Abstract
Background
Cognitive theory and empirical evidence both suggest that cognitive reactivity (the tendency to think more negatively when in a sad mood) is an important marker of depression vulnerability. Research has not yet determined whether genetic factors contribute to the expression of cognitive reactivity.
Methods
The present study examined associations between the 5-HTTLPR polymorphism of the SLC6A4 gene, the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene, and cognitive reactivity in a never depressed, unmedicated, young adult sample (N = 151).
Results
The interaction between 5-HTTLPR and Val66Met polymorphisms significantly predicted change in dysfunctional thinking from before to after a standardized sad mood provocation. Cognitive reactivity increased among S/LG 5-HTTLPR homozygotes if they were also homozygous for the Val Val66Met allele. In contrast, presence of a Met Val66Met allele was associated with attenuated cognitive reactivity among S/LG 5-HTTLPR homozygotes.
Limitations
The sample size of the current study is relatively small for modern genetic association studies. However, results are consistent with previous research demonstrating biological epistasis between SLC6A4 and BDNF for predicting connectivity among neural structures involved in emotion regulation.
Conclusions
The BDNF Met allele may protect S/LG 5-HTTLPR homozygotes from increased dysfunctional thinking following a sad mood provocation. Study results are the first to demonstrate an epistatic genetic relationship predicting cognitive reactivity and suggest the need for more complex and integrative models of depression vulnerability.
Keywords: 5-HTTLPR, BDNF, cognitive reactivity, depression vulnerability, genetics, serotonin transporter
Introduction
Cognitive theories of depression vulnerability (Beck, 1967) suggest that underlying negative beliefs and attitudes characterize individuals vulnerable to depression. Furthermore, these negative beliefs and attitudes may not be apparent when vulnerable individuals are in a euthymic state, but will be best observed in the context of sad mood states (Segal & Ingram, 1994; Teasdale, 1988). The increase in dysfunctional thinking from euthymic to dysphoric mood is termed cognitive reactivity.
Cognitive reactivity, typically measured as self-reported dysfunctional attitudes, has been associated with depression vulnerability. For example, Miranda and Persons (Miranda & Persons, 1988) found that women who had previously experienced an episode of depression were more likely to endorse dysfunctional attitudes when in a sad mood state than never depressed women in a similarly sad mood. Several other studies, many using standardized methods to provoke a sad mood, have found similar changes in dysfunctional thinking among depression vulnerable populations (Lewinsohn et al., 1999; Miranda et al., 1990; Van der Does, 2002a).
More recently, longitudinal studies have documented that cognitive reactivity prospectively predicts depressive relapse in previously depressed patients (Segal et al., 2006; Segal et al., 1999). In these two important studies, remitted depressed patients were administered the dysfunctional attitude scale (Weissman & Beck, 1978) before and after a negative mood provocation consisting of sad music combined with autobiographical recall of a sad event. In both studies, Segal and colleagues found that increased dysfunctional attitudes following the sad mood provocation predicted depressive relapse during the follow-up period, even when controlling for number of previous depressive episodes (Segal et al., 2006). Further, preventative treatments designed to reduce cognitive reactivity have been shown to decrease depressive relapse, particularly among individuals with three or more episodes of depression (Coelho et al., 2007; Teasdale et al., 2000). In summary, there is considerable evidence that cognitive reactivity is an important marker of vulnerability to depression (Lau et al., 2004; Scher et al., 2005).
Despite growing evidence supporting cognitive reactivity as a risk factor for depression, few studies have examined mechanisms that increase cognitive reactivity. Recently there has been an increase in interest and research examining the link between genetic risk for depression and cognitive vulnerability factors such as cognitive reactivity (Beevers & Wells, 2009). Variants of the serotonin transporter-linked polymorphic region (5-HTTLPR) of the SLC6A4 gene and the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene are two well-studied genes implicated in the pathophysiology of depression (Wray et al., 2009; Schumacher et al., 2005). In addition, variation in these genes has been associated with neural function in brain structures involved in the processing of emotional information (Schofield et al., 2009; Paaver et al., 2007; Pezawas et al., 2005). As such, these are intriguing candidate genes for association with cognitive vulnerability to depression.
The 5-HTTLPR is characterized by two common variants: a long (L) allele and a short (S) allele (Heils et al., 1996). The S allele is associated with decreased serotonin (5-HT) transporter expression and serotonin uptake compared to the L allele (Lesch et al., 1996). A recently discovered single nucleotide polymorphism (SNP) rs25531 results in two L variants (LA or LG) with the LG allele acting as a functional equivalent to the S allele (Hu et al., 2005). This results in a triallelic classification of the 5-HTTLPR (LA, LG, S). Caspi and colleagues (Caspi, 2003) found that the S allele of the 5-HTTLPR was associated with increased risk for depression in the context of life stress. Although a number of studies replicated or partially replicated this effect across a variety of paradigms (Uher & McGuffin, 2008), recent meta-analyses have called this gene by environment effect into question (Munafo et al., 2009; Risch et al., 2009). Given the inconsistent findings, more research is needed to better understand the relationship between the 5-HTTLPR and depression (Rutter et al., 2009).
One helpful strategy to better understand the relationship between candidate genes and psychiatric outcome is to identify intermediate phenotypes (Meyer-Lindenberg & Weinberger, 2006). A potential intermediate phenotype for depression is cognitive vulnerability to depression and there is an increasing amount of evidence that 5-HTTLPR variability is associated with cognitive factors associated with depression. For example, individuals with S/LG alleles of the 5-HTTLPR show increased attention to anxious word stimuli (Beevers et al., 2007) and increased difficulty disengaging attention from happy, sad, and fearful facial stimuli (Beevers, Wells, Ellis et al., 2009). In addition, S allele-carriers may lack an attentional bias for positive information that is found in individuals homozygous for the L allele (Fox et al., 2009). Children with S/LG 5-HTTLPR alleles also show greater depressogenic attributional styles than children with LA 5-HTTLPR alleles (Sheikh et al., 2008).
Several small studies have also found an interaction between genetic status, mood, and cognitive factors related to depression. For example, after a sad mood provocation, 7-year-old children homozygous for the S allele were found to endorse and recall more negative words in a self-referent encoding task compared to L allele-carriers (Hayden et al., 2008). Similarly, young adults homozygous for the S 5-HTTLPR allele endorsed more negative thinking after viewing a sad film clip than L allele carriers but there were no differences between allele groups after viewing a neutral film clip (Beevers et al., 2009). In short, the presence of S/LG 5-HTTLPR alleles appears to be associated with increased negative thinking in response to a sad mood. However, no studies to date have examined the relationship between the 5-HTTLPR and change in dysfunctional attitudes elicited by a sad mood provocation.
The effects of BDNF Val66Met variation on depression may be complex and the overall contribution of Val66Met variation on the development of major depression may be small (Chen et al., 2008; Martinowich et al., 2007). However, Val66Met Val/Val genotype status has been associated with increased levels of cognitive and affective symptoms of depression in healthy participants (Duncan et al., 2009). In addition, Val66Met variation has been associated with rumination, a risk factor for depression (Spasojevic & Alloy, 2001). Among adolescent girls at risk for depression, Val/Val genotype status was associated with increased depressive symptoms and increased rumination (Hilt et al., 2007). In contrast, among mothers with a history of adult-onset depression, Val/Met genotype status was associated with increased depressive symptoms and increased rumination (Hilt et al., 2007). In healthy young adults, Val/Met genotype is associated with increased rumination compared to individuals with Val/Val genotype (Beevers, Wells & McGeary, 2009). Clearly, more research is needed to determine the impact of Val66Met allele status on factors related to depression.
In addition to the individual effects of 5-HTTLPR and Val66Met variability on risk factors associated with depression, there is evidence of interaction between serotonin and BDNF systems at a neural and genetic level. For example, BDNF regulates the development and function of serotonergic neurons while increases in extracellular serotonin increase BDNF expression (Martinowich & Lu, 2008). Furthermore, there is evidence from both mutant mouse studies and human studies of a genetic epistasis between 5-HTTLPR and BDNF (Martinowich & Lu, 2008). In a recent study, Pezawas and colleagues demonstrated epistatic effects on neural structure. In humans, the 5-HTTLPR S allele is associated with decreased amygdala and anterior cingulate volume compared to L allele homozygotes (Pezawas et al., 2005). However, the presence of the Val66Met Met allele protects against the structural volume reduction associated with the 5-HTTLPR S allele (Pezawas et al., 2008). The expected reduction in amygdala and anterior cingulate volume is observed in individuals with Val66Met Val/Val genotype with the 5-HTTLPR S allele, but is absent in individuals with Val/Met genotype and the 5-HTTLPR S allele.
Given previous findings of increased negative thinking and negative self-referent processing after a sad mood provocation among 5-HTTLPR S allele carriers (Hayden et al., 2008; Beevers et al., 2009) we predicted that 5-HTTLPR S/LG allele carriers, compared to LA allele homozygotes, would show greater change in dysfunctional attitudes (i.e., cognitive reactivity) following a sad mood provocation. In line with Pezawas and colleagues (Pezawas et al., 2008), we also hypothesized that the presence of the Val66Met Met allele would protect against increased cognitive reactivity among S/LG 5-HTTLPR allele-carriers.
Methods
Participants
Participants were 151 non-depressed young adults recruited from introductory psychology classes at the University of Texas at Austin. All participants had Beck Depression Inventory – II (BDI-II; (Beck et al., 1996) scores of 9 or less, had no history of depression, and were unmedicated at the time of testing (see Table 1 for descriptive statistics). Participants partially fulfilled a research requirement by completing this study.
Table 1. Demographic Characteristics.
| Allele Status | |||||
|---|---|---|---|---|---|
| 5-HTTLPR | Val66Met | ||||
| Demographic | L′L′ | S′L′ | S′S′ | Val/Val | Met-carrier |
| n | 29 | 88 | 34 | 92 | 59 |
| Age (Years) | 19.4 (1.73) | 19.0 (1.03) | 18.9 (1.04) | 19.1 (1.36) | 19.0 (0.88) |
| Gender (F/M) | 36%/64% | 62%/38% | 56%/44% | 63%/37% | 45%/55% |
| Race (Caucasian/“other”) | 86%/14% | 73%/27% | 59%/41% | 71%/29% | 64%/36% |
| Depressive symptoms (BDI-II) | 3.79 (2.28) | 2.95 (2.73) | 2.98 (2.91) | 3.18 (2.16) | 3.02 (2.85) |
Note. BDI-II = Beck Depression Inventory – II
Assessments
Beck Depression Inventory II
The Beck Depression Inventory II (BDI-II; (Beck et al., 1996) is a 21-item self-report questionnaire that assesses symptoms of depression. The BDI-II is one of the most widely used self-report measures of depressive symptomology and has demonstrated adequate internal consistency, test-retest reliability and construct validity (Dozois et al., 1998).
Inventory to Diagnose Depression – Lifetime Version
The Inventory to Diagnose Depression – Lifetime Version (IDD-L; (Zimmerman et al., 1986) is a 22-item self-report inventory designed to assess the presence and severity of the diagnostic criteria for a major depressive episode. The IDD-L has good agreement with diagnostic interview on presence of a previous major depressive episode (Goldston et al., 1990).
Dysfunctional Attitudes Scale Short Form
The original Dysfunctional Attitudes Scale (DAS; (Weissman & Beck, 1978) was originally a 100-item scale that is most often divided into two 40-item forms (A & B). The DAS-A is used more frequently and has since been refined into two equivalent 9-item short forms (DAS-SF1 and DAS-SF2; (Beevers et al., 2007). Both short forms were highly correlated with the DAS-A (>.9) and have demonstrated good reliability, convergent validity, and predictive validity (Beevers et al., 2007). Examples of items on the DAS-SF include, “If others dislike you, you cannot be happy” and “If I do not do as well as other people, it means I am an inferior human being.” Participants indicate the degree to which they agree with each item on a 4-point Likert scale (1 = “totally agree” to 4 “totally disagree”). Responses are summed and higher scores reflect higher levels of dysfunctional attitudes.
Current Mood
Current mood was measured with a single item scale that ranged from 1 (Very Sad) to 9 (Very Happy). This measure served as a manipulation check following the sad mood provocation, so it was intended to be a brief and face valid assessment of mood. This approach is commonly used in mood provocation studies (Van der Does, 2002a; Van der Does, 2002b).
Genotyping
Genomic DNA were isolated from buccal cells using a modification of published methods (Lench, 1988; Meulenbelt et al., 1995; Spitz et al., 1996; Freeman et al., 1997). The cheeks and gums are rubbed for 20 s with three sterile, cotton-tipped wooden swabs. The swabs are placed in a 50-ml capped polypropylene tube containing lysis buffer (500 μl of 1 M Tris-HCl; pH 8.0; 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride). The subjects then rinse out the mouth vigorously with 10 ml of distilled water for 20 sec and this was added to the 50-ml tube. The tubes were stored at 4°C until the DNA was extracted.
Serotonin transporter promoter region polymorphism (5-HTTLPR)
The 5-HTTLPR gene, which maps to 17q11.1-17q12, contains a 43 bp insertion/deletion in the 5′ regulatory region of the gene (Heils et al., 1996). This polymorphism in the promoter appears to be associated with variations in transcriptional activity: the long variant (528 bp) has approximately three times the basal activity of the shorter promoter (484 bp) with the deletion (Lesch et al., 1996). The assay is a modification of the method of Lesch and colleagues (Lesch et al., 1996). The primer sequences are: forward, 5′- GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled), and reverse, 5′-GAGGGACTGAGCTGGACAACCAC-3′. These primer sequences yield products of 484 or 528 bp. Allele sizes are scored by two investigators independently and inconsistencies were reviewed and rerun when necessary. The frequency of the 5-HTTLPR alleles (SS, n = 33 (21.9%); SL, n = 77 (51%); LL, n = 41 (27.2%)) did not differ from the Hardy-Weinberg equilibrium, χ2 = 0.08, p = 0.78.
To distinguish between the S, LA, and LG fragments, the PCR fragment was digested with MspI according to the methods found in Wigg et al. (Wigg et al., 2006). The resulting polymorphic fragments were separated using an ABI 377 DNA sequencer (S: 297, 127, 62 bp, LA: 340, 127, and 62 bp, LG: 174, 166, 127, and 62 bp). Using this approach, allele frequencies were S: n = 143 (47.4%), LA: n = 146 (48.3%), LG: n = 13 (4.3%). Genotype distribution for the A/G SNP was in Hardy-Weinberg equilibrium, χ2 = 0.38, p = .53. Consistent with previous research, the S and LG alleles were designated S′ and the LA allele was designated L′. We therefore formed three groups: (a) S′S′ (i.e., SS: n = 33 (21.9%), SLG: n = 1 (0.7%), LGLG: n = 0 (0%)), (b) S′L′ (i.e., SLA: n = 76 (50.3%), LGLA: n = 12 (7.9%)), and (c) L′L′ (i.e., LALA: n = 29 (19.2%)).
Brain derived neurotrophic factor (BDNF)
The Val66Met polymorphism (rs6265) was genotyped using Taqman assay C___11592758_10 (Applied Biosystems) using an ABI 7300 Real time PCR system. The frequency of the BDNF alleles (Val/Val, n = 92; Val/Met, n = 48; Met/Met, n = 11) did not differ from the Hardy-Weinberg equilibrium (χ2 = 1.74, p = 0.19). Consistent with previous research (Pezawas et al., 2008) we combined the Val/Met and Met/Met groups to form a MET-carrier group (n = 59). Cell sizes for combined 5-HTTLPR and Val66Met groups were as follows: L′L′-Val/Val (n = 20), L′L′-Met-carrier (n = 9), S′L′-Val/Val (n = 54), S′L′-Met-carrier (n = 34), S′S′-Val/Val (n = 18), S′S′-Met-carrier (n = 16).
Sad Mood Provocation
As in previous work (Segal et al., 2006; Segal et al., 1999; Van der Does, 2002b), participants listened to sad music while imagining a time in their life when they were very sad. The music was Samuel Barber's Adagio for Strings, which has been used effectively in previous mood provocation research (Hunt & Forand, 2005). The mood induction was computer controlled. Instructions were presented on the computer screen and the prompt to imagine a sad time in their life remained on the screen for the duration of the song (approximately 7 minutes). Mood was measured before and after the provocation. This type of mood provocation is effective in eliciting a temporary sad mood (Van der Does, 2002b).
Procedure
Mass pre-testing identified participants who scored less than 4 on the short-form of the Beck Depression Inventory (BDI-SF; (Beck et al., 1974). Upon arrival to the laboratory, depression severity was re-assessed using the BDI-II and history of depression was assessed with the IDD-L. Participants with BDI-II scores greater than 9, a history of depression according to the IDD-L, or anyone currently taking psychiatric medications were excluded from the experiment. Participants who qualified were oriented to the laboratory and completed a demographics form. Participants provided buccal cells via a cheek swab and mouthwash for genotyping. Next, participants filled out one DAS-SF. They then completed a sad mood provocation and completed the alternate DAS-SF. Order of DAS-SF equivalent forms was counterbalanced across participants. Consistent with Segal and colleagues (Segal et al., 2006; Segal et al., 1999), cognitive reactivity was assessed with a change score by subtracting pre-mood provocation DAS-SF total score from post-mood provocation DAS-SF total score. Current mood was also assessed immediately before and after the mood provocation with a single item scale that ranged from 1 (Very Sad) to 9 (Very Happy). The internal review board at the University of Texas at Austin approved all procedures.
Results
Sample Characteristics
Descriptive statistics for the sample are presented in Table 1 stratified by 5-HTTLPR and Val66Met allele groups. There were no significant differences as a function of 5-HTTLPR allele grouping for age, F(2, 149) = 1.38, p = .26, race, χ2(2, 146) = .757, p = .69, or depressive symptoms, F(2, 150) = .894, p = .41. Similarly, there were no significant differences for demographic variables as a function of BDNF Val66Met allele grouping for age, F(1, 149) = .581, p = .52, race, χ2(1, 146) = .707, p = .4, or depressive symptoms, F(1, 150) = .112, p = .74. However, there were significant differences for gender as a function of 5-HTTLPR allele grouping, χ2(2, 151) = 6.34, p = .04, and BDNF Val66Met allele grouping, χ2(1, 151) = 4.62, p = .03.
Manipulation Check
A paired samples t-test indicated a significant increase in sadness following the sad mood provocation, t(150) = 14.41, p < .001, Cohen's d = 2.35, with participants experiencing a mean change of 1.86 points (SD = 1.57) on the 9-point scale. Mean pre-provocation mood was 6.31 (“Somewhat happy”) and mean post-provocation mood was 4.45 (“Somewhat sad”). There were no significant main effects or interaction of genetic status on mood change, Fs < 1, ps = ns.
Genetic Effects on Cognitive Reactivity
A 3 (5-HTTLPR genotype: L′L′, S′L′, S′S′) × 2 (Val66Met genotype: Val/Val, Met-carrier) × 2 (time: pre-mood provocation, post-mood provocation) repeated measures analysis of variance (ANOVA) was conducted with DAS-SF score at pre and post-mood provocation as the within subjects factor. There was no significant main effect for 5-HTTLPR, F(2, 145) = .36, p = .7, or Val66Met, F(1, 145) = 1.28, p = .26, on change in DAS-SF. However, there was a significant main effect for time, F(1, 145) = 7.73, p = .006, partial η2 = .051, with a mean increase of 0.88 (SD = 3.34) on the DAS-SF after the sad mood provocation. In addition, the 3-way interaction was statistically significant, F(2, 145) = 3.17, p = .045, partial η2 = .042, indicating an interaction between 5-HTTLPR and Val66Met genotypes.
Due to differences in allele frequency by gender, the 3 (5-HTTLPR genotype: L′L′, S′L′, S′S′) × 2 (Val66Met genotype: Val/Val, Met-carrier) × 2 (time: pre-mood provocation, post-mood provocation) repeated measures ANOVA was conducted for men and women separately. The significant gene by gene interaction for change in DAS remained significant for both women, F(2, 77) = 5.66, p = .005, partial η2 = .128, and men, F(2, 62) = 4.84, p = .011, partial η2 = .135 indicating that participant gender did not moderate the effect. Furthermore, the effect remained significant when using the bi-allelic (i.e., ignoring the LG/LA distinction) rather than tri-allelic classification of the 5-HTTLPR, F(2, 145) = 3.24, p = .042, partial η2 = .043.
Follow-up analyses indicated a significant effect of Val66Met genotype on change in DAS-SF for the S′S′ 5-HTTLPR genotype, F(1, 32) = 5.40, p = .027, partial η2 = .144, but no significant effect of Val66Met genotype for S′L′, F(1, 86) = .01, p = .918, or L′L′, F(1, 27) = .42, p = .525, genotype groups. Within the S′S′ 5-HTTLPR genotype group, the Val/Val Val66Met genotype resulted in greater cognitive reactivity compared to the Met-carriers (see Figure 1). Increase in dysfunctional attitudes was significantly greater than 0 for participants with S′S′ and Val/Val genotypes, t(17) = 3.13, p = .006, d = 1.52. Change in dysfunctional attitudes did not differ from 0 for any other 5-HTTLPR × BDNF allele group, all t < 1.43, all p > .15.
Figure 1.
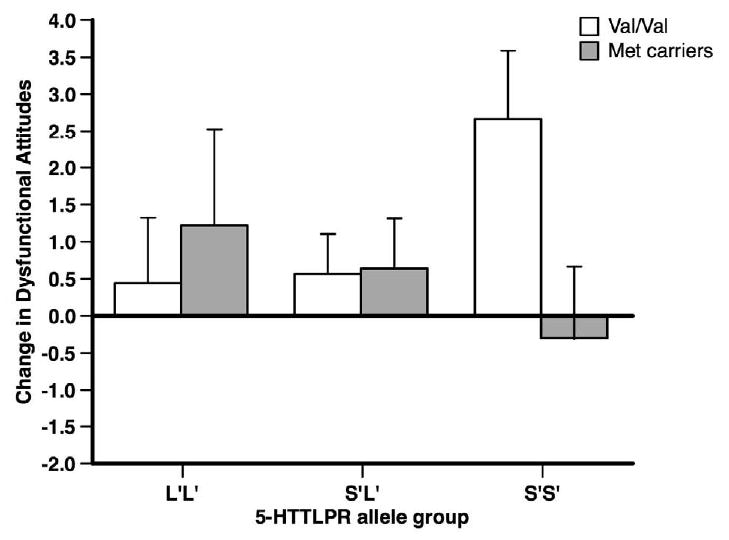
Change in Dysfunctional Attitudes Scale – Short Form (DAS-SF) score from pre to post-mood provocation as a function of allele grouping. Error bars represent the standard error of the mean.
Discussion
This study examined whether genetic variants of the 5-HTTLPR and BDNF predicted individual differences in dysfunctional thinking from before to after a sad mood provocation (i.e., cognitive reactivity). As expected, 5-HTTLPR and BDNF Val66Met variation interacted to predict cognitive reactivity. Specifically, when compared with Val/Val participants, the Val66Met Met allele was associated with attenuated cognitive reactivity in the S′S′ 5-HTTLPR genotype group. Individuals heterozygous or homozygous for the LA 5-HTTLPR allele reported low levels of cognitive reactivity regardless of BDNF allele status.
The epistatic relationship between 5-HTTLPR and BDNF Val66Met observed in this study is consistent with previous research demonstrating interaction between these polymorphisms at the neural level (Pezawas et al., 2008). The current study provides further evidence for this epistatic relationship and extends the effects to cognitive vulnerability factors associated with depression. The fact that the association between 5-HTTLPR variation and cognitive vulnerability was only observed in the context of an interaction with Val66Met variation suggests that future studies investigating the association between 5-HTTLPR variation and cognitive, behavioral, or psychiatric outcomes should consider the potential effects of this epistatic relationship. While speculative, epistasis of 5-HTTLPR and Val66Met might account for some of the variability in studies investigating the effects of 5-HTTLPR variation and depression (Risch et al., 2009; Munafo et al., 2009). Nevertheless, findings from the current study are consistent with suggestions that the genetic basis of depression vulnerability is nuanced, complex, and likely involves interactions between numerous polymorphisms across neurotransmitter systems (Hasler et al., 2004).
This is the first study to examine the genetic basis of cognitive reactivity, an important risk factor for predicting future onset of depression (Lau et al., 2004; Scher et al., 2005). The current study suggests that the interaction between 5-HTTLPR and Val66Met contributes to the development of cognitive reactivity. This is consistent with previous research demonstrating associations between 5-HTTLPR and Val66Met variation and cognitive factors associated with depression, such as negative thinking, difficulty disengaging from emotional information, rumination, and negative attributional style (Sheikh et al., 2008; Beevers et al., 2009; Beevers, Wells & McGeary, 2009; Beevers, Wells, Ellis et al., 2009). It is also consistent with previous research suggesting an association between self-reported cognitive reactivity and the serotonin system (Booij & Van der Does, 2007) as well as dysfunctional attitudes and the serotonin system (Meyer et al., 2004; Meyer et al., 2003). Furthermore, genetic effects on cognitive reactivity in the current study were found despite the absence of differences in mood reactivity. This is also consistent with previous research (Beevers et al., 2009).
It is interesting that 5-HTTLPR genotype effect was most apparent for S′ 5-HTTLPR homozygotes. Despite similar putative serotonin reuptake among S/LG 5-HTTLPR allele homozygotes and heterozygotes (Lesch et al., 1996), several studies have found group differences between individuals with one or two copies of the short 5-HTTLPR allele in a variety of domains, including neural responses to emotional images (Surguladze et al., 2008), selective attention for emotional stimuli (Beevers, Wells, Ellis et al., 2009), and stress response (Gotlib et al., 2008). This finding is also consistent with previous studies examining the interaction between life stress and depression, where risk for depression among people who have experienced life stress increases with number of short 5-HTTLPR alleles (Caspi, 2003; Kendler et al., 2005). Thus, evidence suggests that some aspects of cognitive vulnerability may be parametrically associated with number of short 5-HTTLPR alleles. Given this possibility, it seems important to examine 5-HTTLPR allele groups separately whenever possible.
It is important to note that the interaction between the 5-HTTLPR and BDNF explained approximately 5% of the variance in cognitive reactivity. While noteworthy, this leaves much of the variance to be explained. As suggested by a number of researchers (Beck, 2008), integrating genetic, neural factors, and the effects of negative events will build a more comprehensive model of cognitive vulnerability to depression. To build such a comprehensive model it will be necessary to continue to investigate the relationships between the 5-HTTLPR and other candidate genes and potential intermediate phenotypes of depression such as cognitive reactivity, affective temperament (Gonda et al., 2006; Rihmer et al., 2007), stress reactivity (Gotlib et al., 2008), and rumination (Beevers, Wells & McGeary, 2009; Hilt et al., 2007). Future studies should continue to integrate these factors and explore the relationships between them.
The results of this study should be interpreted with some limitations in mind. The sample size of the current study is relatively small for modern genetic association studies and behavioral genetics results are often not replicated when initial studies involve small sample sizes (Ioannidis et al., 2001). However, as mentioned above, the current study replicated previous findings of an epistatic relationship between these two genes (Pezawas et al., 2008). This convergence across studies despite strong methodological differences is promising and suggestive of a reliable effect. Nevertheless, future replication studies of the epistatic relationship between 5-HTTLPR and Val66Met are needed to further elucidate the nature and strength of this association.
Population stratification is a potential concern in any genetic association study (Hutchison et al., 2004). This confound is unlikely as 5-HTTLPR and Val66Met allele frequencies did not significantly differ across race. In addition, the possibility that the 5-HTTLPR or Val66Met polymorphism is in linkage disequilibrium with another functional genetic marker should be considered as a possible explanation for the observed effects. Though not a limitation, the current study made use of a healthy, never-depressed sample with few current symptoms of depression. This group was selected in order to recruit a homogenous sample of participants and to minimize the impact of individual differences (other than the genetic variants of interest) on cognitive reactivity.
Despite these limitations, we believe this study makes an interesting and important contribution to understanding the genetic basis of cognitive vulnerability to depression. This study is the first to demonstrate an epistatic effect between BDNF Val66Met and 5-HTTLPR polymorphisms for the prediction of cognitive reactivity, an established vulnerability for depression. This study highlights the need to study multiple genes and their interaction when investigating genetic associations with vulnerability to depression. Studying the additive and interactive effects of multiple polymorphisms on cognitive, behavioral, and neural markers of vulnerability to depression may facilitate development of more comprehensive and predictive models of depression vulnerability.
Acknowledgments
We thank the research assistants from the Mood Disorders Laboratory at the University of Texas for their assistance with data collection.
Role of Funding Source: Preparation of this article was facilitated by a grant (R01MH076897) from the National Institute of Mental Health (NIMH) to Christopher Beevers, a shared equipment grant (S10RR023457) from the National Center for Research Resources (NCRR) and U.S. Department of Veteran Affairs (VA) shared equipment program to John E. McGeary, and by a Liberal Arts Graduate Research Award from the University of Texas at Austin to Tony Wells. The NIMH, NCRR, VA, and University of Texas had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of interest: The authors report no potential conflicts of interest.
Contributors: Tony Wells and Christopher Beevers designed the study. John McGeary undertook all genetic analyses. Tony Wells wrote the study protocol, managed data collection, undertook the statistical analyses, and wrote the first draft of the manuscript. Christopher Beevers provided significant revisions to the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tony T. Wells, University of Texas at Austin.
Christopher G. Beevers, University of Texas at Austin
John E. McGeary, Providence Veterans Affairs Medical Center and Center for Alcohol and Addiction Studies, Brown University
References
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rial WY, Rickels K. Short form of the Beck Depression Inventory: cross-validation. Psychol Rep. 1974;34:1184–1186. [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown GK. Beck Depression Inventory (BDI-II) San Antonio (TX): Psychological Corporation; 1996. [Google Scholar]
- Beck A. Depression: causes and treatment. Philadelphia: University of Pennsylvania Press; 1967. [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J Abnorm Psychol. 2007;116:208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Scott WD, McGeary C, McGeary JE. Negative cognitive response to a sad mood induction: Associations with polymorphisms of the serotonin transporter (5-HTTLPR) gene. Cognition & Emotion. 2009;23:726–738. [Google Scholar]
- Beevers CG, Strong DR, Meyer B, Pilkonis PA, Miller IW. Efficiently assessing negative cognition in depression: An item response theory analysis of the Dysfunctional Attitude Scale. Psychol Assess. 2007;19:199–209. doi: 10.1037/1040-3590.19.2.199. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT. Genetic associations with cognitive vulnerability to depression. In: Ingram R, editor. The International Encyclopedia of Depression. New York: Springer Publishing Company; 2009. [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, McGeary JE. The BDNF Val66Met polymorphism is associated with rumination in healthy adults. Emotion. 2009;9:579–584. doi: 10.1037/a0016189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Van der Does AJW. Cognitive and serotonergic vulnerability to depression: Convergent findings. J Abnorm Psychol. 2007;116:86–94. doi: 10.1037/0021-843X.116.1.86. [DOI] [PubMed] [Google Scholar]
- Caspi A. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen L, Lawlor DA, Lewis SJ, Yuan W, Abdollahi MR, Timpson NJ, et al. Genetic association study of BDNF in depression: Finding from two cohort studies and a meta-analysis. Am J Med Genet. 2008;147B:814–821. doi: 10.1002/ajmg.b.30686. [DOI] [PubMed] [Google Scholar]
- Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: Evaluating current evidence and informing future research. J Consult Clin Psychol. 2007;75:1000–1005. doi: 10.1037/0022-006X.75.6.1000. [DOI] [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychol Assess. 1998;10:83–89. [Google Scholar]
- Duncan LE, Hutchison KE, Carey G, Craighead WE. Variation in brain-derived neurotrophic factor (BDNF) gene is associated with symptoms of depression. J Affect Disord. 2009;115:215–219. doi: 10.1016/j.jad.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin transporter gene. Proc R Soc Lond B Biol Sci. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Goldston DB, O'Hara MW, Schartz HA. Reliability, validity, and preliminary normative data for the Inventory to Diagnose Depression in a college population. Psychol Assess. 1990;2:212–215. [Google Scholar]
- Gonda X, Rihmer Z, Zsombok T, Bagdy G, Akiskal KK, Akiskal HS. The 5HTTLPR polymorphism of the serotonin transporter gene is associated with affective temperaments as measured by TEMPS-A. J Affect Disord. 2006;91:125–131. doi: 10.1016/j.jad.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering Endophenotypes for Major Depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hayden E, Dougherty L, Maloney B, Olino T, Sheikh H, Durbin C, et al. Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. J Affect Disord. 2008;107:227–230. doi: 10.1016/j.jad.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hilt L, Sander L, Nolen-Hoeksema S, Simen A. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neurosci Lett. 2007;429:12–16. doi: 10.1016/j.neulet.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An Expanded Evaluation of the Relationship of Four Alleles to the Level of Response to Alcohol and the Alcoholism Risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hunt M, Forand N. Cognitive vulnerability to depression in never depressed subjects. Cognition & Emotion. 2005;19:763–770. [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population Stratification in the Candidate Gene Study: Fatal Threat or Red Herring? Psychol Bull. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The Interaction of Stressful Life Events and a Serotonin Transporter Polymorphism in the Prediction of Episodes of Major Depression. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Lau M, Segal ZV, Williams JMG. Teasdale's differential activation hypothesis: implications for mechanisms of depressive relapse and suicidal behaviour. Behav Res Ther. 2004;42:1001–1017. doi: 10.1016/j.brat.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lench N. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;331:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lesch K, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of Anxiety-Related Traits with a Polymorphism in the Serotonin Transporter Gene Regulatory Region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: Differential processes of psychosocial risk. J Abnorm Psychol. 1999;108:483–489. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and Serotonin: Role in Mood Disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJ, Boomsma DI, Slagboom PE. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. Am J Hum Genet. 1995;57:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Hussey DF, Goulding V, Wilson AA, et al. Brain Serotonin Transporter Binding Potential Measured With Carbon 11-Labeled DASB Positron Emission Tomography: Effects of Major Depressive Episodes and Severity of Dysfunctional Attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, Brown GM, Wilson AA, Eynan-Harvey R, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Miranda J, Persons JB, Byers CN. Endorsement of dysfunctional beliefs depends on current mood state. J Abnorm Psychol. 1990;99:237–241. doi: 10.1037//0021-843x.99.3.237. [DOI] [PubMed] [Google Scholar]
- Munafo M, Durrant C, Lewis G, Flint J. Gene × Environment Interactions at the Serotonin Transporter Locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Paaver M, Nordquist N, Parik J, Harro M, Oreland L, Harro J. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacology. 2007;194:545–554. doi: 10.1007/s00213-007-0867-z. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Rihmer Z, Gonda X, Akiskal KK, Akiskal HS. Affective Temperament: A Mediating Variable Between Environment and Clinical Depression? Arch Gen Psychiatry. 2007;64:1096-a–1097. doi: 10.1001/archpsyc.64.9.1096-b. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, et al. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression: A Meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Thapar A, Pickles A. Gene-Environment Interactions: Biologically Valid Pathway or Artifact? Arch Gen Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- Scher C, Ingram R, Segal ZV. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clin Psychol Rev. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Williams LM, Paul RH, Brown K, Cooper N, Dobson-Stone C, et al. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: Evidence from cognition, the P300 and fronto-hippocampal systems. Biol Psychol. 2009;80:176–188. doi: 10.1016/j.biopsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, et al. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Gemar M, Williams S. Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. J Abnorm Psychol. 1999;108:3–10. doi: 10.1037//0021-843x.108.1.3. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Ingram R. Mood priming and construct activation in tests of cognitive vulnerability to unipolar depression. Clin Psychol Rev. 1994;14:663–695. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry. 2006;63:749. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Sheikh H, Hayden E, Singh S, Dougherty L, Olino T, Durbin C, et al. An examination of the association between the 5-HTT promoter region polymorphism and depressogenic attributional styles in childhood. Personality and Individual Differences. 2008;45:425–428. doi: 10.1016/j.paid.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojevic J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1:25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, et al. Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behav Genet. 1996;26:55–63. doi: 10.1007/BF02361159. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Elkin A, Ecker C, Kalidindi S, Corsico A, Giampietro V, et al. Genetic variation in the serotonin transporter modulates neural system-wide response to fearful faces. Genes Brain Behav. 2008;7:543–551. doi: 10.1111/j.1601-183X.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- Teasdale J. Cognitive Vulnerability to Persistent Depression. Cognition & Emotion. 1988;2:247–274. [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Van der Does AJW. Cognitive reactivity to sad mood: structure and validity of a new measure. Behav Res Ther. 2002a;40:105–119. doi: 10.1016/s0005-7967(00)00111-x. [DOI] [PubMed] [Google Scholar]
- Van der Does AJW. Different types of experimentally induced sad mood? Behav Ther. 2002b;33:551–561. [Google Scholar]
- Weissman AN, Beck AT. Development and Validation of the Dysfunctional Attitude Scale: A Preliminary Investigation 1978 [Google Scholar]
- Wigg KG, Takhar A, Ickowicz A, Tannock R, Kennedy JL, Pathare T, et al. Gene for the serotonin transporter and ADHD: No association with two functional polymorphisms. Am J Med Genet. 2006;141B:566–570. doi: 10.1002/ajmg.b.30247. [DOI] [PubMed] [Google Scholar]
- Wray NR, James MR, Gordon SD, Dumenil T, Ryan L, Coventry WL, et al. Accurate, Large-Scale Genotyping of 5HTTLPR and Flanking Single Nucleotide Polymorphisms in an Association Study of Depression, Anxiety, and Personality Measures. Biol Psychiatry. 2009;66:468–476. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Corenthal C, Wilson S. A self-report scale to diagnose major depressive disorder. Arch Gen Psychiatry. 1986;43:1076–1081. doi: 10.1001/archpsyc.1986.01800110062008. [DOI] [PubMed] [Google Scholar]


