Abstract
Burn induces a sustained catabolic response which causes massive loss of muscle mass after injury. A better understanding of the dynamics of muscle wasting and its impact on muscle function is necessary for the development of effective treatments. Male Sprague-Dawley rats underwent either a 40% total body surface area (TBSA) scald burn or sham burn, and were further assigned to subgroups at four time points after injury (days 3, 7, 14 and 21). In situ isometric contractile properties were measured including twitch tension (Pt), tetanic tension (Po) and fatigue properties. Body weight decreased in burn and sham groups through day 3, however, body weight in the sham groups recovered and increased over time compared to burned groups, which progressively decreased until day 21 after injury. Significant differences in muscle wet weight and protein weight were found between sham and burn. Significant differences in muscle contractile properties were found at day 14 with lower absolute Po as well as specific Po in burned rats compared to sham. After burn, the muscle twitch tension was significantly higher than the sham at day 21. No significant difference in fatigue properties was found between the groups. This study demonstrates dynamics of muscle atrophy and muscle contractile properties after severe burn; this understanding will aid in the development of approaches designed to reduce the rate and extent of burn induced muscle loss and function.
Keywords: atrophy, thermal injury, skin, tetanic tension
Introduction
Severe trauma and burn induce a profound hypermetabolic response characterized by increased protein catabolism causing severe loss of lean body mass [1,2], immunologic compromise [3] and slowed wound healing and growth delays [4], all of which contribute to increased morbidity and mortality and prolonged periods of recovery. Catabolism with sustained loss of muscle mass leads to long-term loss of muscle strength and delayed return to customary pre-injury activities. Although administration of nutrient support during hospitalization has been shown to reduce weight loss in severely burned patients [5] and to decrease loss of body mass in other critically ill patients [6], these reductions are only partial and severely injured patients still undergo massive wasting of the peripheral musculature with loss of lean body mass [7].
Restoration of normal function is an important outcome after severe burn [8,9]. The time required for burned patients to resume normal function is excessive, dramatically interfering with return to normal activities. Even patients with burns <20% of total body surface area (TBSA) require an average of 13 weeks convalescence before they are capable of returning to normal activities and employment [10]. As the percent of TBSA burned increases, so does the amount of time required before returning to normal activities [11]. Retrospective studies indicate that although many burned patients eventually return to normal function, up to one fourth cannot return to previous levels of productivity [9,12], primarily due to the loss of physical capacity for standing or walking. For this reason, successful rehabilitation strategies must optimize muscle strength and function.
While all severely burned patients currently receive directed rehabilitation programs to improve functional outcome during convalescence [13], these efforts are usually begun weeks to months after the wound is healed completely, which is likely to be hampered by loss of muscle mass and therefore strength due to sustained catabolism. We don’t currently know whether early treatments devised to improve muscle weight and protein mass benefit muscle function. It is also not clear whether decreased muscle function is associated with proportional loss of muscle mass, or whether injury has independent effects on the contractile properties of muscle. We developed the hypothesis that severe burn in rats is associated with loss of muscle contractile function that is greater than the proportion attributable to loss of muscle mass alone. The ability to understand the dynamics of burn induced muscle loss on muscle function, as well as the ability to develop strategies to reduce early muscle wasting following burn would be aided by a clinically relevant animal model. The goal of this study was to characterize the changes in muscle mass and function following 40% TBSA of burn in a rat model.
Methods
Male Sprague-Dawley rats weighing approximately 300 grams were used. The study protocol was reviewed and approved by Institutional Animal Care and Use Committees (IACUC) at the United States Army Institute of Surgical Research (USAISR), and the animal study was accomplished in the animal lab facility at the USAISR. The rats were housed in a temperature-controlled environment with a 12-hour light/dark cycle. After acclimatization for one week, the animals were pair-fed a liquid diet (Boost, Mead Johnson Nutritionals, Evansville, Indiana) and water ad libitum for three days prior to the experiment, and at daily intervals before the end of experiment. Sixty-four animals were randomly and evenly assigned into burn or sham burn groups. The animals were further assigned to subgroups of four time points at days 3, 7, 14 and 21 after burn (n=8 per subgroup).
Rats were anesthetized by continuous 1.5–3% isoflurane (Forane®, Baxter Healthcare Corp. IL 60015) in 100% oxygen using a face cone/mask. The rats in both sham and burn groups were shaved on the dorsal and ventral surface of the trunk. The rats in burn group received a 40% total burn surface area (TBSA) by immersing the dorsum in 100°C heated water for 10 seconds and ventral surface for 2 seconds according to the previously established models [15, 16]. Burned rats were resuscitated immediately with 20 ml Ringer’s lactate solution given intraperitoneally. After burn, the rats were returned to individual wire-bottom cages, and received analgesia (0.1 mg/kg buprenorphine, Buprenex®, Hospira, Inc., Lake Forest, IL) subcutaneously twice a day for 3 days. The animals in the sham group received the same analgesic injections. All animals were returned to cages after awakening from anesthesia and pair-fed BOOST® at 50 cc of (0.17 kcal/kg body weight) on the first day after burn, 75 cc (0.25 kcal/kg body weight) on the second day, and 100 cc (0.33 kcal/kg) on the third day. Graded daily intake was in accord with the previously defined intake of burned rats [17] which has been verified in several subsequent studies [18–19].
Muscle contractile properties were observed at day 3, 7, 14 and 21 after burn. One leg was randomly chosen from each animal for the test. Following anesthesia by continuous inhalation of isoflurane (1.5–3%), the common sciatic nerve was isolated and implanted into an electrode cuff with ends connected to a pulse stimulator (A-M Systems, Inc, Mod. 2100). After securing the electrode cuff, the proximal end of the nerve was carefully severed. The distal tendon of the tibialis anterior (TA) was isolated and cut at the annular retinaculum dorsal to the hock, and the distal 1/3 TA was gently dissected free from the surrounding musculature leaving the origin and neurovascular pedicle intact. The distal tendon was threaded through a hole in the lever arm of a dual-mode servo muscle lever system (Aurora Scientific, Inc., Mod. 309b) and secured with 4-0 silk suture. The lower leg was secured and stabilized on the working platform with pins at the knee and ankle joints. Core temperature was monitored using a rectal thermistor and maintained at 36.5°C to 37. 5°C by manually adjusting the temperature of circulating water on the surgical platform. The temperature of the peroneus longus muscle was monitored with a needle thermistor, and acted as a surrogate for the TA with maintenance at 36.0±1.0°C. The isometric contractile properties of the TA muscle were then evaluated using an in situ preparation and test battery described previously [20,21]. All measurements were made with the muscles set at optimal length (Lo), which was determined from Pt using an automated routine as follows: at a slack position the muscle was stimulated at 1 Hz for a set of 8 twitches; the last two twitches were averaged and the Pt was stored. The lever was then moved 0.1 mm, and the routine was repeated 2 seconds later. Each twitch set including lever movement took 10 seconds. This continued until the average Pt did not change more than 2% between 3 consecutive twitch sets. Optimal length was defined as the second of the three twitch sets. Stimulus frequency for Po was set at 150 Hz. Following establishment of Lo, Pt was determined from the average of 3 unpotentiated twitches (1 min between each twitch); Po was determined from the average of 3 tetani separated by 2 minutes. Lastly, the isometric fatigue properties were determined [22] by stimulation at 40Hz for 330msec every second for four minutes. Muscle fatigability was defined by the fatigue index calculated by the percent rate of the last tetanic force against initial peak force.
The TA from contra-lateral leg was isolated immediately before muscle function testing for measurement of total muscle wet weight. For wet weight/dry weight determination, a small piece of muscle sample was dissected, weighed and placed in a drying oven at 50°C for 5 days and weighed again for its dry weight. Another sample of TA (about 100gm) was processed for whole protein extraction. Briefly, the muscle was snap-frozen in liquid nitrogen and pulverized in BioPulverizer™. The sample was then immediately homogenized in lysis buffer (Cell Signaling Technology®, Danvers, MA) containing 20 mM Tris-HCL, 150 mM Nacl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1ug/ml leupeptin. The protein suspension was extracted after centrifugation, and protein concentration was determined by BCA Protein Assay (Thermo Scientific, Rockford, IL). The total amount of protein and percentage of protein content (protein weight vs. muscle weight) in TA were then calculated.
All data are expressed by mean ± standard error of mean (SEM). Two-way ANOVA was used to analyze differences between groups. Student’s t-test was used to analyze differences between groups at single time points only where mentioned. Significance was established at p<0.05.
Results
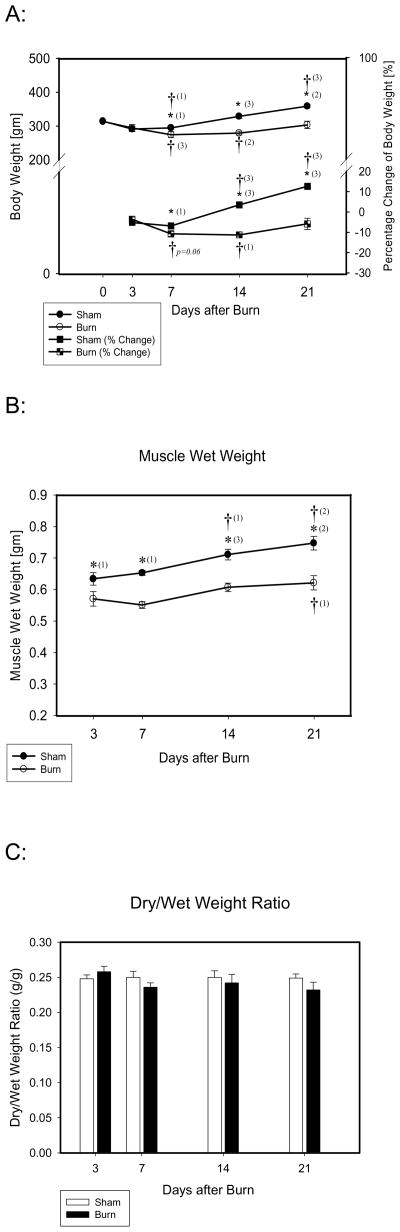
Both burn and sham animals lost approximately 5% of their original weight by day 3. Sham animals began to regain weight after day 7. Weight loss continued in burned animals by 10% to 15% until day 14 after burn after which they began to regain weight. At day 21, weights were still approximately 5% less than original weights (Fig. 1A). Sham animals lost less weight than burn rats at day 7, and the body weight increased thereafter following the normal growth pattern, increasing by 10% to 15% over original weight at day 21. Therefore, both body weight and percentage of body weight change differed significantly between sham and burn groups at 7, 14 and 21 day after burn (Fig. 1-A).
Fig. 1.
A, B, C: A). Body weight/Percentage Change of Body Weight at days 3, 7, 14 and 21 after burn; Circles represent body weight at days 0, 3, 7, 14, and 21; squares represent percent change in body weight at days 3, 7, 14 and 21. Body weight in burned rats was significantly lower than that of sham rats at days 7, 14 and 21. Significant differences were found in body weight percent change between groups at days 7, 14 and 21. B). TA muscle wet weight at days 3, 7, 14 and 21 after burn: muscle wet weight in burned rats was significantly lower than that of the sham rats at days 3, 7, 14 and 21. C). Muscle dry/wet weight ratio, no significant difference was found between groups.
*: Significant difference between the groups of burn and sham; †: Significant difference at days 7, 14 or 21 compared to day 3 respectively. (1): p<0.05; (2): p<0.01; (3): p<0.001.
TA wet weights in burned rats decreased after burn (Fig. 1-B). Significant differences in wet weights between burn and sham groups were found from day 3 to day 21. Compared to the sham group, percent reduction in TA muscle wet weight after burn was −9.7%, −7.4%, −16.0%, and −9.9% at days 3, 7, 14, and 21 respectively. In addition, no significant difference was found in TA dry/wet-weight ratios at any time point (Fig 1-C) suggesting no significant difference in tissue water content between the groups. However, a significant difference in dry weights between burn and sham was found at day 7 though day 21.
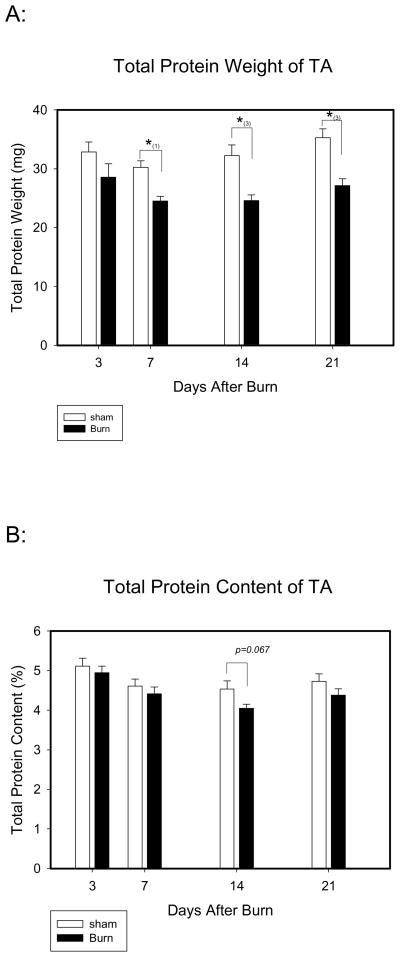
Total TA protein amount was significantly lower in burn compared to sham at days 7, 14 and 21 after injury (Fig. 2). However, we found no significant difference in TA protein content percent between the groups. At day 14, an 11% reduction in protein content percent was found in burn compared to sham, but this was not statistically significant (p<0.067 by two way ANOVA) (Fig. 2).
Figure 2.
The total muscle protein weight and protein content of TA: A). Total muscle protein weight: total muscle protein weight after burn was significantly lower than sham at days 7, 14 and 21. *: Significant difference between the groups. (1): p<0.05; (2): p<0.01; (3): p<0.001. B). Total muscle protein content: no significant difference of total muscle protein content between groups was found. *: p=0.067 comparison between burn and sham at day 14.
Absolute Pt (twitch tension), Po (tetanic tension), and fatigue index were measured, and the specific force of Pt and Po were expressed by the ratio of Pt or Po to the muscle wet weight of TA (N/gm). Pt was not significantly different between the groups from day 3 through 14, but a significantly higher Pt was found at day 21 in the burn group compared to the sham in both absolute and specific force (Table 1). In addition, we found a slower time to peak tension (greater TPT) and longer time to relaxation in the burn group (greater ½ RT) compared to sham at day 21 (Table 1). Although there did not reach statistical significance, the velocity to the peak tension (+dt/dp) was also 17% lower in burn group compared to sham at day 21. This may imply a higher percentage of slow twitch fiber content in TA of burn than sham at this late time point.
Table 1.
Muscle contractile properties (twitch tension).
| Day 3 | Day 7 | Day 14 | Day 21 | |||||
|---|---|---|---|---|---|---|---|---|
| Sham | Burn | Sham | Burn | Sham | Burn | Sham | Burn | |
| Pt (N) | 4.29±0.26 | 4.16±0.33 | 4.33±0.33 | 3.95±0.26 | 4.75±0.32 | 4.29±0.43 | 3.94±0.22 | 5.26±0.5*(2) †(1) |
| Pt/MW (N/gm) | 7.04±0.57 | 6.38±0.43 | 6.50±0.49 | 6.27±0.36 | 6.27±0.39 | 6.70±0.61 | 5.26±0.83 | 7.17±0.93*(2) |
| TPT (ms) | 22.0±0.8 | 22.4±0.9 | 19.8±0.8 | 21.0±0.8 | 20.5±0.7 | 20.0±0.8 | 19.5±0.7 | 22.4±0.8*(1) |
| RT1/2 (ms) | 16.8±1.1 | 16.0±1.2 | 14.9±1.1 | 17.2±1.0 | 14.5±1.0 | 15.5±1.0 | 14.0±0.9 | 17.0±1.1*(1) |
| +dp/dt | 324±23 | 299±20 | 336±20 | 302±18 | 357±14 | 334±27 | 306±20 | 366±27 |
| −dp/dt | −187±19 | −185±15 | −202±8 | −163±11 | −229±6 | −191±11 | −201±18 | −213±19 |
| Po (N) | 12.34±0.58 | 11.79±0.64 | 12.29±0.59 | 11.26±0.54 | 14.56±0.50Δ(1)‡(1) | 10.64±0.54*(3)†(1) | 13.39±0.50 | 12.65±0.58 |
| Po/MW (N/gm) | 17.73±0.67 | 19.99±0.37 | 18.42±0.16 | 17.94±0.48 | 19.22±0.17 | 16.49±1.02*(2)Δ(3) | 17.55±1.07 | 18.10±0.51 |
| +dp/dt | 470±43 | 439±30 | 514±16 | 441±20 | 552±8 | 457±30*(1) | 498±31 | 432±27 |
| −dp/dt | −560±32 | −508±41 | −402±40 | −470±22 | −573±40 | −422±39*(2) | −482±44 | −522±15 |
| Pt-Po Ratio | 0.350±0.03 | 0.353±0.03 | 0.353±0.03 | 0.353±0.03 | 0.326±0.02 | 0.409±0.03*(1) | 0.297±0.02 | 0.411±0.03*(2) |
All values are mean ± SEM. Pt: twitch tension; Pt/MW: ratio of twitch tension/muscle weight; TPT: time to the peak tension; RT1/2: half time to complete relaxation; +dp/dt: velocity to the peak tension; −dp/dt: velocity to muscle relaxation. Po: tetanic tension; Po/MW: ratio of titanic tension vs. muscle weight; TPT: time to the peak; +dp/dt: velocity to the peak tension; −dp/dt: velocity to muscle relaxation; Pt/Po: Twitch-Tetanic Ratio.
Significant difference between burn and sham at the same time point
Significant difference between day 14 and day 3;
Significant difference between day 14 and day 7;
Significant difference between day 14 and day 21.
p<0.05;
p<0.01;
p<0.001.
The absolute Po progressively decreased in burned rats from day 3 to day 14 after injury with a significant difference between sham and burn at day 14 (burn: 10.64±0.88 N and vs. sham: 14.56±0.22 N, p<0.01) (Table 1). Compared to the absolute Po of burn to sham, the percent reduction was −4.5%, −8.4%, −26.2%, and −5.5% at days 3, 7, 14, and 21 respectively. The absolute Po was 18.0% increased in shams, but was −9.8% decreased compared to the initial level respectively at day 3 when the biggest deficient was found between burn and sham. Similar to the absolute Po, the specific Po (proportion of Po to TA muscle weight) was also significantly lower in the burn group compared to sham at day 14 (burn: 16.49±1.02 N/gm vs. sham: 19.22±0.17 N/gm, p<0.01) (Table 1). This suggests that the muscle strength of TA was not only proportionally reduced by loss of muscle mass, but was also affected by the burn itself. Both absolute and specific Po recovered following burn at day 21 with no significant difference to sham at that time (Table 1). As a result of the increase in Pt, the Pt/Po ratio was significantly higher in the burn group at days 14 and 21. In addition, a significant correlation between tetanic tension and muscle weight was found (r=0.72 and r2=0.51, p<0.001) (Fig. 3).
Figure 3.
Linear Regression between muscle wet weight and absolute tetanic tension. There was a linear regression between muscle wet weight and absolute tetanic tension. r=0.72, r2=0.51, p<0.001 Figure 1A and B and C: A: Body Weight, B: Muscle Wet Weight (TA), C: Dry/Wet Weight Ratio
Fatigue index was not significantly different between groups.
Discussion
Using an established model of hypermetabolism after severe burn in rats, we found a continuous loss of body weight with a peak low appearing between 7 to 14 days after injury with an associated loss of muscle mass in individual hind-limb muscles. As expected, this loss of muscle mass resulted in a reduction of Po (absolute). However, the novel finding of the study was that the loss of absolute Po was also associated with reduction in specific Po (N/gm of muscle). Thus, the loss of peak tension was greater proportionally than can be accounted simply by loss of muscle mass. Therefore, we show here for the first time a decreased in strength per muscle mass unit after severe burn, indicating an associated intrinsic loss of muscle function over and above that associated with simple muscle mass loss.
Burn induces a hypermetabolic response and causes sustained negative net protein balance of skeletal muscle in human subjects up to almost one year after injury even though the wounds had healed [2]. Animal studies have also shown apoptotic loss of myofibrils in response to burn [23]. In this rat model, similar to the burned subjects, we found that muscle protein weight was significantly decreased after burn compared to sham, suggesting burn induced muscle protein catabolism. However, the muscle tetanic force of burn animals recovered to values near the sham animals at day 21 before the burn wound was completely healed. A likely explanation is that burned rats in this study were not restricted in movement after injury, and appeared to display normal cage activity. The attenuating affect of muscular activity on protein sparing, even during starvation has been well established [24]. In contrast, patients with severe burn are often undergoing long-term bed rest in the hospital, and have limited mobility once leaving the hospital while convalescing. Although exercise has been recommended to patients during their rehabilitation period, it is not clear whether long-term bed-rest and inactivity also contribute to muscle catabolism seen after severe injury, and may play a role in delaying or prolonging the process of recovery for muscle mass or muscle strength.
Sham animals also had a drop in body weight and muscle weight (unlike no intervention controls, data not shown), which could have been due to responses caused by the stress of anesthesia. This was a temporary response, however, as usually within 3 days the animals quickly returned to normal growth patterns. In burned rats, body weight and muscle weight continuously decreased after 7 days, and remained below initial values until day 14 (muscle weight) and day 21 (body weight).
The predominant reason for burn related loss of force production was the loss of muscle mass as indicated by the linear regression between muscle weight and Po (r=0.71, p<0.001). However the greater loss in Po relative to the reduction in muscle mass suggests specific tension of muscle and/or neural input to muscle is reduced. Interestingly hindlimb unloading in rats [25,26] and bed rest in humans [27] has also been shown to result in a reduction in specific tension. This has been attributed to relative reduction in contractile proteins per individual fiber, resulting in a reduction in available cross-bridges. In this study, we found that a decrease in protein content percent per muscle mass in burn compared sham which peaked at 11% reduction in burn compared to sham at day 14. This may have contributed to the significant reduction in specific force in the burn group at that time. Future studies will determine whether the reduction of specific force is associated with loss of muscle motor protein without proportional muscle protein loss after burn. In addition, burn has recently been shown to exert a systemic effect on peripheral nerves [28,29]. While these studies only measured conduction velocity, it is possible that other aspects of the nerve-muscle interaction may be affected.
In this standard burn model, it was reported that only fast muscle fibers are affected and slow muscle fibers were mostly preserved [30,31,32]. We found similar results in that the muscle weight decreases were only shown in predominantly fast twitch muscles such as TA, plantaris, extensor digitorum longus and gastrocnemius, and minimal change was found in predominantly slow twitch muscles such as sloeus (data not shown). In rats, the TA contains predominantly fast twitch fibers (type IIB) [33, 34], but is mixed with about 15% type I and type IIA fibers. We found that TPT, ½ RT, +dp/dt, and Pt/Po were increased in burn compared to sham at day 21, suggesting a potential fiber type shifting from fast to slow in TA after burn. It is not fully understood why twitch tension (Pt) was significantly higher in burn groups at day 21 compared to the groups of sham. The likely explanation is that burn induces a massive increase of Ca2+ influx in skeletal muscle [35,36], which may be associated with increased twitch tension after burn, however, whether this is the cause of the change of sarcoplasmic reticulum Ca2+ uptake and Ca2+-ATPase activity and then increase twitch tension was not determined in this study.
Muscle fatigue is defined as muscle failure to maintain expected force output. The cause of fatigue is a complex process which involves multiple factors. The relative importance of an individual factor is dependent on the fiber type composition of the contracting muscles. Slow-twitch fibers, including type 1 and fast oxidative glycolytic type IIa fibers, have high mitochondrial content and thus are relatively fatigue resistant compared to the fast glycolytic fibers [37]. In this study, although there was a temporarily increase in fatigue index of the TA at day 3 and 7 in both burn and sham, the levels were both back to normal at day 21, and no significant difference was seen between groups. As discussed previously, the contractile property results suggested a fiber type shifting from fast to slow at day 21 after burn, so we might have expected to see some fatigue resistance increase at day 21 in response to burn. However, the fatigue index of burn returned to normal levels at day 21, suggesting a potential fiber type shift only from type IIb (fast oxidative glycolytic) to type I (slow oxidative) without changing the relative amount of fatigue resistant fibers (type IIa+type I) while decreasing the total number of fast-twitch fibers (type IIa+type IIb). This could explain why muscle contraction was slower than sham, but fatigue resistance was unchanged.
In conclusion, we demonstrated that the muscle catabolism decreases muscle strength and is initiated early after burn. These decreases in strength appear to be related not only to loss of muscle mass but also to decreases in general muscle fiber strength related to the injury. Resolution occurs in the first three weeks of injury, and seems to be related to a switch in fiber type from fast twitch to slow twitch.
Table 2.
Fatigue Index. All values are mean ± SEM. No significant difference was found between the groups.
| Day 3 | Day 7 | Day 14 | Day 21 | |||||
|---|---|---|---|---|---|---|---|---|
| Sham | Burn | Sham | Burn | Sham | Burn | Sham | Burn | |
| Fatigue Index | 0.171±0.015 | 0.164±0.014 | 0.196±0.037 | 0.207±0.013 | 0.130±0.006 | 0.163±0.014 | 0.151±0.011 | 0.152±0.009 |
Acknowledgments
Grant Information: Combat Casualty Care Division United States Army Medical Research and Materiel Command and The National Institutes of Health (1 R01 GM063120-04)
Footnotes
The opinions or assertions contained herein are the private views of the author and are not to be construed as official or reflecting the views of the US Department of Defense or the US Government. The author is an employee of the US Government. This work was prepared as part of his official duties and, as such, there is no copyright to be transferred.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newsome TW, Mason AD, Jr, Pruitt BA., Jr Weight loss following thermal injury. Ann Surg. 1973;178(2):215–7. doi: 10.1097/00000658-197308000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo R, Herndon DN, Kobayashi M, Pollard RB, Suzuki F. CD4-CD8-TCR alpha/beta+ suppressor T cells demonstrated in mice 1 day after thermal injury. J Trauma. 1997;42(4):635–40. doi: 10.1097/00005373-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study. Lancet. 1999;354(9192):1789. doi: 10.1016/s0140-6736(99)02741-5. [DOI] [PubMed] [Google Scholar]
- 5.Gore DC, Rutan RL, Hildreth M, Desai MH, Herndon DN. Comparison of resting energy expenditures and caloric intake in children with severe burns. J Burn Care Rehabil. 1990;11(5):400–4. doi: 10.1097/00004630-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bower RH. Nutrition during critical illness and sepsis. New Horiz. 1993;1(2):348–52. [PubMed] [Google Scholar]
- 7.Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987;27(3):262–6. doi: 10.1097/00005373-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wrigley M, Trotman BK, Dimick A, Fine PR. Factors relating to return to work after burn injury. J Burn Care Rehabil. 1995;16(4):445–50. doi: 10.1097/00004630-199507000-00012. discussion 444. [DOI] [PubMed] [Google Scholar]
- 9.Cheng S, Rogers JC. Changes in occupational role performance after a severe burn: a retrospective study. Am J Occup Ther. 1989;43(1):17–24. doi: 10.5014/ajot.43.1.17. [DOI] [PubMed] [Google Scholar]
- 10.Engrav LH, Covey MH, Dutcher KD, Heimbach DM, Walkinshaw MD, Marvin JA. Impairment, time out of school, and time off from work after burns. Plast Reconstr Surg. 1987;79(6):927–34. doi: 10.1097/00006534-198706000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Fletchall S, Hickerson WL. Quality burn rehabilitation: cost-effective approach. J Burn Care Rehabil. 1995;16(5):539–42. doi: 10.1097/00004630-199509000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Xiao J, Cai BR. Functional and occupational outcome in patients surviving massive burns. Burns. 1995;21(6):415–21. doi: 10.1016/0305-4179(95)00010-9. [DOI] [PubMed] [Google Scholar]
- 13.Edgar Dale. Megan Brereton. Rehabilitation after burn injury. BMJ. 2004 Aug 7;329(7461):343–5. doi: 10.1136/bmj.329.7461.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001 Oct;56(10):B443–8. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- 15.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968 Nov;8(6):1049–51. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Herndon DN, Wilmore DW, Mason AD., Jr Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res. 1978 Nov;25(5):394–403. doi: 10.1016/s0022-4804(78)80003-1. [DOI] [PubMed] [Google Scholar]
- 17.Jeschke MG, Debroy MA, Wolf SE, Rajaraman S, Thompson JC. Burn and starvation increase programmed cell death in small bowel epithelial cells. Dig Dis Sci. 2000;45(2):415–420. doi: 10.1023/a:1005445501016. [DOI] [PubMed] [Google Scholar]
- 18.Wu XW, Spies M, Chappell VL, Herndon DN, Thompson JC, Wolf SE. Effect of bombesin on gut mucosal impairment after severe burn. Shock. 2002 Dec;18(6):518–22. doi: 10.1097/00024382-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Jeschke MG, Wolf SE, DebRoy MA, Jarrar D, Herndon DN. Recombinant human growth hormone (rhGH) downregulates hepatocyte growth factor (HGF) in burns. J Surg Res. 1998 Apr;76(1):11–6. doi: 10.1006/jsre.1998.5261. [DOI] [PubMed] [Google Scholar]
- 20.Walters TJ, Sweeney HL, Farrar RP. Aging does not affect contractile properties of type IIb FDL muscle in Fischer 344 rats. Am J Physiol. 1990 Jun;258(6 Pt 1):C1031–5. doi: 10.1152/ajpcell.1990.258.6.C1031. [DOI] [PubMed] [Google Scholar]
- 21.Walters TJ, Sweeney HL, Farrar RP. Influence of electrical stimulation on a fast-twitch muscle in aging rats. J Appl Physiol. 1991 Nov;71(5):1921–8. doi: 10.1152/jappl.1991.71.5.1921. [DOI] [PubMed] [Google Scholar]
- 22.Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234(3):723–48. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan H, Chai J, Sheng Z, et al. Effect of burn injury on apoptosis and expression of apoptosis-related genes/proteins in skeletal muscles of rats. Apoptosis. 2009 Jan;14(1):52–65. doi: 10.1007/s10495-008-0277-7. [DOI] [PubMed] [Google Scholar]
- 24.Goldspink G, Ward PS. Changes in rodent muscle fibre types during post-natal growth, undernutrition and exercise. J Physiol. 1979 Nov;296:453–469. doi: 10.1113/jphysiol.1979.sp013016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardetto PR, Schluter JM, Fitts RH. Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol. 1989 Jun;66(6):2739–2749. doi: 10.1152/jappl.1989.66.6.2739. [DOI] [PubMed] [Google Scholar]
- 26.Booth FW, Seider MJ. Recovery of skeletal muscle after 3 mo of hindlimb immobilization in rats. J Appl Physiol. 1979 Aug;47(2):435–439. doi: 10.1152/jappl.1979.47.2.435. [DOI] [PubMed] [Google Scholar]
- 27.Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. 1997 Jan;82(1):182–188. doi: 10.1152/jappl.1997.82.1.182. [DOI] [PubMed] [Google Scholar]
- 28.Higashimori H, Whetzel TP, Mahmood T, Carlsen RC. Peripheral axon caliber and conduction velocity are decreased after burn injury in mice. Muscle Nerve. 2005 May;31(5):610–20. doi: 10.1002/mus.20306. [DOI] [PubMed] [Google Scholar]
- 29.Higashimori H, Carlsen RC, Whetzel TP. Early excision of a full-thickness burn prevents peripheral nerve conduction deficits in mice. Plast Reconstr Surg. 2006 Jan;117(1):152–64. doi: 10.1097/01.prs.0000186537.62939.07. [DOI] [PubMed] [Google Scholar]
- 30.Fang CH, Li BG, Tiao G, Wang JJ, Fischer JE, Hasselgren PO. The molecular regulation of protein breakdown following burn injury is different in fast- and slow-twitch skeletal muscle. Int J Mol Med. 1998 Jan;1(1):163–9. doi: 10.3892/ijmm.1.1.163. [DOI] [PubMed] [Google Scholar]
- 31.Hasselgren PO, James JH, Benson DW, et al. Total and myofibrillar protein breakdown in different types of rat skeletal muscle: effects of sepsis and regulation by insulin. Metabolism. 1989;38(7):634–40. doi: 10.1016/0026-0495(89)90100-5. [DOI] [PubMed] [Google Scholar]
- 32.Fang CH, James HJ, Ogle C, Fischer JE, Hasselgren PO. Influence of burn injury on protein metabolism in different types of skeletal muscle and the role of glucocorticoids. J Am Coll Surg. 1995;180(1):33–42. [PubMed] [Google Scholar]
- 33.Staron RS, Kraemer WJ, Hikida RS, Fry AC, Murray JD, Campos GE. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol. 1999 Feb;111(2):117–23. doi: 10.1007/s004180050341. [DOI] [PubMed] [Google Scholar]
- 34.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996 Jan;80(1):261–70. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 35.Turinsky J, Gonnerman WA, Loose LD. Impaired mineral metabolism in post burn muscle. J Trauma. 1981;21(6):417–23. [PubMed] [Google Scholar]
- 36.Sayeed MM. Signaling mechanisms of altered cellular responses in trauma, burn, and sepsis. Role of Ca2+ Arch Surg. 2008;135:1432–1442. doi: 10.1001/archsurg.135.12.1432. [DOI] [PubMed] [Google Scholar]
- 37.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]