Abstract
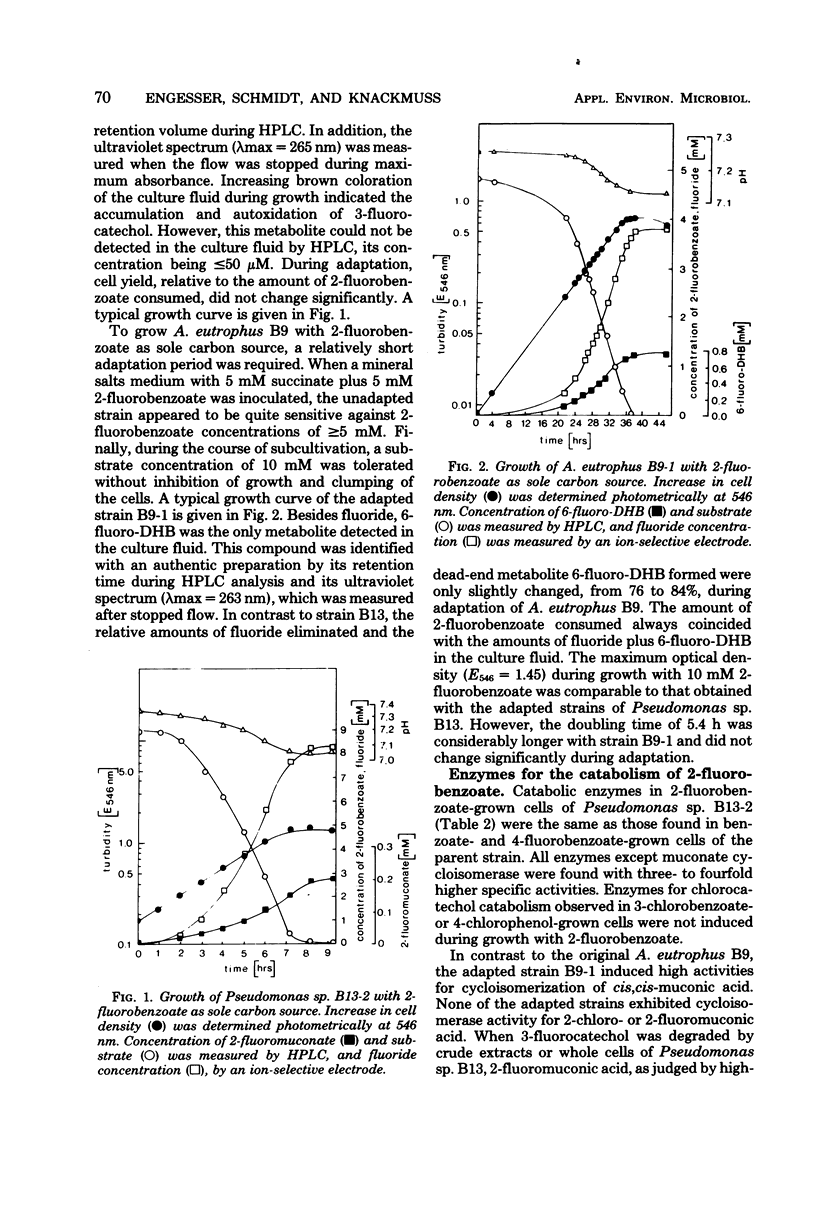
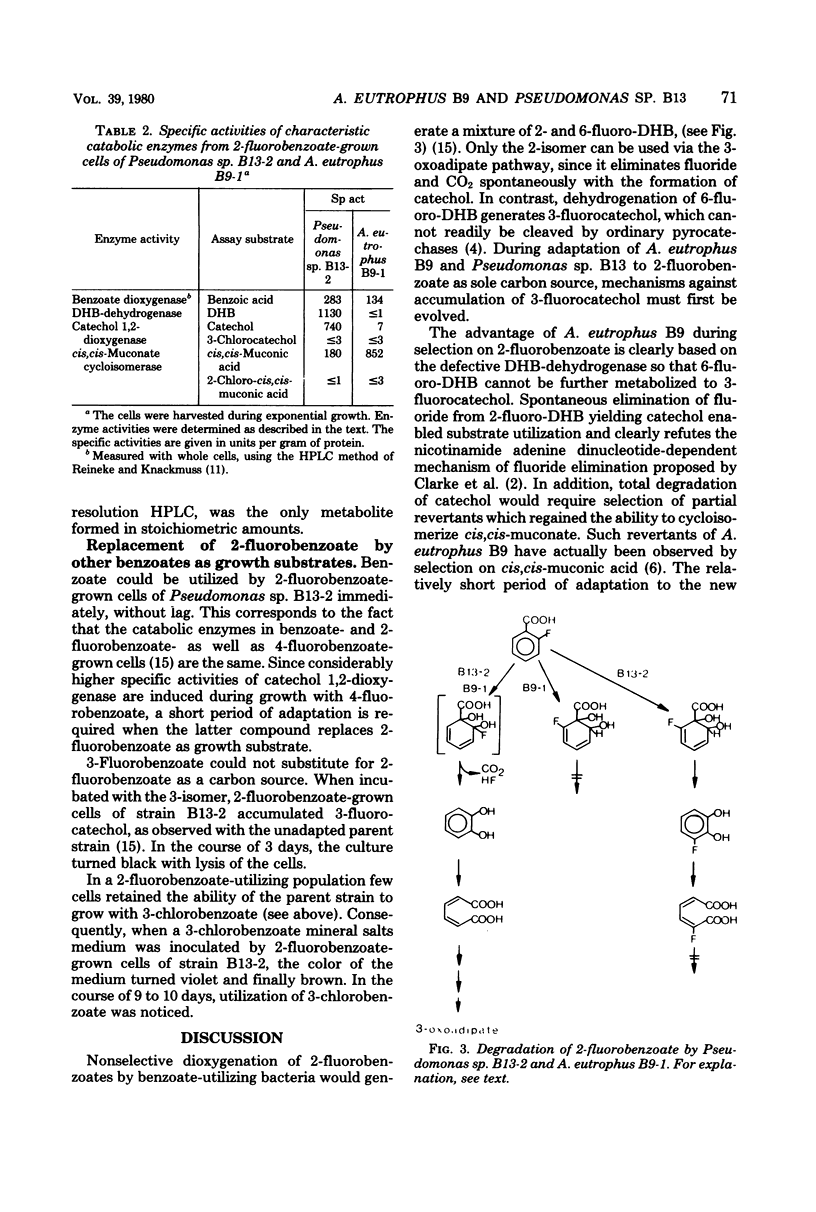
Alcaligenes eutrophus B9 and Pseudomonas sp. B13 could be adapted to 2-fluorobenzoate as the sole source of carbon and energy. The ability of the A. eutrophus B9 to use this new substrate is clearly based on the defective dihydrodihydroxybenzoate dehydrogenase. Nontoxic 6-fluoro-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid is accumulated instead of 3-fluorocatechol. About 84% of the substrate is dioxygenated to catechol and utilized via the 3-oxoadipate pathway. During continuous adaptation of Pseudomonas sp. B13 regioselectivity of dioxygenation of 2-fluorobenzoate is drastically changed in favor of a 1,2-attack. Consequently, approximately 97% of the substrate is utilized via catechol. A three- to fourfold overproduction of key enzymes of the 3-oxoadipate pathway compensates for the slower turnover rates of the fluorinated substrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clarke K. F., Callely A. G., Livingstone A., Fewson C. A. Metabolism of monofluorobenzoates by Acinetobacter calcoaceticus N.C.I.B. 8250. Formation of monofluorocatechols. Biochim Biophys Acta. 1975 Oct 9;404(2):169–179. doi: 10.1016/0304-4165(75)90323-2. [DOI] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Regulation of the -ketoadipate pathway in Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):476–485. doi: 10.1128/jb.107.2.476-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackmuss H. J., Hellwig M. Utilization and cooxidation of chlorinated phenols by Pseudomonas sp. B 13. Arch Microbiol. 1978 Apr 27;117(1):1–7. doi: 10.1007/BF00689343. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Chemical structure and biodegradability of halogenate aromatic compounds. Substituent effects on 1,2-dioxygenation of benzoic acid. Biochim Biophys Acta. 1978 Sep 6;542(3):412–423. doi: 10.1016/0304-4165(78)90372-0. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of aromatic compounds in bacteria. Purification and properties of the catechol-forming enzyme, 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (NAD + ) oxidoreductase (decarboxylating). J Biol Chem. 1972 Aug 25;247(16):4960–4965. [PubMed] [Google Scholar]
- Rigby P. W., Burleigh B. D., Jr, Hartley B. S. Gene duplication in experimental enzyme evolution. Nature. 1974 Sep 20;251(5472):200–204. doi: 10.1038/251200a0. [DOI] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Schreiber A., Hellwig M., Dorn E., Reineke W., Knackmuss H. J. Critical Reactions in Fluorobenzoic Acid Degradation by Pseudomonas sp. B13. Appl Environ Microbiol. 1980 Jan;39(1):58–67. doi: 10.1128/aem.39.1.58-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



