9 is still unknown. The mutant fibroblasts used in this study harbor mutations in COQ9, p.244R>X (P1) [18], COQ2, p.197R>H, p.228N>S (P2) [14] and p.297Y>C (P3) [17], and PDSS2, p.322Q>X, p.382S>L (P4) [20].
Results
Cellular CoQ10 levels after treatment with compounds for 24 hours
Fibroblasts from the four patients with different molecular defects in the CoQ10 biosynthetic pathway used in this study showed significantly decreased levels of CoQ10 relative to controls (P<0.001) (Fig. 2). When control and patients' cells were treated for 24 h with 5 µM of CoQ10, cellular levels of ubiquinone increased significantly in all cells (P<0.001), resulting in values 20–85-fold higher than in control cells (Fig. 2). When the cells were treated with idebenone, CoQ2, or vitamin C, cellular levels of CoQ10 remained unchanged (Fig. 2).
Figure 2. Cellular CoQ10 levels after 24 hours of treatment.
Cultured skin fibroblasts from controls and CoQ10 deficiency patients were treated with 5 µM CoQ10, idebenone, CoQ2 or vitamin C. After 24 hours, fibroblasts were collected and cellular CoQ10 were determined by EQ-HPLC. Results are expressed as a mean ± SD of three experiments. P1 = COQ9 mutant; P2 = COQ2 mutant; P3 = COQ2 mutant; P4 = PDSS2 mutant. ***P<0.001 compared with untreated control; ###P<0.001 compared with untreated P1; +++P<0.001 compared with untreated P2; φφφ P<0.001 compared with untreated P3; ΩΩΩ P<0.001 compared with untreated P4.
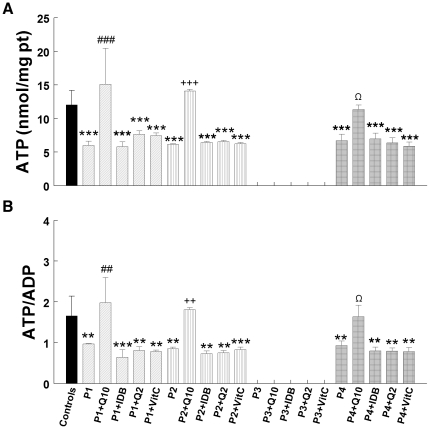
Bioenergetic status: 24 hours versus 1 week of treatment
The four CoQ10 deficient fibroblasts showed significant decreases in cellular ATP levels (P<0.001) (Fig. 3A) and ATP/ADP ratios (P<0.001) (Fig. 3B). Treatment with 5 µM CoQ10, idebenone, CoQ2, or vitamin C for 24 h did not increase ATP levels or ATP/ADP ratios. However, treatment with 5 µM CoQ10 for 1 week increased significantly levels of cellular ATP in P1 (P<0.001), P2 (P<0.001), and P4 (P<0.05) to normal levels (Fig. 4A). At the same time, ATP/ADP ratios were significantly increased in P1 (P<0.01), P2 (P<0.01), and P4 (P<0.05) (Fig. 4B) thus bringing to normal the bioenergetics status of the cells. In contrast, idebenone, CoQ2, and vitamin C treatment failed to alter ATP levels or ATP/ADP ratios (Fig. 4).
Figure 3. Bioenergetic status of cells after 24 hours of treatment.
Cultured skin fibroblasts from controls and CoQ10 deficient patients were treated with 5 µM CoQ10, idebenone, CoQ2, or vitamin C. After 24 h, fibroblasts were collected and cellular ATP and ADP were determined by UV-HPLC. Data are represented as nmol of ATP/mg prot (A) and ATP/ADP ratio (B). Results are expressed as mean ± SD of three experiments. P1 = COQ9 mutant; P2 = COQ2 mutant; P3 = COQ2 mutant; P4 = PDSS2 mutant. ***P<0.001 compared with untreated control.
Figure 4. Bioenergetic status of cells after 1 week of treatment.
Cultured skin fibroblasts from controls and CoQ10 deficient patients were treated with 5 µM CoQ10, idebenone, CoQ2, or vitamin C. After 1 week, fibroblasts were collected and cellular ATP and ADP were determined by UV-HPLC. Data are represented as nmol of ATP/mg prot (A) and ATP/ADP ratio (B). Results are expressed as mean ± SD of three experiments. P1 = COQ9 mutant; P2 = COQ2 mutant; P3 = COQ2 mutant; P4 = PDSS2 mutant. ***P<0.001 and **P<0.01 compared with untreated control; ##P<0.01 and ###P<0.001 compared with untreated P1; ++P<0.01 and +++P<0.001 compared with untreated P2; Ω P<0.05 and ΩΩ P<0.01 compared with untreated P4.
The effect of compound administration on superoxide anion levels: 24 hours versus 1 week of treatment
After incubation for 24 h in galactose medium supplemented with dialyzed FBS, P3 cells showed increased MitoSOX Red stain indicating elevated levels of superoxide anions (P<0.001) (Fig. 5A). Under the same culture conditions, the other three CoQ10 deficient fibroblasts did not show any significant changes in MitoSOX stain. After 24 h of treatment with CoQ10, idebenone, CoQ2, or vitamin C, superoxide anion levels decreased significantly in P3 cells (P<0.01) (Fig. 5A). Cells from controls and from the three other patients did not show significant changes in MitoSOX stain after 24 hours of treatment with any of the four compounds; however, there was a trend towards increased superoxide anions in P4 cells treated with CoQ2 for 24 h (Fig. 5A).
Figure 5. Superoxide anion production in cells treated with antioxidant compounds for 24 hours (A) or 1 week (B).
Cultured skin fibroblasts from controls and CoQ10 deficient patients were treated with 5 µM CoQ10, idebenone, CoQ2, or vitamin C. After 24 h (A) or 1 week (B), fibroblasts were collected and superoxide anion generation was assessed by MitoSOX staining and quantified by flow cytometry The Y axis represents the fluorescence intensity of the sample relative to the fluorescence intensity of the control sample (FP/FC), after subtracting the background intensity; the X axis represent the sample. 20,000 cells were analyzed in every experiment. Results are expressed as mean ± SD of three experiments. P1 = COQ9 mutant; P2 = COQ2 mutant; P3 = COQ2 mutant; P4 = PDSS2 mutant. ***P<0.001 compared with untreated control; ##P<0.01 and ###P<0.001 compared with untreated P1; +++P<0.001 compared with untreated P2; φφ P<0.01 and φφφ P<0.001 compared with untreated P3.
After one week of incubation in galactose medium plus dialyzed FBS, P2 and P3 cells showed increased MitoSOX Red staining (Fig. 5B). After 1 week of treatment with CoQ10, idebenone, CoQ2, or vitamin C, superoxide anion levels were decreased significantly in both P2 and P3 cells (P<0.001) (Fig. 5B).
The effect of compound administration on cell death: 24 hours versus 1 week treatment
In adherent cells, cell death was significantly higher in untreated P2 and P3 cells than in control cells (P<0.05 and P<0.01, respectively) (Fig. 6A). Cell death was significantly reduced in both P2 and P3 treated for 24 h with CoQ10 (P<0.01) or idebenone (P<0.05). Vitamin C also reduced cell death in P3 cells (P<0.05) (Fig. 6A). Control, P1, and P4 cells showed similar proportions of dead cells with and without treatment (Fig. 6A).
Figure 6. Dead cells after 24 hours (A) or 1 week of treatment (B).
Cultured skin fibroblasts from controls and CoQ10 deficiency patients were treated with 5 µM CoQ10, idebenone, CoQ2, or vitamin C. After 24 h (A) or 1 week (B), fibroblasts were collected and Trypan blue assays were performed. Results are expressed as mean ± SD of three experiments. P1 = COQ9 mutant; P2 = COQ2 mutant; P3 = COQ2 mutant; P4 = PDSS2 mutant. *P<0.05, **P<0.01 and ***P<0.001 compared with untreated control; +P<0.05 and ++P<0.01 compared with untreated P2; φP<0.05 and φφφ P<0.001 compared with untreated P3.
Untreated P2 and P3 cells also showed significantly higher cell death than controls when incubated for 1 week in galactose medium supplemented with dialyzed FBS (P<0.01 and P<0.001, respectively) (Fig. 6B). Cell death was reduced in P2 cells by 24 h treatment with CoQ10 (P<0.05), idebenone (P<0.05), CoQ2 (P<0.01), or vitamin C (P<0.05); and in P2 cells by one week treatment with CoQ10 (P<0.001), idebenone (P<0.05), CoQ2 (P<0.001), or vitamin C (P<0.001) (Fig. 6B). While the P3 cell death levels were significantly reduced by treatment with idebenone for 1 week (P<0.05), they were still significantly higher than those of control cells (P<0.001) (Fig. 6B).
Because we observed abundant floating COQ2 mutant cells cultured in galactose medium with dialyzed FBS, we performed Trypan blue staining of P2 cells and found that 15% of the cells were dead after 24 h and 63% were dead after 1 week. Percentages of floating dead cells were significantly decreased after one week of treatment with CoQ10 (36% dead cells), CoQ2 (45% dead cells), or idebenone (43% dead cells).
Discussion
Over the last 4 years, studies of CoQ10 deficient patients have focused on two major issues: identifying the molecular genetic basis of ubiquinone deficiencies and characterizing pathogenic mechanisms [24]. We identified the two first mutations in genes encoding proteins required for CoQ10 biosynthesis, COQ2 [17] and PDSS2 [20], in patients with primary CoQ10 deficiency. Our studies of cultured fibroblasts from these patients revealed that both CoQ10 biosynthetic disorders caused bioenergetic defects, but only COQ2 mutant fibroblasts showed increased ROS production and signs of oxidative stress [23]. In the present study, we have evaluated the in vitro efficacy of CoQ10 supplementation in normalizing the bioenergetic status and the oxidative balance in fibroblasts of CoQ10 deficient patients harboring mutations in PDSS2, COQ2, and COQ9 [14], [17], [18], [20], [23]. We chose a final concentration of 5 µM CoQ10 because this is the approximate concentration reached in the plasma of patients treated with oral supplementation of CoQ10 [3], [6], [25]–[27]. We have also evaluated the efficacy of short-tail ubiquinone analogs (idebenone and CoQ2), which are less lipophilic, and vitamin C, a well-known hydrophilic antioxidant. It is noteworthy that 5 µM concentrations of ubiquinone analogs exceed the EC50 of idebenone (∼0.4–0.5 µM) and CoQ2 (∼0.03 µM) in cultured fibroblasts under oxidative stress [28], [29].
After 24 h of 5 µM CoQ10 supplementation, cellular CoQ10 levels increased dramatically. The magnitudes of the increases were independent of the molecular defects of the cells since both control and patients' fibroblasts showed similar degrees of CoQ10 uptake. However, after 24 h of CoQ10 treatment, none of the cell lines showed significant improvement in ATP levels or in ATP/ADP ratios, which are markers of respiratory chain function. In contrast to our results, López-Martín and colleagues [30] noted normalization of mitochondrial complexes I+III and II+III activities in COQ2 mutant fibroblasts after 24 h of 10 µM CoQ10 supplementation. Paradoxically, however, the same authors using the same COQ2 mutant cells and other genetically undefined CoQ10 deficient fibroblasts found that the activities of complex II+III activities increased only slightly and remained below control values after 72 hours of 100 µM CoQ10 supplementation [31]. This discrepancy may be due to the fact that in the second study, but not in the first, respiratory chain enzyme activities were normalized to the activity of citrate synthase [31], a marker of mitochondrial mass [32]. In another study, embryonic cells from COQ7 knockout mice treated with 25 µM of water-soluble CoQ10 for 5 h only showed slight increase of ATP [33]. The negligible effects of CoQ10 supplementation on the bioenergetic status of CoQ10 deficient cells can be explained by the strong lipophilic nature of CoQ10, which accumulates in membranes reaching saturated concentrations [34], [35]. Thus, exogenous CoQ10 is mainly distributed in lysosomes, endoplasmic reticulum, and plasma membrane and only a small proportion (∼11%) reaches the mitochondria [36], [37]. In addition, much of the CoQ10 in mitochondria is likely to be trapped in the outer membrane, not available to the respiratory chain, which is located in the mitochondrial inner membrane [38].
Because 5 µM CoQ10 did not increase the ATP levels after 24 h of supplementation, we tried two other strategies: 1) increasing the duration of CoQ10 supplementation to 1 week; and 2) using less-lipophilic ubiquinone analogs. In marked contrast to treatment for 24 h of CoQ10, incubation of ubiquinone-deficient fibroblasts for 1 week with 5 µM CoQ10 increased ATP levels and ATP/ADP ratios significantly, indicating normalization of the bioenergetic status. Similar to these results, yeast coq mutants show inefficient uptake of exogenous CoQ6 to the mitochondrial inner membrane, which is reflected in a low succinate cytochrome c reductase activity after 2–15 µM CoQ6 supplementation for 48 h [37], [39]. The complete rescues of growth of the yeast coq mutants' supplemented with 15 µM CoQ6 were only possible after 6–8 days [37], [39], [40]. These results suggest that the pharmacokinetic constraints of CoQ10 in reaching the mitochondrial respiratory chain are key limiting factors in determining its efficacy in CoQ10 deficient patients. Furthermore, an in vivo study of PDSS2 mutant mice treated for 4 months with 100 mg/kg body weight (b.w.)/day or 200 mg/kg b.w./day of water-soluble CoQ10 showed that only the highest dose of CoQ10 improved the renal function in these CoQ deficient animals [41]. Thus, the dose of CoQ10 is another important factor influencing the effectiveness of CoQ10 supplementation in ubiquinone deficient patients. Nevertheless, the gold standard to evaluate the effectiveness of CoQ10 treatment in ubiquinone deficiency is in vivo measurements of mitochondrial function, bioenergetics, and oxidative stress.
In contrast to CoQ10, short-tail ubiquinone analogs and vitamin C failed to increase ATP levels and ATP/ADP ratio in patients' cells after both 24 h and 1 week treatment. These results are in agreement with previous studies showing that hydrophilic ubiquinone analogs (CoQ2 and idebenone) or mitochondrial-targeted ubiquinone (MitoQ) are less efficient than hydrophobic ubiquinone analogs in enhancing energy production by the mitochondrial respiratory chain [42]–[44], since the specific effects of ubiquinones depend on their interaction with hydrophilic and hydrophobic (or physiological) binding sites [42], [43]. Accordingly, hydrophobic and hydrophilic ubiquinone analogs are not interchangeable. Experience with two patients with CoQ10 deficiency and cerebellar ataxia due to ADCK3/CABC1 mutations is relevant. Both patients were treated with CoQ10 (5–10 mg/kg/b.w.) [21], [45]. One patient, who also had exercise intolerance and hyperlactatemia, improved after 3 months of treatment whereas the other, who had only ataxia, did not benefit from 3 years of therapy. The ataxia did not improve in either patient and for this reason CoQ10 was replaced with low-dose idebenone in both patients (5–10 mg/kg/b.w.). However, their symptoms worsened, prompting reinstitution of CoQ10 therapy [21], [45]. Based on our in vitro results showing improvements of bioenergetics by ubiquinone in CoQ10 deficient cells, we postulate that CoQ10 therapy improved the first patient's exercise intolerance and may have stabilized symptoms in the second patient because of enhanced ATP production. The lack of improvement of cerebellar symptoms may be due to poor transfer of CoQ10 across the blood brain barrier, irreversible structural alterations in the cerebellum, or both factors [36], especially considering the low dose used (5–10 mg/kg/b.w.). Our finding that high doses of idebenone in vitro failed to increase ATP levels in CoQ10 deficient fibroblasts or the low-dose used for therapy may explain ineffectiveness of idebenone therapy compared to CoQ10 treatment in both patients.
Because oxidative stress is another important pathogenic factor in CoQ10 deficiencies, we evaluated also the effect of CoQ10 and three other antioxidants on superoxide anion production. After both 24 h and after 1 week of treatment, we noted that all four compounds significantly reduced MitoSox staining in P3 cells (the cell line with the most prominent MitoSox fluorescence). Reduction of superoxide anion levels correlated with decreased cell death, suggesting that ROS generation and oxidative damage are the main causes of death in COQ2 mutant fibroblasts. This notion is supported by our observation that P3 COQ2 fibroblast line had the highest levels of superoxide anion production and protein oxidation and showed the most robust features of apoptosis and the highest proportion of cell death [46]. In contrast to COQ2 mutant fibroblasts, COQ9 and PDSS2 mutant fibroblast do not have increased ROS, a difference that may be related to the disparate CoQ-dependent flow of electrons in the mitochondrial respiratory chain in the different mutant cell lines. As we recently reported, 10–15% residual CoQ10 are not associated with significant ROS production, whereas 30–50% residual CoQ10 is accompanied by increased ROS production and cell death [23], [46]. Interestingly, there was a trend towards increased ROS production in P4 cells supplemented with CoQ2 for 24 h and in P1 cells supplemented with idebenone for 1 week. In contrast, idebenone decreased ROS levels in the other 3 cell lines after one week of treatment. Our data suggest that these compounds may have pro-oxidant and anti-oxidant properties under certain in vitro conditions [47] and confirm in cultured fibroblasts observations previously reported in isolated mitochondria [48].
In summary, our in vitro study tackles important issues regarding treatment of CoQ10 deficiency and, more in general, of mitochondrial diseases. First, the prolonged pharmacokinetics of CoQ10 to reach the mitochondrial respiratory chain is critical to the restoration of energy status of human CoQ10 deficient cells. This may explain the delayed clinical response of CoQ10 deficiency patients to oral supplementation with CoQ10 [16] and suggest that high doses of CoQ10 are needed. Second, short tail ubiquinone analogs cannot substitute for CoQ10 in the mitochondrial respiratory chain of human CoQ10 deficient cells revealing the importance of the decaprenyl tail. Third, oxidative stress and cell death can be attenuated by the administration of lipophilic and hydrophilic antioxidants.
Thus, our results confirm that in CoQ10 deficient patients: (i) early treatment based on early diagnosis is critical to maximize the efficacy of ubiquinone supplementation; (ii) short-tail ubiquinone analogs (i.e. idebenone or CoQ2) are not suitable substitutes for CoQ10; and (III) complementary administration of antioxidants with high bioavailability may be helpful in CoQ10 deficiency patients. Further studies on the pathogenesis of CoQ10 deficiency in patients with different molecular defects and in animal models will lead to more rational and improved therapies.
Materials and Methods
Ethics Statement
Skin biopsies, to generate cultured fibroblasts, were performed after obtaining written informed parental consents under study protocols approved by the Institutional Review Boards of the Columbia University Medical Center, University of Padova, and Charité University Hospital as well as Local Research Ethics committees of Great Ormond Street Hospital for Children NHS Trust and Institute of Child Health. The informed consents and clinical studies were in compliance with the principles expressed in the Declaration of Helsinki.
Cells culture and treatment
All experiments were performed in human skin fibroblasts from 5 controls and 4 CoQ10 deficient patients (Fig. 1): P1 with a homozygous mutation in COQ9 (p.R244X) [18]; P2 with compound heterozygous mutations in COQ2 (p.R197H and p.N228S) [14]; P3 with a homozygous mutation in COQ2 (p.Y297C) [17]; and P4 with compound heterozygous mutations in PDSS2 (p.Q322X and p.S382L) [20]. Cells were grown in RPMI high-glucose medium supplemented with 10% fetal bovine serum, 5 ml MEM non-essential amino acids, 1 ml fungizone, and 5 ml penicillin-streptomycin until 80% confluent.
For the 24 h treatment experiments, 75% confluent cells were grown in RPMI-1640 glucose-free medium with 10% dialyzed fetal bovine serum, 25 mM HEPES, 1.5 mM Glutamax, 25 mM galactose, 1 ml fungizone, and 5 ml penicillin-streptomycin for 2 days. Twenty-four hours before the experiments, cells were incubated in fresh medium supplemented with idebenone (Santhera Pharmaceuticals, Liestal, Switzerland), CoQ2 (Sigma–Aldrich Corp., St. Louis, MO, USA), CoQ10 or vitamin C (Sigma-Aldrich Corp., St. Louis, MO, USA) (5 µM final concentration).
For the 1 week treatment experiments, 60% confluent cells were incubated for 7 days in RPMI-1640 glucose-free medium with 10% dialyzed fetal bovine serum, 25 mM HEPES, 1.5 mM Glutamax, 25 mM galactose, 1 ml fungizone, 5 ml penicillin-streptomycin and one of the following: idebenone, CoQ2, CoQ10, or vitamin C (5 µM final concentration).
Regular FBS contains 85–245 µg glucose/ml and dialyzed FBS less than 5 µg glucose/ml. Because our experiments required glucose-free media supplemented with galactose to force energy production through oxidative phosphorylation rather than glycolysis [23], [49], [50], we used 10% dialyzed FBS. This was particularly important for the 24 h treatment experiments since cells can use the glucose contained in 10% regular FBS during the first 24–48 h.
Idebenone, CoQ2, CoQ10, and vitamin C were dissolved in dialyzed fetal bovine serum prior to addition to culture medium.
All cells culture reagents were obtained from Invitrogen Corp. (Carlsbad, CA, USA). Patients and control cell lines at passage 7–10 were grown to replicate every experiment at least 3 times.
Cellular CoQ10 levels
One week after treatment, fibroblast pellets were collected and washed 4 times with phosphate buffered saline (PBS). After the fourth wash, no CoQ10 was detected in the PBS supernatant. CoQ10 from fibroblasts was extracted in a hexane:ethanol mixture [20]. The lipid component of the extract was separated by HPLC on a reverse phase Symmetry® C18 3.5 µm, 4.6×150 mm column (Waters), using a mobile phase consisting of methanol, ethanol, 2-propanol, acetic acid (500∶500∶15∶15) and 50 mM sodium acetate at a flow rate of 0.9 ml/min. The electrochemical detector consisted of an ESA Coulochem II with the following setting: guard cell (upstream of the injector) at +900 mV, conditioning cell at –600 mV (downstream of the column), followed by the analytical cell at +500 mV. CoQ10 concentration was estimated by comparison of the peak area with those of standard solutions of known concentration [20].
Mitochondrial bioenergetics assessment
Fibroblasts cultured under glucose-free media supplemented with galactose possess ATP and ADP pools that are predominantly dependent on the oxidative phosphorylation [23], [49]; therefore, using these culture conditions, ATP levels and ATP/ADP ratio are reliable markers of oxidative phosphorylation and correlate with the rates of ATP synthesis in CoQ10 deficient cells, as reported [23]. To determine the adenine nucleotides levels, cells were grown in 15-cm-diameter plates to 90% confluency and the adherent cells were collected in ice-cold PBS using a scraper. After centrifugation at 3,000 g for 3 min at 4°C, pellets were suspended in 200 µl of ice-cold 0.5 M perchloric acid, vortexed for 30 s, and centrifuged at 11,000 g for 10 min at 4°C. Pellets were stored at −80°C for protein measurement. Adenine nucleotides were measured in the supernatants injected into an Alliance HPLC (Waters Corporation, Milford, MA, USA) with an Alltima C18 NUC reverse-phase column (Alltech Associates, Deerfield, IL, USA) [23]. Adenine nucleotide levels were expressed in nmol/mg protein. Because of poor growth of P3 cells, the 1 week experiments were performed only in P1, P2 and P4 fibroblasts.
Oxidative stress analyses
Previously, we demonstrated that oxidative stress can be detected by MitoSOX Red assay, lipid peroxidation assay, and carbonyl groups detection in CoQ10 deficient cells due to COQ2 mutation [23]; and results of the three assays were highly concordant [23]. Here, to evaluate the effect of the proposed treatments in those and others CoQ10 deficient cells, we estimated mitochondrial matrix oxidant levels in control and mutant fibroblasts using MitoSOX Red, a fluorochrome specific for mitochondrial matrix reactive oxygen species (ROS) burden (Molecular Probes, Invitrogen Corp., Carlsbad, CA, USA) as described [23], [51]. Approximately 1×106 adherent cells (90% confluence) were trypsinized, incubated with 5 µM MitoSox for 20 min at 37°C in the dark, washed twice with PBS and re-suspended in 500 µl of PBS. Cytofluorometric analysis was performed using a FACSCalibur cell analyzer equipped with a 488 nm At-kt laser and fluorescence was measured using the FL2 channel. Fluorescence was background subtracted and normalized to the signal of control values (FP/FC). Data were acquired using Cell Pro Quest and analyzed using Flowjo software (Becton Dickinson, NJ, USA). Before to start with the set of experiments for the cytofluorometric analysis, the MitoSOX staining was evaluated and monitored by fluorescence microscopy (IX70 inverted system microscope, Olympus, Tokyo, Japan). Additionally, a control cell line treated with 0.5 µM antimycin A for 15 h before the MitoSOX staining was used as a positive control for increased ROS production [23].
Oxidative stress-induced death cells studies
Measurement of cell viability was performed with the Trypan blue exclusion method. Numbers of living and dead adherent cells were determined by light microscopy using a hemocytometer. We also determined the proportion dead non-adherent P2 cells. Healthy nuclei from viable cells appeared round and phase bright, whereas nuclei from dead or dying cells appeared blue and irregularly shaped. All cells were counted and results were expressed as percentage of dead cells relative to total cells [52].
Statistical analysis
Control data are expressed as mean ± SD of 5 different samples in triplicate experiments. Patients' data are expressed as mean ± SD of triplicate experiments. One-way analysis of variance (ANOVA) followed by Student-Newman-Keuls multiple comparisons test was used. A P value of less than 0.05 was considered statistically significant.
Acknowledgments
The authors would like to thank Dr. Eric Schon (Columbia University Medical Center), Dr. Nuri Güevan (Santhera Pharmaceuticals, Liestal, Switzerland) and members of Schon laboratory for comments and critical review of the manuscript. We also appreciate the technical support of Ms. Saba Tadesse.
Footnotes
Competing Interests: Santhera Pharmaceuticals (Switzerland) Ltd supplied idebenone for this study. In addition, Dr. Hirano has a research contract with Santhera for a clinical trial of idebenone for a mitochondrial disease (Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-like episode [MELAS]). Dr. Hirano receives no salary support from Santhera. Other than supplying idebenone, Santhera was not involved in the research described in this manuscript. The work of Dr. Hirano and colleagues was performed independently of Santhera. In fact, the results of this study may be viewed as negative for Santhera, because the findings suggest that idebenone cannot replace coenzyme Q10 in the mitochondrial respiratory chain.
Funding: This work was supported by U.S. National Institutes of Health (NIH) grants HD32062 and NS11766, by grants from the Muscular Dystrophy Association (MDA), and by the Marriott Mitochondrial Disorders Clinical Research Fund (MMDCRF). LCL was supported by a post-doctoral fellowship from the Ministerio de Educación y Ciencia, Spain. CMQ is supported by the MDA. LS is supported by Telethon grant GGP09207. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Beal MF. Therapeutic effects of coenzyme Q10 in neurodegenerative diseases. Methods Enzymol. 2004;382:473–487. doi: 10.1016/S0076-6879(04)82026-3. [DOI] [PubMed] [Google Scholar]
- 2.Hart PE, Lodi R, Rajagopalan B, Bradley JL, Crilley JG, et al. Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol. 2005;62:621–626. doi: 10.1001/archneur.62.4.621. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann P, Thompson JL, Levy G, Buchsbaum R, Shefner J, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancuso M, Orsucci D, Volpi L, Calsolaro V, Siciliano G. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr Drug Targets. 2010;11:111–121. doi: 10.2174/138945010790031018. [DOI] [PubMed] [Google Scholar]
- 5.Schulz JB, Di Prospero NA, Fischbeck K. Clinical experience with high-dose idebenone in Friedreich ataxia. J Neurol. 2009;256(Suppl 1):42–45. doi: 10.1007/s00415-009-1008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 7.Ogasahara S, Engel AG, Frens D, Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc Natl Acad Sci U S A. 1989;86:2379–2382. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rötig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- 9.Salviati L, Sacconi S, Murer L, Zaccello G, Franceschini L, et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology. 2005;65:606–608. doi: 10.1212/01.wnl.0000172859.55579.a7. [DOI] [PubMed] [Google Scholar]
- 10.Lamperti C, Naini A, Hirano M, De Vivo DC, Bertini E, et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology. 2003;60:1206–1208. doi: 10.1212/01.wnl.0000055089.39373.fc. [DOI] [PubMed] [Google Scholar]
- 11.Musumeci O, Naini A, Slonim AE, Skavin N, Hadjigeorgiou GL, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–855. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- 12.Horvath R, Schneiderat P, Schoser BG, Gempel K, Neuen-Jacob E, et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology. 2006;66:253–255. doi: 10.1212/01.wnl.0000194241.35115.7c. [DOI] [PubMed] [Google Scholar]
- 13.Lalani SR, Vladutiu GD, Plunkett K, Lotze TE, Adesina AM, et al. Isolated mitochondrial myopathy associated with muscle coenzyme Q10 deficiency. Arch Neurol. 2005;62:317–320. doi: 10.1001/archneur.62.2.317. [DOI] [PubMed] [Google Scholar]
- 14.Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 15.Mollet J, Giurgea I, Schlemmer D, Dallner G, Chretien D, et al. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;117:765–772. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med. 2008;358:2849–2850. doi: 10.1056/NEJMc0800582. [DOI] [PubMed] [Google Scholar]
- 17.Quinzii C, Naini A, Salviati L, Trevisson E, Navas P, et al. A Mutation in Para-Hydroxybenzoate-Polyprenyl Transferase (COQ2) Causes Primary Coenzyme Q10 Deficiency. Am J Hum Genet. 2006;78:345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan AJ, Bitner-Glindzicz M, Meunier B, Costello H, Hargreaves IP, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagier-Tourenne C, Tazir M, Lopez LC, Quinzii CM, Assoum M, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mollet J, Delahodde A, Serre V, Chretien D, Schlemmer D, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman S, Hargreaves I, Clayton P, Heales S. Neonatal presentation of coenzyme Q10 deficiency. J Pediatr. 2001;139:456–458. doi: 10.1067/mpd.2001.117575. [DOI] [PubMed] [Google Scholar]
- 23.Quinzii CM, Lopez LC, Von-Moltke J, Naini A, Krishna S, et al. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. FASEB J. 2008;22:1874–1885. doi: 10.1096/fj.07-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinzii CM, Lopez LC, Naini A, DiMauro S, Hirano M. Human CoQ10 deficiencies. Biofactors. 2008;32:113–118. doi: 10.1002/biof.5520320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhagavan HN, Chopra RK. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 26.Ferrante KL, Shefner J, Zhang H, Betensky R, O'Brien M, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- 27.Miles MV. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7(Suppl):S72–77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 29.Jauslin ML, Wirth T, Meier T, Schoumacher F. A cellular model for Friedreich Ataxia reveals small-molecule glutathione peroxidase mimetics as novel treatment strategy. Hum Mol Genet. 2002;11:3055–3063. doi: 10.1093/hmg/11.24.3055. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Martin JM, Salviati L, Trevisson E, Montini G, DiMauro S, et al. Missense mutation of the COQ2 gene causes defects of bioenergetics and de novo pyrimidine synthesis. Hum Mol Genet. 2007;16:1091–1097. doi: 10.1093/hmg/ddm058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Hernández A, Cordero MD, Salviati L, Artuch R, Pineda M, et al. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009;5:19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- 32.Kirby DM, Thorburn DR, Turnbull DM, Taylor RW. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:93–119. doi: 10.1016/S0091-679X(06)80004-X. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi M, Shimizu T, Moriizumi E, Shirasawa T. Clk-1 deficiency induces apoptosis associated with mitochondrial dysfunction in mouse embryos. Mech Ageing Dev. 2008;129:291–298. doi: 10.1016/j.mad.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Cornell BA, Keniry MA, Post A, Robertson RN, Weir LE, et al. Location and activity of ubiquinone 10 and ubiquinone analogues in model and biological membranes. Biochemistry. 1987;26:7702–7707. doi: 10.1021/bi00398a025. [DOI] [PubMed] [Google Scholar]
- 35.Lenaz G, Samori B, Fato R, Battino M, Parenti Castelli G, et al. Localization and preferred orientations of ubiquinone homologs in model bilayers. Biochem Cell Biol. 1992;70:504–514. doi: 10.1139/o92-078. [DOI] [PubMed] [Google Scholar]
- 36.Bentinger M, Dallner G, Chojnacki T, Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- 37.Santos-Ocaña C, Do TQ, Padilla S, Navas P, Clarke CF. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem. 2002;277:10973–10981. doi: 10.1074/jbc.M112222200. [DOI] [PubMed] [Google Scholar]
- 38.Geromel V, Darin N, Chretien D, Benit P, DeLonlay P, et al. Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol Genet Metab. 2002;77:21–30. doi: 10.1016/s1096-7192(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 39.Do TQ, Hsu AY, Jonassen T, Lee PT, Clarke CF. A defect in coenzyme Q biosynthesis is responsible for the respiratory deficiency in Saccharomyces cerevisiae abc1 mutants. J Biol Chem. 2001;276:18161–18168. doi: 10.1074/jbc.M100952200. [DOI] [PubMed] [Google Scholar]
- 40.Jonassen T, Marbois BN, Faull KF, Clarke CF, Larsen PL. Development and fertility in Caenorhabditis elegans clk-1 mutants depend upon transport of dietary coenzyme Q8 to mitochondria. J Biol Chem. 2002;277:45020–45027. doi: 10.1074/jbc.M204758200. [DOI] [PubMed] [Google Scholar]
- 41.Saiki R, Lunceford AL, Shi Y, Marbois B, King R, et al. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol. 2008;295:F1535–1544. doi: 10.1152/ajprenal.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degli Esposti M, Ngo A, Ghelli A, Benelli B, Carelli V, et al. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Arch Biochem Biophys. 1996;330:395–400. doi: 10.1006/abbi.1996.0267. [DOI] [PubMed] [Google Scholar]
- 43.Degli Esposti M, Ngo A, McMullen GL, Ghelli A, Sparla F, et al. The specificity of mitochondrial complex I for ubiquinones. Biochem J. 1996;313(Pt 1):327–334. doi: 10.1042/bj3130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 45.Aure K, Benoist JF, Ogier de Baulny H, Romero NB, Rigal O, et al. Progression despite replacement of a myopathic form of coenzyme Q10 defect. Neurology. 2004;63:727–729. doi: 10.1212/01.wnl.0000134607.76780.b2. [DOI] [PubMed] [Google Scholar]
- 46.Quinzii CM, López LC, Gilkerson RW, Dorado B, Coku J, et al. FASEB J in press; 2010. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gille L, Rosenau T, Kozlov AV, Gregor W. Ubiquinone and tocopherol: dissimilar siblings. Biochem Pharmacol. 2008;76:289–302. doi: 10.1016/j.bcp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, et al. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta. 2009;1787:384–392. doi: 10.1016/j.bbabio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson BH, Petrova-Benedict R, Buncic JR, Wallace DC. Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem Med Metab Biol. 1992;48:122–126. doi: 10.1016/0885-4505(92)90056-5. [DOI] [PubMed] [Google Scholar]
- 50.Soderberg K, Nissinen E, Bakay B, Scheffler IE. The energy charge in wild-type and respiration-deficient Chinese hamster cell mutants. J Cell Physiol. 1980;103:169–172. doi: 10.1002/jcp.1041030121. [DOI] [PubMed] [Google Scholar]
- 51.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 52.Blanchard-Fillion B, Prou D, Polydoro M, Spielberg D, Tsika E, et al. Metabolism of 3-nitrotyrosine induces apoptotic death in dopaminergic cells. J Neurosci. 2006;26:6124–6130. doi: 10.1523/JNEUROSCI.1038-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]