Abstract
Oximes are commonly used nucleophilic reactivators of alkyl phosphorylated and alkyl methylphosphonylated acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). Covalent inhibition of these enzymes by organophosphate (OP) pesticides results typically in phosphorylated enzymes, while covalent inhibition by nerve agent OPs results in methyl phosphonylated cholinesterases. In this study we determined kinetic constants for interaction of three triazole containing oximes with native human AChE, enzyme diethylphosphorylated by paraoxon, enzyme phosphonylated by VX and cyclosarin as well as enzyme aged upon phosphonylation by soman. Stopped-flow kinetics of oxime interaction was monitored using quenching of intrinsic tryptophan fluorescence of AChE as an indicator of oxime binding. Triazole oximes were efficiently synthesized using copper catalyzed cycloaddition between azide and alkyne building blocks (“Click chemistry”). Equilibrium dissociation constants determined for both native enzymes were in low micromolar range for all three oximes, while dissociation constants for phosphylated (phosphorylated and phosphonylated) enzymes were typically one to two orders of magnitude larger. Dissociation constants for interaction with aged enzymes were similar or smaller than those determined for native enzymes. Similar results were obtained with reference oximes, 2PAM and HI6. Association rate constants for formation of oxime complexes were similar for both native, phosphylated and aged enzymes. In summary our data suggest that modification of active site gorge in AChEs by phosphylation of the active serine compromises oxime binding. Dealkylation of phosphonylated enzyme, however opens space in the gorge allowing oximes to bind tighter.
Introduction
Not long after synthesis of the first organophosphates (OPs), it became evident that the cholinesterases (ChEs), and specifically acetylcholinesterase (AChE), are physiologically most important targets in vertebrate OP poisoning [1]. Today according to the World Health Organization between thirty thousand and two hundred thousand people die annually, worldwide, from acute OP poisoning [2]. Most of those deaths occur in the third world countries. At the same time more than 95% of US population are found to carry OP residuals in their plasma, presumably resulting from more chronic exposure to sublethal doses of OP based pesticides [3]. This clear evidence of constant and continuing intoxication of large human populations by OPs is often overshadowed in the public press by prospects of insidious use of nerve gases in terrorism. OP based nerve agents are a documented terrorist weapon of convenience due to straightforward synthesis and ease of deployment. A clear mechanism at a molecular level can be ascertained where the OPs, by virtue of their structural resemblance of the tetrahedral transition state for acyl ester hydrolysis, react covalently with the active center serine in the enzyme rendering it non-functional with respect to catalyzing the hydrolysis of the neurotransmitter, acetylcholine [1].
Therapy of acute OP poisoning typically includes administration of atropine as a muscarinic acetylcholine receptor antagonist, together with an oxime, pralidoxime (2-PAM), HI6 or congeneric bis-quaternary structures. Oximes are commonly used nucleophilic reactivators of phosphorylated and phosphonylated ChEs typically resulting from covalent inhibition by OP pesticides and nerve agent OPs, respectively. In this study we describe three novel triazole oximes synthesized by copper catalyzed cycloaddition between azide and alkyne building blocks (“click chemistry”) using well known oxime reactivators, 2PAM and HI6, as reference compounds. Their interaction with native human AChE, as well as with phosphorylated and phosphonylated AChE conjugates, was studied directly by monitoring quenching of intrinsic tryptophan fluorescence of AChE upon oxime binding. Stopped-flow techniques for monitoring fast reactions in the millisecond time range were used to deconstruct the kinetic constants. Rate constants of oxime association and dissociation with AChE, as well as the resulting equilibrium dissociation constants, were determined for native AChE and its OP conjugates and analyzed in the context of the enzyme reactivation mechanisms.
Material and Methods
Human AChE was prepared by purification from cell media of HEK-293 cells stably transfected with hAChE cDNA construct containing a coding sequence for a FLAG peptide inserted between the leader peptide and the amino terminus of the processed protein. The protein was purified in mg quantities by adsorption and desorption from an antiFLAG peptide resin.
Oximes were purchased from Sigma (2PAM), US Biological, Swampscott, MA, USA (HI6) or synthesized in this study. In general, isomerically pure anti- substituted triazole oximes were prepared by combining an azide and alkyne building blocks in aqueous solution at room temperature with catalytic amount of Cu(I), as described [4].
OPs were purchased from Sigma (paraoxon) or kindly provided by Dr. Gabi Amitai, Israeli Institute for Biological Research, Ness Ziona, Israel.
The hAChE-OP conjugates were prepared by allowing hAChE stock (in the μM concentration range) to react with at least a four fold excess of a VX or cyclosarin analogue [5]. Inhibited enzyme was passed through two consecutive Sephadex G-50 spin columns to remove excess unreacted inhibitor.
Association (k1) and dissociation (k-1) kinetic rate constants of oxime interaction with native and OP conjugated hAChE were measured at 50 – 100 nM enzyme and multiple micromolar oxime concentrations in a stopped-flow apparatus by monitoring quenching of intrinsic hAChE Trp fluorescence in a millisecond time frame during the course of formation of oxime*hAChE complex [6].
Equilibrium binding of oximes to the native or OP-conjugated hAChE was measured from the dependence of the pseudo-first order association rate, kobs, of the reversible inhibitors 9-amino acridine, ambenonium or decidium (1 or 2 μM) on ligand concentration (from 100 nM to 300 mM). Rates were monitored in a millisecond time frame by stopped-flow measurements of intrinsic Trp fluorescence quenching of hAChE and dissociation constants (Kd) determined as described [6, 7].
Reversible inhibition of native hAChE by oximes was studied using Lineweaver & Burk analysis of control and oxime inhibited hAChE activities determined by the spectrophotometric Ellman method [8].
All experiments were done in 0.1M Phosphate buffer pH 7.4 at 22 °C. Final concentrations of organic solvents were less than 1% in enzyme assays.
Results and Discussion
Dissociation constants of five oximes with native and OP conjugated hAChE are summarized in the Table 1. Constants for interaction with native hAChE determined in both, substrate competition experiments measuring enzyme activity and stopped-flow experiments measuring direct binding, agreed well and were therefore averaged and presented as one constant in the table. The binding of three triazole oximes to native AChE with dissociation constants in low micromolar range was significantly, one to two orders of magnitude, greater than that of 2PAM and HI6 reflecting a better fit of elongated and slightly bent triazole structures (Figure 1) within the long, narrow and slightly curved active center gorge of AChE. Conjugation of OP moieties with the active serine upon AChE inhibition by VX, paraoxon (POX) and cyclosarin (CS) resulted in an increase in Kd values for all oximes reflecting a general reduction in the available binding space or distortion [9,10] in the active center gorge of phosphylated hAChE. The extent of Kd increase was consistent with the increase of molecular volume of covalently attached moieties (calculated using DS Visualizer by Accelrys as a volume of solvent accessible surface generated with a sphere of 1.4 Å radius), in the following order : VX-cojugate (∼ 79 Å3), POX-conjugate (∼ 95 Å3) and CS-conjugate (∼ 115 Å3). Dealkylation of VX or CS inhibited enzyme yields an aged form of hAChE where ∼ 54 Å3 of the gorge volume is taken by an anionic OP moiety. Binding of all five oximes to aged hAChE was much tighter than to any other OP-hAChE conjugate (Table 1). In fact Kds for oximes 28B, 2PAM and HI6 were one order of magnitude lower than corresponding Kds for binding to non-conjugated, native hAChE. This decrease in Kds in spite of ∼ 54 Å3 reduction of the gorge volume available for oxime binding points to significant stabilizing role of electrostatic interaction between electron deficient pyridinium oxime moieties and negatively charged methylphosphono anion of the aged hAChE. Only for oximes 81A and 153 that bound to native hAChE tightest of all five oximes, binding to aged hAChE was not further improved. The aged OP-hAChE conjugate thus provides tightest stabilization for oxime binding, narrowing down 375-fold range of Kd values (0.4 uM – 150 uM) observed for binding to the native hAChE ten–fold, to a 38-fold range (0.23 uM – 8.7 uM for the aged hAChE). Recent structural studies are consistent with this observation showing that both 2PAM and HI6 bind to aged AChE in orientations different from those found bound to native AChE (Fig 2). HI6 for example binds only to the upper half of the native mouse AChE gorge, above the “choke point” (Fig.2A), while it was found bound to the very base of the aged mouse AChE gorge (Fig.2B). Also, 2PAM orientation bound to native Torpedo californica AChE (Fig.2C) was different from the one found bound to the aged Torpedo AChE (Fig.2D). The inability of 2PAM and HI6 to reactivate aged AChE was thus not due to the lack of oxime binding in the proximity of the aged OP moiety in the AChE active center gorge. In addition to the inherent stability of the dealkylated OP conjugates, the presumed unproductive oxime group orientations directed away from phosphorus atom in both aged enzyme * oxime complexes (Figs 2B and 2D) account for the lack of reactivation.
Table 1.
Dissociation constants (Kd) for binding of oximes to native and OP-conjugated hAChE. Constants were determined by one or more different experimental approaches described in Material and Methods section, and averaged with standard deviation lower than of 50% of Kd values.
| Kd (μM) | |||||
|---|---|---|---|---|---|
| oxime | h AChE | ||||
| native | aged | VX-inhibited | POX-inhibited | CS-inhibited | |
| 28B | 2.0 | 0.23 | 2.5 | 16 | ≥ 100 |
| 81A | 0.40 | 1.6 | 27 | 63 | 4.8 |
| 153 | 1.1 | 5.8 | 16 | 91 | ≥ 300 |
| 2PAM | 150 | 8.7 | 350 | ≥ 1000 | ≥ 300 |
| HI6 | 38 | 7.9 | 260 | ≥ 1000 | nd |
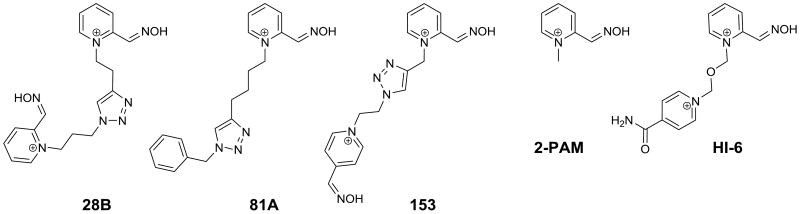
Figure 1.
Structures of oxime AChE reactivators used in this study.
Counterion for all compounds was chloride except methiodide for 2PAM.
Figure 2.
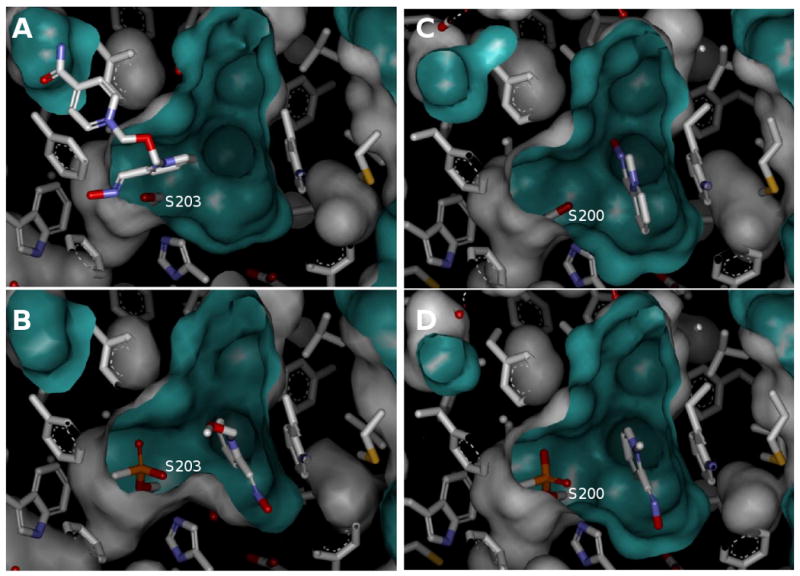
Reversible complexes of 2PAM and HI6 with native and aged AChE. Crystal structures of HI6 bound to the active center gorge of A) native mouse AChE (pdb ID 2GYU, [11]) and B) aged mouse AChE at S203 (pdb ID 2WHQ, [12]). Crystal structures of 2PAM bound to the base of the active center gorge of C) native Topedo californica AChE (pdb ID 2VQ6, [13]) and D) aged Topedo californica AChE at S200 (pdb ID 2WG1, [14]). The oxime structures are shown as a stick model. The AChE active center gorge is represented by Connolly solvent accessible surface.
Kinetics of oxime binding to native and OP conjugated hAChE was also studied, but only for hAChE*oxime complexes with Kd values lower than 8 μM, since the approach to equilibrium for other, weaker complexes could not be resolved in the stopped-flow apparatus. The second order association rate constants were therefore determined for binding of three triazole oximes with native, VX inhibited and aged hAChE. The constants varied between 1.2 and 7.7 × 109 M-1min-1, characteristic for protonated ligands such as tacrine and 9-aminoacridine, and slower than quaternary ligands carrying a permanent positive charge [6]. Since oximes 28B and 153 are bisquaternary and 81A monoquaternary, permanently charged ligands, their slower association rates could be attributed to the influence of deprotonated oxime moieties. These oximes thus may associate with hAChE with their oxime groups deprotonated and positioned for nucleophilic attack on the conjugated phosphorus atom. On the other hand static, structural data obtained typically in crystallization media of pH lower than 7.0 point to possibility of hydrogen bond formation between oxime and catalytic triad His [12,14] suggesting that at pH ≤ 7 oximes bind to OP conjugated AChE in their protonated state. The association rate constants of oximes 28B and 81A show modestly diminished rates for aged and VX-inhibited hAChE. The influence of decrease in the available binding space in the hAChE active center gorge on ligand association kinetics was also manifested in the association rate constants of 9-aminoacridine (Table 3).
Table 3.
Interaction kinetics of 9-aminoacridine with native and OP-conjugated hAChE. The second order association constants (k1) were determined as described in Material and Methods section. The calculated standard deviation was lower than of 30% of the constant values.
| hAChE |
k1 (109 M-1min-1) |
|---|---|
| native | 6.6 |
| aged | 4.9 |
| VX-inhibited | 1.6 |
| POX-inhibited | 1.6 |
| CS-inhibited | 1.9 |
In summary results of our study indicate that conjugation of hAChE with large OPs results in altered geometry of the active center gorge and compromised binding of oxime reactivators leading to loss of their reactivating potency. Dealkylation of OP-conjugated hAChE resulting in aged hAChE enhanced oxime binding, but according to structural studies, in completely nonproductive orientation for reactivation. The electrostatic interactions between anionic aged hAChE and cationic oximes thus resulted in further stabilization rather than in destabilization and breakdown of otherwise nonreactivatable aged hAChE.
Table 2.
Interaction kinetics of oximes with native and OP-conjugated hAChE. The second order association constants (k1) were determined as described in Material and Methods section. The calculated standard deviation was lower than of 30% of the constant values.
| k1 (109 M-1min-1) | |||
|---|---|---|---|
| oxime | h AChE | ||
| native | aged | VX-inhibited | |
| 28B | 5.9 | 5.6 | 3.8 |
| 81A | 6.9 | 3.1 | - |
| 153 | 1.2 | 1.9 | 7.7 |
Acknowledgments
Supported by U01 NS58046 grant to PT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson IB, Bergmann F, Nachmansohn D. Acetylcholinesterase. X. Mechanism of the catalysis of acylation reactions. J Biol Chem. 1950;186:781–790. [PubMed] [Google Scholar]
- 2.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Q J Med. 2000;93:715–731. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 3.Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, Nguyen J, Udunka S, Walden D, Walker RD, Weerasekera G, Whitehead RD, Jr, Schober SE, Needham LL. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res. 2005;99:314–326. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 5.Amitai G, Adani R, Yacov G, Yishay S, Teitlboim S, Tveria L, Limanovich O, Kushnir M, Meshulam H. Asymmetric fluorogenic organophosphates for the development of active organophosphate hydrolases with reversed stereoselectivity. Toxicology. 2007;233:187–198. doi: 10.1016/j.tox.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Radić Z, Taylor P. Interaction kinetics of reversible inhibitors and substrates with acetylcholinesterase and its fasciculin 2 complex. J Biol Chem. 2001;276:4622–4633. doi: 10.1074/jbc.M006855200. [DOI] [PubMed] [Google Scholar]
- 7.Bourne Y, Radic Z, Sulzenbacher G, Kim E, Taylor P, Marchot P. Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding. J Biol Chem. 2006;281:29256–29267. doi: 10.1074/jbc.M603018200. [DOI] [PubMed] [Google Scholar]
- 8.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 9.Grosfeld H, Barak D, Ordentlich A, Velan B, Shafferman A. Interactions of oxime reactivators with diethylphosphoryl adducts of human acetylcholinesterase and its mutant derivatives. Mol Pharmacol. 1996;50:639–649. [PubMed] [Google Scholar]
- 10.Shi J, Boyd AE, Radic Z, Taylor P. Reversibly bound and covalently attached ligands induce conformational changes in the omega loop, Cys69-Cys96, of mouse acetylcholinesterase. J Biol Chem. 2001;276:42196–42204. doi: 10.1074/jbc.M106896200. [DOI] [PubMed] [Google Scholar]
- 11.Ekström F, Pang YP, Boman M, Artursson E, Akfur C, Börjegren S. Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: structural basis for differences in the ability to reactivate tabun conjugates. Biochem Pharmacol. 2006;72:597–607. doi: 10.1016/j.bcp.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Ekström F, Hörnberg A, Artursson E, Hammarström LG, Schneider G, Pang YP. Structure of HI-6*sarin-acetylcholinesterase determined by X-ray crystallography and molecular dynamics simulation: reactivator mechanism and design. PLoS One. 2009;4:e5957. doi: 10.1371/journal.pone.0005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harel M, Silman I, Sussman JL. The Structure of Torpedo Californica Acetylcholinesterase Complexed with 2-Pam. PDB id 2VQ6 2008 [Google Scholar]
- 14.Sanson B, Nachon F, Colletier JP, Froment MT, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime. J Med Chem. 2009;52:7593–7603. doi: 10.1021/jm900433t. [DOI] [PubMed] [Google Scholar]