Abstract
The understanding of how environmental exposures interact with genetics in central nervous system dysfunction has gained great momentum in the last decade. Seminal findings have been uncovered in both mammalian and non-mammalian model in large result of the extraordinary conservation of both genetic elements and differentiation processes between mammals and non-mammalians. Emerging model organisms, such as the nematode and zebrafish have made it possible to assess the effects of small molecules rapidly, inexpensively, and on a miniaturized scale. By combining the scale and throughput of in vitro screens with the physiological complexity and traditional animal studies, these models are providing relevant information on molecular events in the etiology of neurodegenerative disorders. The utility of these models is largely driven by the functional conservation seen between them and higher organisms, including humans so that knowledge obtained using non-mammalian model systems can often provide a better understanding of equivalent processes, pathways, and mechanisms in man. Understanding the molecular events that trigger neurodegeneration has also greatly relied upon the use of tissue culture models.
The purpose of this summary is to provide-state-of-the-art review of recent developments of non-mammalian experimental models and their utility in addressing issues pertinent to neurotoxicity (Caenorhabditis elegans and Danio rerio). The synopses by Aschner and Levin summarize how genetic mutants of these species can be used to complement the understanding of molecular and cellular mechanisms associated with neurobehavioral toxicity and neurodegeneration. Next, studies by Suñol and Olopade detail the predictive value of cultures in assessing neurotoxicity. Suñol and colleagues summarize present novel information strategies based on in vitro toxicity assays that are predictive of cellular effects that can be extrapolated to effects on individuals. Olopade and colleagues describe cellular changes caused by sodium metavanadate (SMV) and demonstrate how rat primary astrocyte cultures can be used as predicitive tools to assess the neuroprotective effects of antidotes on vanadium-induced astrogliosis and demyelination.
Keywords: Caenorhabditis elegans, zebrafish, tissue culture, neurotoxicity, astrocyte, vanadium, in vitro
C. Elegans as an Alternative Model in Toxicology
Caenorhabditis elegans in Neurotoxicology Studies
The search for experimental models that allow live in vivo analysis in the toxicological field has led to the use of several invertebrate organisms. Caenorhabditis elegans (C. elegans) has demonstrated great advantages in providing insights into the mechanisms of toxicity and neuronal injury. The codification of the complete genome revealed the extraordinary conservation of its genome with mammals (60–80% homology) [1] and studies indicating that worms and mammals share similar biosynthetic and metabolic pathways provide justification for its relevance in the toxicological field.
C. elegans has a small size (adults are ~1 mm long), ease of maintenance, ability to be frozen and stored, speedy generation time (3 days), short life span, and large brood size (>300 progeny per hermaphrodite). All these features provide a limitless supply of worms for cellular, molecular, and genetic analyses. The transparency of C. elegans along with the ease of constructing reporter gene fusions facilitates the visualization of neuronal morphology and protein expression patterns within the living system. Furthermore, the intensely studied genome, complete cell lineage map, knock-out libraries, and established genetic methodologies, including mutagenesis, RNAi and transgenesis, provide a variety of options for manipulating and performing molecular analyses in C. elegans.
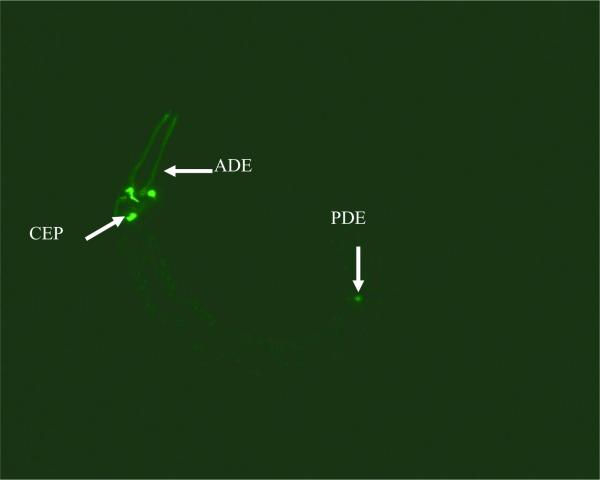
At its first inception, the C. elegans platform was developed as an experimental model to study nervous system development [2]. A complete three-dimensional map of the 302-cell nervous system provides the resources for identifying most synapses between neurons [3] and green fluorescent protein (GFP) reporter strains have been generated to allow for the observation of different neuronal populations, for example the dat-1∷GFP fusion allows for the observation of all 8 dopaminergic neurons in the worm (Figure 1). The extensive knowledge of the nervous system has been exploited in the investigation of C. elegans and has allowed for the study of various neurodegenerative disorders, such as Duchenne muscular dystrophy (DMD), Parkinson's disease, Huntington's disease and Alzheimer's disease. An example is the DYSTROPHIN gene, responsible for DMD, which is conserved in C. elegans and the study of mutants has revealed progressive muscle degeneration [4]. Exposure of C. elegans to 1-methyl-4-phenylpyridinium (MPP+) [5] or 6-hyrdroxydopamine (6-OHDA) [6] mimics Parkinson's disease as both of these induce damage to the dopaminergic nervous system. Expression of polyQ Huntingtin variants in C. elegans has led to discoveries revealing gene interactions with Huntingtin, axonal defects and protein aggregation, thereby furthering our understanding of Huntington's disease [7,8]. The investigation into Alzheimer's disease has been advanced with mutant worms expressing human beta-amyloid precursor protein (betaAPP) or TAU, allowing researchers to develop insights into disease progression and discover how other genes are involved [9].
Figure 1.
DA neurons visualized in adult C. elegans with DAT-1∷GFP transcriptional fusions. Arrows point to cephalic sensilla (CEP), anterior deirids (ADE) and posterior deirids (PDE) neurons.
C. elegans has also been used as a model to elucidate the toxicity and toxicological mechanisms of a variety of metals [10]. The toxicity of aluminum, arsenic, barium, cadmium, copper, chromium, lead, mercury, uranium, and zinc has been investigated in the C. elegans model system, in which behavioral, morphological, and developmental alterations were observed upon toxicant exposure [11–13]. For instance, feeding behavior, thrashing, and pharyngeal pumping are behavioral endpoints that allow examination of the involvement of specific neuronal networks affected by different metals and studies have indicated a decrease in these endpoints. Various GFP reporters have been used to demonstrate changes in expression. For example, metallothioneins are upregulated upon exposure to cadmium [14].
Paraquat, rotenone, organophosphates, and other pesticides have also been assessed in the C. elegans model, enlightening researchers to the novel neurotoxic mechanisms through which these agents exert their effects [11]. Strains of C. elegans have been generated that have either increased [15] or decreased [16] sensitivity to paraquat. Although the identities of many of the proteins encoded by the genes that cause these alterations are yet unknown, researchers have found that alterations in antioxidants such as superoxide dismutase can cause alterations in the response of C. elegans to paraquat. Specifically, it has been found that mutants that lack these enzymes display increased sensitivity [17] while mutants that overexpress these enzymes display decreased sensitivity [18,19].
C. elegans is an ideal model to use to address the increased interest in the use of high-throughput approaches to reproducibly and efficiently screen various chemicals. High-throughput screening tools in C. elegans studies include computer tracking software to assess behavior and biosorters and microfluidics to manipulate large numbers of worms with great speed, efficiency, and precision [20,21].
In summary, C, elegans is a valuable model for use in biological research, especially toxicology. The use of worms in the toxicological field demonstrates great potential for revealing toxicity mechanisms which elude investigators using other models. The findings of numerous C. elegans studies have been reported and reviewed for the scientific community [11,21,22]. Understanding mechanisms of toxicity will help to elucidate ways in which new therapeutics can be used to mitigate the adverse health effects of various toxicants.
Behavioral Analysis of Xenobiotics in Zebrafish
Introduction
Zebrafish can provide a useful complementary model for developmental neurotoxicology to provide important mechanistic and screening information in combination with in vitro cell-based models, invertebrate models such as c. Elegans and drosophila as well as classic mammalian rodent models and epidemiological studies. Zebrafish have a rich array of tools with which determine the molecular biology of neurodevelopment and mal-development. There are a great many zebrafish mutants with which to determine key genomic factors for the developmental process. The use of morpholino techniques to transiently suppress specific molecular components during early development is particularly powerful in studies of developmental neurotoxicology. In addition, since zebrafish have a clear chorion reporter systems can be used to highlight individual components of the nervous system nervous system development can be visualized continuously and the impact of developmental neurotoxicants on developing neural processes can be determined. These elegant tools to study molecular and cellular processes in a temporally and anatomically intact model can be powerful for determining critical mechanisms of neurobehavioral toxicity if valid, sensitive, reliable and efficient measures of neurobehavioral function can be developed.
Recently, a variety of laboratories including ours have developed tests of learning and memory, stress response and sensorimotor reactivity for zebrafish, which are sensitive to drug and toxicant effects, similar to those seen in classic rodent assays and humans. The neurobehavioral test battery developed to date in our laboratory includes tests of swimming activity in newly hatched fish, and in adults' sensorimotor response, exploratory behavior and stress response as well as learning and memory. These tests have proven to be sensitive to toxicant and pharmacological effects and with some the efficiency of the tests are enhanced by automated computerized video analysis.
Behavioral Analyses in Zebrafish
Swimming Activity in Newly Hatched Fish
Spontaneous swimming activity can be quantified in zebrafish during the early period after hatching for a rapid read on behavioral consequences of developmental toxicant exposure. We found that low-dose (0.029 and 0.29 μM, 10 and 100 ml/l) exposure during the first five days after fertilization to the organophosphate pesticide chlorpyrofos caused significant reductions in locomotor activity in young zebrafish tested on days 6 and 9 after fertilization [23]. That study used hand scoring of videotapes of the fish swimming activity in a three-minute session. More recently we have reproduced the same result using a computerized video tracking system (Noldus EthoVision, Wageningen, The Netherlands).
Sensorimotor Response
A rapid, intense sensory stimulus will in most species produce a sudden motor reaction. The same is true in zebrafish. We have developed a test in which a sudden tactile stimulus from a tap produced by a solenoid on the bottom of the test environment produces a flurry of swimming activity in the fish, an effect, which shows habituation or lessened response with repeated exposure. This sensorimotor response test has proven to be sensitive to the persisting effects of developmental exposure to the organophosphate pesticide chlorpyrifos. Since chlorpyrifos inhibits acetylcholinesterase (AChE) and thus acts as an indirect cholinergic agonist we also tested the effects of developmental exposure to direct cholinergic agonists for nicotinic and muscarinic acetylcholine receptors nicotine and pilocarpine [24]. All three of these treatments during the first five days after fertilization produce significant increase in sensorimotor response when the fish were later tested as adults but chlorpyrifos was effective at a much lower dose (0.29 μM) than either nicotine (15 μM) or pilocarpine (1,000 μM) [24].
Exploratory Behavior and Stress Response
When introduced into a novel environment zebrafish will dive to the bottom of the tank in what is likely a predation escape response. Gradually, over time the fish swim increasingly to the higher levels of the tank [25]. This effect is specific to the fish being introduced into a novel tank. Placement into a familiar tank does not produce this effect [26]. The novel tank diving response is reduced by anxiolytic drugs diazepam and buspirone, but not by chlordiazepoxide [26]. Nicotine, which has been shown to have anxiolytic effects in mammals including humans also significantly reduces the novel tank diving response, an effect which is reversed by co-administration of nicotinic antagonists [25,27]. Recently, we have found that 0.29 μM of chlorpyrifos during the first five days after fertilization caused a significant increase in swimming activity in the novel tank diving task as well as significantly less of the normal diving response when the fish were tested as adults after early developmental exposure [26].
Learning and Memory
Zebrafish learn spatial and color discrimination as well as spatial alternation as assessed in a three-chamber test tank we have developed [23,28]. Nicotine significantly improves both spatial learning and memory in this test paradigm [23,24,29]. The nicotine-induced learning improvement has been shown to be related to increases in brain dopamine activity [24]. The nicotinic antagonist mecamylamine blocks both the nicotine-induced learning improvement and the nicotine-induced increase in dopamine metabolite [24].
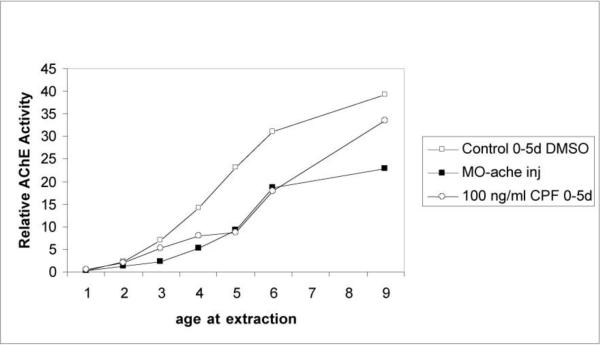
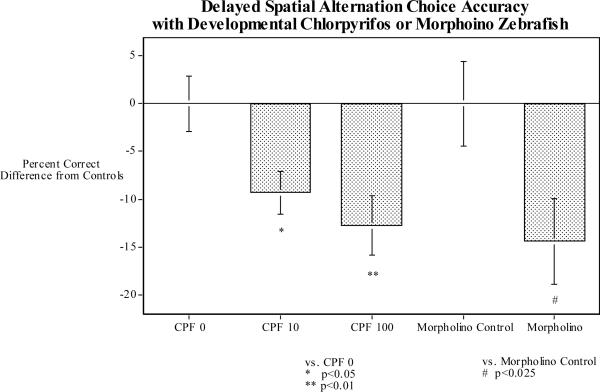
With regard to developmental neurotoxicity, we have found that 0.029 or 0.29 μM of chlorpyrifos in the tank water of zebrafish for the first five days after fertilization causes long-term impairment in memory assessed by the delayed spatial alternation task when the fish were tested as adults [30]. These chlorpyrifos doses had a biphasic effect on swimming speed in the memory test with the lower dose significantly slowing response speed and the higher dose causing significant hyperactivity. To test the impact of inhibition of the AChE inhibition on these behavioral functions we produced a zebrafish morpholino, which roughly matched the degree of AChE inhibition by 0.29μM of CPF in the tank water for the first five days after fertilization (Figure 2). When these fish and their controls were tested in the three-chamber spatial alternation task when they were adults, the AChE morpholino fish had a significant impairment in memory relative to controls, quite similar to the chlorpyrifos exposed fish (Figure 3) indicating that it was the inhibition of AChE by chlorpyrifos which was sufficient to produce the memory impairment. Interestingly, the effect of the AChE morpholino on response speed did not match the effect of 0.29 μM of chlorpyrifos. The morpholino matching the same degree of AChE inhibition as 0.29 μM of chlorpyrifos did not produce hyperactivity as did 0.29 μM chlorpyrifos. If anything there was a slight reduction in speed.
Figure 2.
Effects on acetylcholinesterase activity of 0.29μM (100 ng/ml) of chlorpyrifos for five days post-fertilization and AChE morpholino.
Figure 3.
Effects on delayed spatial alternation choice accuracy of 0.29μM (100 ng/ml) of chlorpyrifos for five days post-fertilization [30] and AChE morpholino, percent correct difference from control mean (mean ± SEM).
These studies show that zebrafish models can provide useful neurobehavioral information concerning the potentially adverse effects of developmental toxicant exposure. Zebrafish can serve as a translational vertebrate model between cell-based and invertebrate and mammalian models providing screening of compounds as well as mechanistic information concerning developmental neurobehavioral toxicity. The development of a neurobehavioral test battery for zebrafish will bring these advantages to bear for functional neurotoxicity.
A Way Forward for Using In Vitro Neurotoxicity Models in Acute Toxicity Testing Strategy: Update on the FP6 Integrated Project ACuteTox
Introduction
Toxicity risk assessment for chemical-induced human health hazards relies mainly on data obtained from animal experimentation, human studies and epidemiology. Accepted toxicity testing since 2001 includes regulations that take into account the concepts of Refining and Reducing animal experimentation: the Fixed Dose Procedure (FDP, TG 420), the Acute Toxic Class Method (ATCM, TG 423), and the Up-and-Down Procedure (UDP, TG 425). In 2007, the US National Academies proposed that toxicity testing in the 21st century should take advantage of the understanding of “toxicity pathways” (the cellular response pathways that underlie adverse health effects when they are sufficiently perturbed). Therefore, they recommend using “a predictive strategy based on in vitro toxicity assays which predict cellular level effects that can be extrapolated to effects on individuals” (NRC, 2007). In 2005, the European Union supported the ACuteTox project, which has set the very ambitious overall objective of developing an in-vitro test strategy sufficiently robust and powerful to replace in-vivo testing for predicting acute toxicity of chemicals. The ACuteTox project aims to improve the previously estimated correlation between in vitro basal cytotoxicity and rodent LD50 values [31], to a level sufficient enough to ensure a valid prediction of human acute toxicity. The core of the ACuteTox project is composed from the generation of a high quality in vivo data base, the generation of a high quality in vitro data base, and the compilation of cell systems and endpoints. In vitro data include those obtained in cytotoxicity assays and in specific in vitro/in silico assays for biokinetics, metabolism and organ toxicity (hepatotoxicity, nephrotoxicity and neurotoxicity) [32]. All this information is stored in a data base that will be publicly available on completion of the project. About 60 reference chemicals including pesticides, pharmaceuticals and industrial chemicals, for which data on their acute human toxicity do exist, have been tested. The linear regression analysis between cytotoxicity data (Neutral red uptake (NRU) assay in the mouse fibroblast 3T3 cell line) and human lethal blood concentration (LC50) gave an explained variance R2 = 0.56 for the reference chemicals [33]. It is foreseen that the integration of biokinetic, metabolism and organ specific toxicity data will provide alerts (against the exclusive use of cytotoxicity data) and correctors (algorithms to be used) for the efficient use of an in vitro-based testing strategy for predicting human acute toxicity.
The ACuteTox Project as a Predictive Tool for Neurotoxicity Assessment
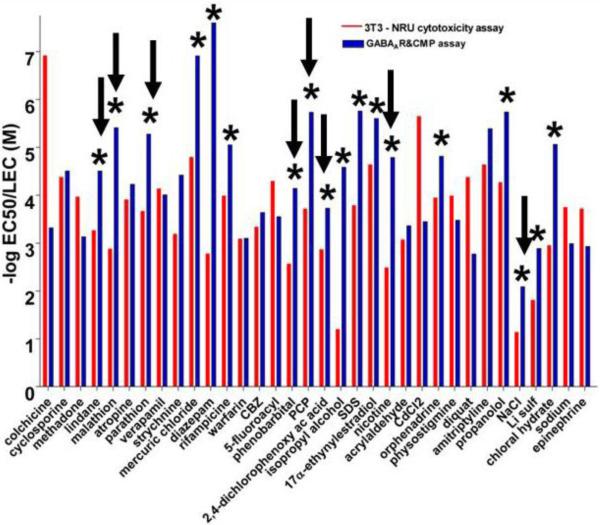
The nervous system is particularly vulnerable to chemical exposure; its complexity results in multiple potential target sites with different toxicity features. Acute human toxicity related to adverse neuronal function is mainly a result of over-excitation or depression of the peripheral or central nervous system (CNS). The major molecular and cellular mechanisms involved in such effects include GABAergic, glutamatergic and cholinergic neurotransmission, regulation of cell and mitochondrial membrane potential, and those critical for maintaining CNS functionality, such as controlling cell energy. Up to 57 neural endpoints, including those related with the above mechanisms and others that specifically identify neural types (neurons, astrocytes and microglia) or a cascade of multiple targets (induction of stress and apoptotic gene biomarkers), have been determined in primary cultured rodent neurons, rodent brain cell aggregates and human neuronal cell lines. Unless otherwise cited, this presentation summarizes published results from the ACuteTox project [34,35]. Cell viability in the neural systems after exposure to the agents for 24 – 48 hours did not differ from that in non neural cell lines, suggesting that the neuronal cytotoxicity model did not correct the outlier chemicals found in the NRU-3T3 cytotoxicity assay versus human LC50 correlation [34]. 28 out of 58 tested chemicals were recognized by the GABAA receptor (GABAAR) assay (a radiometric assay determining 36Cl− influx in primary cultured neurons) and 10 out of 23 chemicals were recognized by the GABA transport assay (a radiometric assay measuring [3H]GABA uptake in primary cultured neurons). In contrast, other neurochemical assays (fluorescence assays for acetylcholine and glutamate receptor function, spectrophotometric assay for acetylcholinesterase activity and radiometric/chromatographic assay for glutamate transport) were very specific and recognized few compounds from the reference list. A more general assay for assessing neuronal excitability (a fluorescence-based assay measuring changes in cell membrane potential (CMP) in the human neuronal cell line SH-SY5Y) recognized 22 out of 58 chemicals. The combination of the CMP and GABAAR assays generated alerts (the neurotoxic endpoints were more sensitive than the cytotoxic endpoint) for outliers (Figure 4) and improved the in vitro predictability of human acute toxicity. The R2 values for the correlation of in vitro data vs. human LC50 values were estimated to 0.57 for only the general cytotoxicity data and 0.64 when considering cytotoxicity data in combination with alerts identified from the CMP+GABAAR assays. By using appropriate agonists and pharmacological agents, the GABAAR and the CMP assays may cover most of the membrane-based neurotoxicity pathways. As an advantage for the use of these in vitro testing of neural endpoints, both the GABAAR and the CMP assays were performed in a short period of time (10 and 2 minutes, respectively). However, the drawback of using a highly energetic radionuclide in the GABAAR assay should be overcome by the use of less energetic isotopes or even, non radiometric techniques.
Figure 4.
Neurotoxicity alerts determined by the combination of the GABAAR and cell membrane potential assay (CMP). Blue bars represent the lowest EC50/LEC values for the GABAAR assay (performed in primary neuronal cultures) or the CMP assay (performed in SH-SY5Y cells) after acutely exposure to the reference chemicals. Red bars represent IC50 values for the NRU cytotoxicity test (performed in 3T3 cells) from Sjöström et al. (2008). Asterisks indicate neurotoxic alerts (significant effects on the GABAAR/CMP assays) were produced at concentrations at least one order of magnitude lower than those producing cytotoxicity. Arrows indicate outlier compounds in the NRU-3T3 cytotoxicity assay vs human LC50 values correlation.
In addition to the GABAAR and CMP assays, genomic and metabolic endpoints were analyzed in a multi-endpoint assay after exposure to rodent brain cell aggregate cultures and the expression of the stress gene marker caspase 3 was determined in primary cultured neurons. The multi-endpoint determining changes in the mRNA of NF-H, GFAP, MBP and HSP32, as well as in the total mRNA level and glucose consumption in the brain cell aggregate cultures that were exposed for 48 hours to the tested chemicals resulted to be the most sensitive endpoints producing alerts for severe outliers like digoxin, lindane, malathion and parathion. Furthermore, the caspase-3 mRNA expression gave a good estimate of the human LC50 for a set of the chemicals tested, correcting severe outliers like atropine, digoxin and malathion [34].
Conclusions
In conclusion, the assays developed in ACuteTox project have provided alerts for all the outliers evolved from the cytotoxicity – human LC50 correlation. The combined genomic and metabolic biomarkers analyzed in the brain cell aggregate cultures were very sensitive endpoints, generating most alerts. The combination of the GABAAR and CMP assays identified correct outliers and improved the prediction of human lethal toxicity. These two endpoints cover most of the membrane-based neurotoxicity. As a whole, it is foreseen that the ACuteTox project will yield “alert” tests to indicate when a cytotoxicity-based test result may not be valid and “corrector” tests to improve the predictive capabilities of a test strategy. A capacity to indicate when a cytotoxicity-based in vitro approach may be insufficient and that additional testing is required or that a certain compound is beyond its predictive capabilities represents an important step forward for in vitro methods.
Erythropoietin, Desferroxamine and Tiron are Protective against Vanadium-Induced Demyelination and Oxidative Stress in the Rat Brain
Introduction
Environmental pollution from fossil fuel burning and the subsequent release of finely particulate vanadium compounds in the Arabian gulf [36] and Nigeria's Niger Delta [37]has been on the increase with minimal studies done on both the short and long term effects on the brain. Hypomyelination and or myelin destruction from lipid peroxidation has been implicated in part to be responsible for the phenotypic expressions of neuromuscular and behavioral deficits seen in vanadium toxicosis [38]. The present study assessed novel cellular changes caused by sodium metavanadate (SMV) in astrocyte culture cells and the neuroprotective effects of antidotes desferroxamine (DFO), tiron and erythropoietin (EPO) on vanadium-induced astrogliosis and demyelination.
Studies on the in vitro and in vivo Neurotoxicity of Vanadium
The effects of vanadium treatment (150 μM for 6 hours) on reactive oxygen species (ROS) generation, cellular hypoxia and erythropoietin (EPO) expression were assessed in primary rat astrocytes cultures. ROS generation was determined with the dichlorofluorescein (DCF) assay [measured as relative fluorescence unit (RFU)]. Protein expression was determined by western blot (WB) analysis, using HIF-1 α (Hypoxia Induction Factor) and EPO antibodies (Santa Cruz). We also treated 15-day-old Sprague-Dawley rats (n=5) with 3mg/kg SMV for seven days; three groups were similarly treated, but were given DFO (300 mg/kg/day), tiron (606 mg/kg/day) or EPO (5,000 U/kg/day) as antidotes concurrently for six days (one day after vanadium treatment) with appropriate controls. Glial fibrillary acidic protein (GFAP) immunohistochemistry was observed in the corpus callosum while myelin basic protein (MBP) was quantified with WB analysis.
There was a significant increase in ROS generation in vanadate treated primary astrocyte cultures compared with controls (Figure 5) including up-regulation in HIF-1 α and EPO expressions as seen by WB analysis (Figure 6). There was also a significant increase in GFAP immunoreactivity in the corpus callosum (Figure 7) and a decrease in MBP quantification in brains of vanadium treated rats compared to controls (Figure 8); these observations were significantly (P<0.05) attenuated in rats receiving the antidotes DFO and EPO, or tiron and EPO.
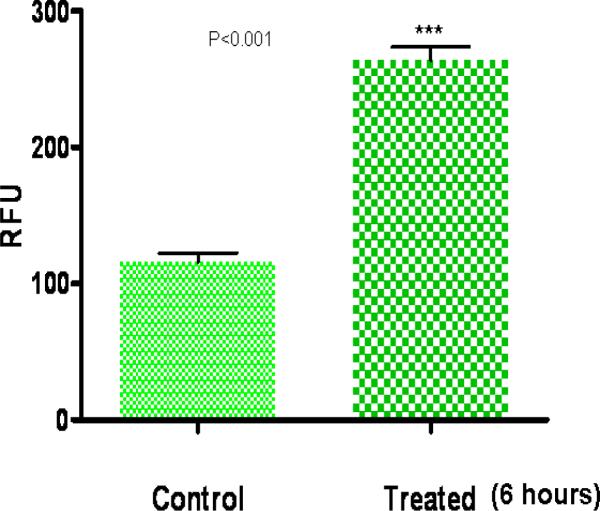
Figure 5.
Analysis of intracellular reactive oxygen (ROS) generation in primary rat astrocytes after vanadium treatment for six hours using the dichlorofluorescein dye. Vanadium treatment resulted in significant increase in detectable ROS generation (P<0.001).
Figure 6.
Primary astrocytes in culture were treated with sodium metavanadate (150 μM) for six hours. Total cellular protein extracts were obtained and transferred to nitrocellulose membranes which were separately incubated with antibodies against HIF-1α (120 kDa) and EPO (37 kDa) (Santa Cruz). Results show up-regulation of HIF-1α (left block) and EPO (right block) when compared to their respective controls.
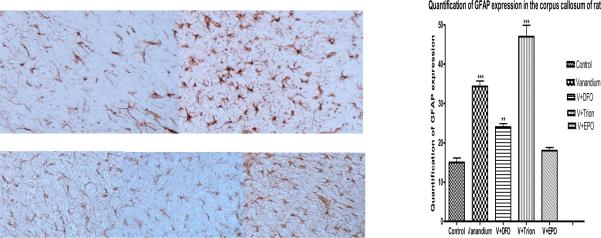
Figure 7.
Treatment of rat cells 3mg /kg of SMV for seven days caused increased GFAP expression (top right) compared to controls (top left). The staining caused by vanadate in was markedly attenuated by antidotes DFO and EPO (bottom left and right respectively) but not by tiron (bottom center).
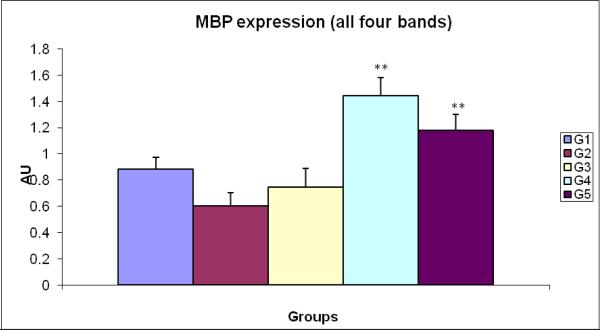
Figure 8.
Treatment of rat with3mg /kg of SMV for seven days led to decreased myelin content (G2) compared to controls (G1). Antidotes tiron and EPO (G4and5) when administered with vanadate caused a significant increase in myelin quantity compared to treated group but not the case with DFO (G3).
In this study, vanadium induced the production of reactive oxygen species (ROS); this causes the cells to undergo hypoxia, the onset of which leads to vanadate-induced expression of HIF-1α [39]]. In other cells, such as the kidney cells and prostate cancer; HIF 1 α upregulation leads to increased EPO expression [40]. EPO has been shown to be expressed in the brain [41]; in mammalian tissue, discrepancy between oxygen supply and utilization leads to hypoxia which induces a variety of specific adaptation mechanisms including the activation of HIF, which, in turn, has a modulatory role on hypoxically-related genes, such as those encoding for EPO [42]. In ischemia, the de novo up regulation of EPO in the brain creates a protective effect against neuronal injury. Though vanadium causes neuronal pathologies and neurobehavioral deficits [38] in vivo, induces oxidative stress and cellular hypoxia in brain cell cultures [43]. The role EPO plays at the cellular, local and systemic level during vanadium neurotoxicosis remains unknown. Our work has shown that vanadium induced HIF-1 α expression can lead to EPO upregulation in primary astrocytes which is indicative of an endogenous protective mechanism in the neuroglia. This current work also shows that systemic administration of antidotes can modulate vanadium-induced neuropathologies. The increased expression of GFAP seen after vanadium treatment was significantly attenuated by the antidotes DFO and EPO, but not by tiron, while the reduced MBP expression caused by vanadium administration was significantly reversed by tiron and EPO, indicating that EPO was effective in both instances. These actions of EPO are consistent with the fact that EPO has anti-inflammatory and antioxidative effects, and prevents lipid peroxidation [44]. While in theory, it is expected that vanadium entry into the brain will lead to increased generation of ROS, which will concurrently be responsible for increased GFAP expression, lipid peroxidation and then demyelination, the fact that DFO in vivo was able to attenuate the increased GFAP expression after vanadate treatment, but not the demyelinating process and vice-versa for tiron may be indicative of other independent pathways between lipid peroxidation and demyelination caused by vanadate neurotoxicosis.
Conclusions
In conclusion, this work demonstrates the utility of primary astrocyte cultures in screening the effects of vandate on cellular responses, as well as novel information on vandate's ability to induce increased expression of EPO in astrocyte cell cultures and that antidotes, such as exogenous EPO can attenuate astrogliosis and demyelination caused by vanadate neurotoxicosis.
Acknowledgements
This review was supported in part by PHS grant NIEHS R01 ES10563 (MA), the Duke University Superfund Basic Research Center NIEHS ES010356 (EDL). Sunol and Forsby wish to acknowledge the contribution by Joan Albert Vericat and the group at Neuropharma (Spain), Victor Rimbau and the group at the University of Barcelona (Spain), Paul Honegger and the group at the University of Lausanne (Switzerland), Sandra Coecke and the group at ECVAM (Italy) and the signed authors and the groups at the CSIC (Spain) and Stockholm University (Sweden). Partial support was also provided by IBRO Research Fellowship (JOO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–98. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- [2].Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hobert O. Specification of the nervous system. WormBook. 2005:1–19. doi: 10.1895/wormbook.1.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gieseler K, Grisoni K, Segalat L. Genetic suppression of phenotypes arising from mutations in dystrophin-related genes in Caenorhabditis elegans. Curr Biol. 2000;10:1092–7. doi: 10.1016/s0960-9822(00)00691-6. [DOI] [PubMed] [Google Scholar]
- [5].Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC. Caenorhabditis elegans MPP+ model of Parkinson's disease for high-throughput drug screenings. Neurodegener Dis. 2004;1:175–83. doi: 10.1159/000080983. [DOI] [PubMed] [Google Scholar]
- [6].Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:3264–9. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holbert S, Dedeoglu A, Humbert S, Saudou F, Ferrante RJ, Neri C. Cdc42-interacting protein 4 binds to huntingtin: neuropathologic and biological evidence for a role in Huntington's disease. Proc Natl Acad Sci U S A. 2003;100:2712–7. doi: 10.1073/pnas.0437967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parker JA, Connolly JB, Wellington C, Hayden M, Dausset J, Neri C. Expanded polyglutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death. Proc Natl Acad Sci U S A. 2001;98:13318–23. doi: 10.1073/pnas.231476398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boyd-Kimball D, Poon HF, Lynn BC, Cai J, Pierce WM, Jr., Klein JB, Ferguson J, Link CD, Butterfield DA. Proteomic identification of proteins specifically oxidized in Caenorhabditis elegans expressing human Abeta(1–42): implications for Alzheimer's disease. Neurobiol Aging. 2006;27:1239–49. doi: 10.1016/j.neurobiolaging.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [10].Berkowitz LA, Hamamichi S, Knight AL, Harrington AJ, Caldwell GA, Caldwell KA. Application of a C. elegans dopamine neuron degeneration assay for the validation of potential Parkinson's disease genes. J Vis Exp. 2008:ii, 835. doi: 10.3791/835. doi: 10.3791/835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Helmcke KJ, Syversen T, Miller DM, 3rd, Aschner M. Characterization of the effects of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2009 doi: 10.1016/j.taap.2009.03.013. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guo Y, Yang Y, Wang D. Induction of reproductive deficits in nematode Caenorhabditis elegans exposed to metals at different developmental stages. Reprod Toxicol. 2009;28:90–5. doi: 10.1016/j.reprotox.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [14].Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicology & Teratology. 2004;26:719–23. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- [15].Ishii N, Takahashi K, Tomita S, Keino T, Honda S, Yoshino K, Suzuki K. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutat Res. 1990;237:165–71. doi: 10.1016/0921-8734(90)90022-j. [DOI] [PubMed] [Google Scholar]
- [16].Fujii M, Tanaka N, Miki K, Hossain MN, Endoh M, Ayusawa D. Uncoupling of longevity and paraquat resistance in mutants of the nematode Caenorhabditis elegans. Biosci Biotechnol Biochem. 2005;69:2015–8. doi: 10.1271/bbb.69.2015. [DOI] [PubMed] [Google Scholar]
- [17].Anantharam V, Lehrmann E, Kanthasamy A, Yang Y, Banerjee P, Becker KG, Freed WJ, Kanthasamy AG. Microarray analysis of oxidative stress regulated genes in mesencephalic dopaminergic neuronal cells: relevance to oxidative damage in Parkinson's disease. Neurochem Int. 2007;50:834–47. doi: 10.1016/j.neuint.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech Ageing Dev. 2002;123:1579–87. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- [19].Burmeister C, Luersen K, Heinick A, Hussein A, Domagalski M, Walter RD, Liebau E. Oxidative stress in Caenorhabditis elegans: protective effects of the Omega class glutathione transferase (GSTO-1) FASEB J. 2008;22:343–54. doi: 10.1096/fj.06-7426com. [DOI] [PubMed] [Google Scholar]
- [20].Hulme SE, Shevkoplyas SS, Samuel A. Microfluidics: streamlining discovery in worm biology. Nat Methods. 2008;5:589–90. doi: 10.1038/nmeth0708-589. [DOI] [PubMed] [Google Scholar]
- [21].Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29:546–55. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Helmcke KJ, Avila DS, Aschner M. Utility of Caenorhabditis elegans in high throughput neurotoxicological research. Neurotoxicol Teratol. 2008 doi: 10.1016/j.ntt.2008.11.005. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- [23].Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicology and Teratology. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- [24].Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Developmental chlorpyrifos causes behavioral and neurochemical defects in zebrafish. Neurotoxicology and Teratology. 2009 doi: 10.1016/j.ntt.2009.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiology and Behavior. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- [26].Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology, Biochemistry and Behavior. 2009 doi: 10.1016/j.pbb.2009.07.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bencan Z, Levin ED. The role of α7 and α4β2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiology & Behavior. 2008;95:408–412. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arthur D, Levin ED. Spatial and non-spatial discrimination learning in zebrafish (Danio rerio) Animal Cognition. 2001;4:125–131. [Google Scholar]
- [29].Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- [30].Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicology & Teratology. 2003;25:51–7. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- [31].Ekwall B, Barile FA, Castano A, Clemedson C, Clothier RH, Dierickx P, Ekwall B, Ferro M, Fiskesjö G, Garza-Ocañas L, et al. MEIC Evaluation of Acute Systemic Toxicity: Part VI. The prediction of human toxicity by rodent LD50 values and results from 61 in vitro methods. Alter Lab Animals. 1988;26:617–658. [PubMed] [Google Scholar]
- [32].Clemedson C. The European ACuteTox project: a modern integrative in vitro approach to better prediction of acute toxicity. Clin Pharmacol Ther. 2008;84:200–2. doi: 10.1038/clpt.2008.135. [DOI] [PubMed] [Google Scholar]
- [33].Sjostrom M, Kolman A, Clemedson C, Clothier R. Estimation of human blood LC50 values for use in modeling of in vitro-in vivo data of the ACuteTox project. Toxicol In Vitro. 2008;22:1405–11. doi: 10.1016/j.tiv.2008.04.017. [DOI] [PubMed] [Google Scholar]
- [34].Forsby A, Bal-Price AK, Camins A, Coecke S, Fabre N, Gustafsson H, Honegger P, Kinsner-Ovaskainen A, Pallas M, Rimbau V, et al. Neuronal in vitro models for the estimation of acute systemic toxicity. Toxicol In Vitro. 2009 doi: 10.1016/j.tiv.2009.07.017. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- [35].Sunol C, Babot Z, Fonfria E, Galofre M, Garcia D, Herrera N, Iraola S, Vendrell I. Studies with neuronal cells: From basic studies of mechanisms of neurotoxicity to the prediction of chemical toxicity. Toxicol In Vitro. 2008;22:1350–5. doi: 10.1016/j.tiv.2008.03.009. [DOI] [PubMed] [Google Scholar]
- [36].Sasi MM, Haider SS, el-Fakhri M, Ghwarsha KM. Microchromatographic analysis of lipids, protein, and occurrence of lipid peroxidation in various brain areas of vanadium exposed rats: a possible mechanism of vanadium neurotoxicity. Neurotoxicology. 1994;15:413–20. [PubMed] [Google Scholar]
- [37].Igado OO, Olopade JO, Onwuka SK, Chukwudi AC, Daramola OA, Ajufo UE. Evidence of environmental pollution in caprine brains obtained from a relatively unindustrialized area in Nigeria. AJBR. 2008;11:305–9. [Google Scholar]
- [38].Soazo M, Garcia GB. Vanadium exposure through lactation produces behavioral alterations and CNS myelin deficit in neonatal rats. Neurotoxicol Teratol. 2007;29:503–10. doi: 10.1016/j.ntt.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [39].Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81:1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- [40].Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- [41].Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, A., Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–9. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–42. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- [43].Olopade JO, Madhankumar AB, Das A, Liu X, Todorich B, Liang JJ, Webb B, Connor JR. Vanadium a possible chemotherapeutic agent against astrocytoma. Proceedings of the 100th annual meeting of the American Association of Cancer.2009. p. 1344. [Google Scholar]
- [44].Siren AL, Ehrenreich H. Erythropoietin--a novel concept for neuroprotection. Eur Arch Psychiat Clin Neurosci. 2001;251:179–84. doi: 10.1007/s004060170038. [DOI] [PubMed] [Google Scholar]