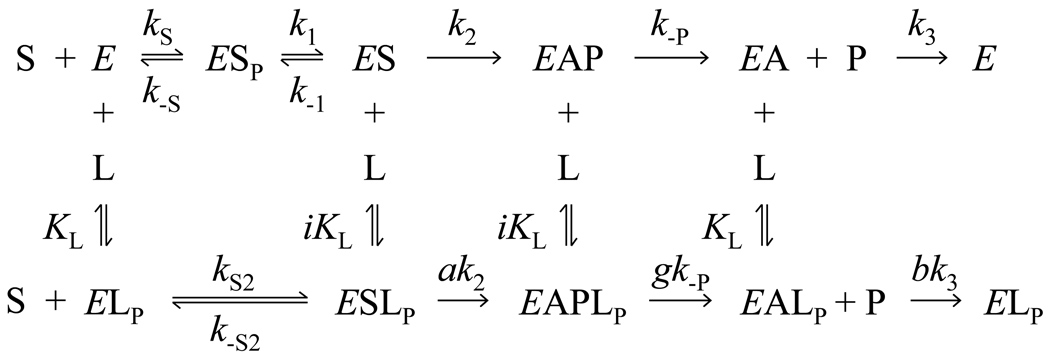Abstract
Acetylcholinesterase (AChE) contains a narrow and deep active site gorge with two sites of ligand binding, an acylation site (or A-site) at the base of the gorge and a peripheral site (or P-site) near the gorge entrance. The P-site contributes to the catalytic efficiency of substrate hydrolysis by transiently binding substrates on their way to the acylation site, where a short-lived acyl enzyme intermediate is produced. Ligands that bind to the A-site invariably inhibit the hydrolysis of all AChE substrates, but ligands that bind to the P-site inhibit the hydrolysis of some substrates but not others. To clarify the basis of this difference, we focus here on second order rate constants for substrate hydrolysis (kE), a parameter that reflects the binding of ligands only to the free form of the enzyme and not to enzyme-substrate intermediates. We first describe an inhibitor competition assay that distinguishes whether a ligand is inhibiting AChE by binding to the A-site or the P-site. We then show that the P-site-specific ligand thioflavin T inhibits the hydrolysis of the rapidly hydrolyzed substrate acetylthiocholine but fails to show any inhibition of the slowly hydrolyzed substrates ATMA (3-(acetamido)-N,N,N-trimethylanilinium) and carbachol. We derive an expression for kE that accounts for these observations by recognizing that the rate-limiting steps for these substrates differ. The rate-limiting step for the slow substrates is the general base-catalyzed acylation reaction k2, a step that is unaffected by bound thioflavin T. In contrast, the rate-limiting step for acetylthiocholine is either substrate association or substrate migration to the A-site, and these steps are blocked by bound thioflavin T.
Keywords: Acetylcholinesterase, peripheral site, thioflavin T, substrate analogs, carbamoylation, enzyme mechanism
1. Introduction
Acetylcholinesterase (AChE) 2 hydrolyzes the neurotransmitter acetylcholine at one of the highest known enzymatic rates (1). Partial inhibition of AChE activity in the brain has been shown to have therapeutic benefits. AChE inhibitors that penetrate the blood-brain barrier elevate the levels of endogenous acetylcholine and are useful in the symptomatic treatment of Alzheimer’s disease (2). However, complete inactivation of AChE, which can occur with organophosphate chemical warfare agents (3), can be lethal.
A better understanding of the AChE catalytic pathway may identify new mechanisms of AChE inhibition that could be therapeutically beneficial. Kinetic and thermodynamic studies have revealed that inhibitors can interact with either or both of two binding sites in AChE (4–7), and X-ray crystallography has provided information about the location of these sites (8–11). A narrow active site gorge some 20 Å deep penetrates nearly to the center of the ~65 kDa catalytic subunits. Near the base of the gorge is the acylation or A-site where H447, E334, and S203 3 participate in a triad that catalyzes the transient acylation and deacylation of S203 during each substrate turnover. The peripheral or P-site, spanned by residues W286 near the mouth of the gorge and D74 near a constriction at the boundary between the P-site and the A-site, specifically binds certain ligands like the neurotoxin fasciculin (12, 13) and the fluorescent probes propidium (5) and thioflavin T (6). The P-site thus far has been shown to contribute to catalytic efficiency by insuring that most substrate molecules that collide with and transiently bind to the P-site proceed on to the A-site (14–16) and, with certain bound cationic substrates, by providing a modest allosteric activation of the acylation step (17).
A series of studies of substrate hydrolysis by AChE has suggested that Scheme 1 is a minimal model for the catalytic pathway (15, 16). This scheme involves three enzyme substrate intermediates at the A-site (ES, EAP, EA) in addition to free enzyme E. An additional ligand corresponding to either a substrate or inhibitor can bind to the P-site (LP) in any of these species, causing the rate constants for successive reaction steps involving the ternary complexes to be altered by the relative factors a, g, or b as indicated in Scheme 1. Formulation and analysis of the rate equation for substrate hydrolysis rates v that arise from Scheme 1 is a challenge, because some of the reversible reactions in Scheme 1 are not at equilibrium. In this case a general solution for v can be obtained only by numerical integration (16, 17). Furthermore, the number of unknown parameters in Scheme 1 often exceeds the number that can be uniquely fitted to experimental data by numerical integration, allowing a number of very different sets of parameter values to fit the data equally well (17, 18). To overcome this uncertainty, we have recently emphasized three overlapping strategies to simplify the analysis of AChE kinetic data (19) and yet measure parameter values unambiguously. These strategies are 1) to choose substrates that eliminate some intermediates in Scheme 1 (17), 2) to group parts of Scheme 1 that are in equilibrium (19), and 3) to determine some of the thermodynamic parameters in Scheme 1 with experiments that are independent of measurements of v (7).
Scheme 1.
In this report we add a fourth strategy to this list, namely to analyze the effects of AChE inhibitors on second order substrate hydrolysis rates. Classic enzymology distinguishes “competitive inhibitors” from “noncompetitive inhibitors”. Competitive inhibitors compete with substrate for a common binding site and do not alter Vmax, the maximum hydrolysis rate at saturating substrate concentration, while noncompetitive inhibitors bind to a site different from the substrate binding site and do not affect the apparent Michaelis constant KM for substrate hydrolysis. These distinctions are of little value for AChE. Many inhibitors of AChE show “mixed” inhibition patterns in which both Vmax and 1/KM are decreased, and X-ray crystallography has revealed that these inhibitors can bind either to the A-site or the P-site. A more useful classical distinction, which applies to AChE and all other enzymes, is that inhibitors which bind to the free enzyme reduce the second order hydrolysis rate constant kcat/KM, while those that bind to an enzyme-substrate intermediate reduce kcat (and thereby Vmax). To emphasize their association with the free enzyme, second order rate constants in the presence or absence of inhibitors here are denoted kE (20). Scheme 1 and the corresponding rate equations for v are simplified when analysis is limited to kE, and here we focus on information about inhibitor site specificity and catalytic mechanism that can be obtained from examination of kE. Structure-activity relationships for ligands that bind specifically to the P-site have yet to be developed, and we reemphasize an inhibitor competition assay that can distinguish whether an inhibitor is binding to the A-site or the P-site. The impact of this assay is increased by the use of inhibitor standards whose sites of binding have been confirmed by x-ray crystallography. We then determine the effects of thioflavin T (Fig. 1), an inhibitor standard specific for the P-site, on substrate hydrolysis by acetylthiocholine and two slowly hydrolyzed analogs, ATMA and carbachol (Fig. 1), that are close structural analogs of acetylcholine. We interpret these effects in the context of substrate equilibration with AChE.
Fig. 1.
Substrates and inhibitors of AChE.
2. Experimental methods
2.1. Inhibition of substrate hydrolysis at low substrate concentrations
Recombinant human AChE was expressed as a secreted, disulfide-linked dimer in Drosophila S2 cells and purified as outlined previously (21). Thioflavin T (Sigma) was recrystallized from water, and concentrations were assigned by absorbance at 412 nm with ε412 nm = 36,000 M−1cm−1. ATMA was synthesized as described, and hydrolysis rates v for ATMA were determined by spectrophotometry at 290 nm (Δε290 nm = 1850 M−1cm−1) (17). For acetylthiocholine, v was measured in a coupled Ellman reaction in which thiocholine generated in the presence of 0.33 mM DTNB (unless otherwise noted) was determined by formation of the thiolate dianion of DTNB at 412 nm (Δε412 nm = 14,150 M−1cm−1) (17). Total AChE concentrations (Etot) were calculated assuming 450 units/nmol (6). Assays were conducted at 25° C in 20 mM sodium phosphate buffer (pH 7.0), 0.02% Triton X-100 unless otherwise noted. Data fitting by nonlinear regression was conducted with SigmaPlot (vers.11.0), and analyses with either eq. 3 or eq. 4 were weighted assuming that the dependent variable had constant percent error.
Steady-state hydrolysis of many substrates by AChE shows either a hyperbolic Michaelis-Menten or a bell-shaped Haldane dependence on substrate concentration (15). In either case, a Michaelis constant KM can be calculated as a measure of the saturation of the enzyme by substrate. Since this saturation includes not just ES but also the other enzyme-substrate intermediates in Scheme 1, KM is often generalized to Kapp. To simplify the analysis of inhibition of substrate hydrolysis arising from the interaction of ligands with either the A- or P-sites, it is useful to focus on the second-order hydrolysis rate constant obtained when the substrate concentration [S] ≪ Kapp. Here we denote this constant kE. In the absence of inhibitors, kE = kcat/Kapp, where kcat is the turnover number for the substrate with AChE (16). Under these conditions, the ESSP complex and all downstream ES intermediates in Scheme 1 become negligible because [S] approaches zero, and Scheme 1 reduces to Scheme 2: Scheme 2 can be applied both to inhibitors that bind at the A-siteLP = I, and 1/i = 0) and to inhibitors that bind at the P-site (LP = IP). Substrate hydrolysis rates v under these second-order conditions are given by eq. 1, where [E]tot is the total enzyme concentration.
| (eq. 1) |
The easiest and most accurate way to measure kE is from the integrated form of eq. 1 at low initial substrate concentration [S]0 (i.e., [S]0 ≤ ~0.2Kapp) (22), as given in eq. 2.
| (eq. 2) |
In eq. 2, z = kE[E]tot and t is the time after mixing E and S. In addition, kE can also be obtained by plotting reciprocal plots of 1/v vs. 1/[S] over a range of fixed [I] and then replotting the slopes of these reciprocal plots vs. [I] (17, 22, 23).
Scheme 2.
When k2 in Scheme 2 is small enough that the substrate and inhibitor reach equilibrium with their enzyme binding site(s), an algebraic expression for v can be written (23). This expression indicates that, when an inhibitor binds to either the A- or P-sites but not both, the ratio of kE in the absence of inhibitor (kE [I]=0) to kE in the presence of inhibitor (kE +I) is given by eq. 3 (23).
| (eq. 3) |
Under these equilibrium conditions, α in eq. 3 is given by ali, the ratio of the relative acylation rate constant a and the relative affinity i of the ligands in the ternary complex in Scheme 2.
2.2 Inhibition of substrate hydrolysis in the presence of two inhibitors
Consider two inhibitors (I1 and I2), each of which show complete inhibition of second order substrate hydrolysis (i.e., α ≅ 0 in eq. 3) when analyzed individually with AChE. If a series of assays are then conducted with a fixed concentration of I1 together with varying concentrations of I2, kE in the presence of both inhibitors (here denoted kE +I2) relative to kE when only I1 is present (here denoted kE [I2] = 0) is given by eq 4 (6).
| (eq. 4) |
In this equation, K1 is the equilibrium dissociation constant for I1 with the free enzyme E, K2 is the equilibrium dissociation constant for I2 with E, and K12 is the equilibrium dissociation constant for I1 with the E I2 complex. The value of [I1]/K12 will be significantly greater than zero only if the ternary complex E I1 I2 can form, and this analysis thus can be used to detect ternary complex formation.
2.3 Inhibition of carbamoylation by a P-site ligand
With a carbamoyl ester substrate of AChE (here denoted a carbamate), both the formation and hydrolysis of the acylated enzyme intermediate, or carbamoyl enzyme (EC), are slow enough to allow equilibrium assumptions. Values of the overall carbamoylation rate constant k12 and the overall decarbamoylation rate constant k21 for a carbamate S1 with AChE were measured by continuous recording with acetylthiocholine as a chromogenic reporter substrate S2 that generates a hydrolysis product P2. Data were fitted to eq. 5, which resolves k12 and k21 (24).
| (eq. 5) |
In eq. 5, ktot = k12 + k21 and v0 is the initial acetylthiocholine hydrolysis rate at t = 0 prior to the formation of any EC. An expression for k12 as a function of [S1] and [S2] (25) can be simplified according to Scheme 2 at low concentrations of both S1 and S2, as shown in eq. 6.
| (eq. 6) |
In eq. 6, Kapp2 refers to Kapp for S2, and k2 and KM = k−Sk−1/kSk1 are parameters for S1. When [S2]/Kapp2 is small, k12/[S1] closely approximates kE and eq. 3 still provides an appropriate framework for analysis of the carbamoylation rate constant k12 in the presence of potential inhibitors.
3. Results
3.1 Inhibition of acetylthiocholine hydrolysis
The inhibition of second order substrate hydrolysis rates provides a convenient framework for examining the interaction of an inhibitor with free AChE. Crystal structure analyses have confirmed that certain inhibitors like edrophonium (Fig. 1) bind specifically to the A-site (26), whereas others like propidium are specific for the P-site (11). Edrophonium binding to the A-site should block substrate binding to this site. In this case, no ternary complex ESIP in Scheme 2 can form (i in this Scheme becomes very large), and α in eq. 3 is zero. This equation then predicts that kE [I]=0/kE +I should have a linear dependence on the edrophonium concentration [I], and this dependence was confirmed in Fig. 2A. On the other hand, propidium binding to the P-site in principle does allow formation of the ternary complex ESIP in which S occupies the A-site, and the expected effect of propidium on kE +I is less clear. However, the data in Fig. 2A clearly show that α also was zero for propidium. In fact, with the rapidly hydrolyzed substrate acetylthiocholine, kE [I]=0/kE +I shows an essentially linear dependence on inhibitor concentration with virtually all inhibitors, whether they bind to the A-site (27, 28), the P-site (6), or bridge both sites (27, 28). Values of α in these cases are not significantly greater than zero.
Fig. 2.
A) Inhibition of acetylthiocholine hydrolysis by the A-site inhibitor edrophonium or the P-site inhibitor propidium. Second order rate constants kE were calculated from data in Fig. 3 of reference (6), and the ratios kE [I]=0/kE +I were fitted to eq. 3. For edrophonium, KI = 0.31 ± 0.01 µM and α = 0.0034 ± 0.0014 (not significantly different from zero). Initial fitting with propidium gave α = −0.008 ± 004 (also not significantly different from zero), and α was fixed at 0 to give KI = 1.61 ± 0.04 µM. B, C) Inhibitor competition assay for assignment of AChE binding site specificity. Values of kE, with inhibitors I1 (thioflavin T) and I2 as indicated, were calculated from data in Fig. 3 of reference (6), and the ratios kE +I2/kE [I2]=0 were fitted to the reciprocal form of eq. 4. K2 values were set as the KIs from Panel A, and K1 for thioflavin T was fixed at 1.0 µM (6). For propidium and thioflavin T (panel B), the affinity in the binary complexes relative to that in the ternary complex was given by K12/K1 = 43 ± 8. For edrophonium and thioflavin T (panel C), K12/K1 = 0.81 ± 0.04. Dotted lines were calculated with the same values of K1 and K2 but with [I1]/K12 fixed at 0.
3.2 Identifying the binding site for an inhibitor
The fact that α is zero for inhibitors of acetylthiocholine hydrolysis allowed us to apply a simplified inhibitor competition analysis (6, 28) to determine whether a new inhibitor binds to the A- and/or P-sites. The analysis is designed to detect any ternary complex formed when both the new inhibitor and an inhibitor with a known binding site are added to AChE simultaneously. The results with thioflavin T as a new inhibitor and propidium or edrophonium as known inhibitors are illustrated in Figs. 2B and 2C. The data with propidium or edrophonium alone reflect the same measurements shown in Fig. 2A, but here plotted in the reciprocal form kE +I/kE [I]=0 to emphasize KI as the IC50, or inhibitor concentration at which kE +I/kE [I]=0 was decreased by 50%. When a fixed concentration of thioflavin T was included in the assays, the IC50 for propidium was shifted to the right, indicating competition between the two inhibitors. One can calculate the extent of the shift that would result from complete competition and no ternary complex formation from the KI values of the inhibitors individually, and these calculated shifts are shown as the dotted lines in Figs. 2B and 2C. Propidium and thioflavin T essentially showed complete competition in Fig. 2B. The affinity of propidium or thioflavin T in the ternary complex decreased by a factor of 43 relative to their affinities in their respective binary complexes with the free enzyme, a large change that was not significantly different from complete competition at the ligand concentrations employed. In contrast, the IC50 for edrophonium in Fig. 2C was not shifted by the addition of thioflavin T, indicating no competition between these two inhibitors. The relative affinities of edrophonium and thioflavin T in the ternary complex with AChE were virtually identical to those in their respective binary complexes. These data indicate that thioflavin T binds to the AChE P-site in a fashion that is essentially competitive with propidium but completely independent of edrophonium bound at the A-site. This location for thioflavin T binding has been confirmed by X-ray crystallography of the complex of thioflavin T with TcAChE, which shows that thioflavin T binds adjacent to the aromatic side chains of W279, Y334 and F330 (TcAChE residue numbering) in the AChE P-site (10).
3.2 Inhibition of AChE by a P-site ligand depends on the substrate
Measurement of second order substrate hydrolysis rate constants kE at low substrate concentrations with eq. 2 has a number of advantages. The fitted constant z in this equation is the product of kE and [E]tot, and its value becomes indirectly dependent on the total substrate concentration [S]0 only when [S]0 is too high to insure strict second order hydrolysis. At lower [S]0, measurement of z tolerates variation in [S]0 and therefore is more accurate than measurement of an initial rate v0, which is proportional to [S]0 as well as [E]tot (eq. 1) and often is not linear over the measurement interval. Furthermore, kE is obtained from a single reaction assay, unlike slopes of reciprocal plots of 1/v vs. 1/[S] that require several assay points, and values of kE +I can be collected at many more inhibitor concentrations than typically are surveyed by reciprocal plots. These features are illustrated in Fig. 3A, where the AChE-catalyzed hydrolysis of 5 µM acetylthiocholine is shown at four thioflavin T concentrations. Since Kapp for acetylthiocholine at the ionic strength employed is 45 µM (23), the necessary condition that [S]0 ≤ ~0.2Kapp is met and the reaction traces are fit precisely as exponential curves as predicted by eq. 2. Analysis of kE +I at various inhibitor concentrations according to eq. 3 (Fig. 3B) confirms that both thioflavin T and edrophonium inhibited acetylthiocholine hydrolysis with KI values in good agreement with those reported in Fig. 2. Furthermore, values of α for both inhibitors were zero, again as expected from Fig. 2.
Fig. 3.
Inhibition of acetylthiocholine hydrolysis by the P-site inhibitor thioflavin T or the A-site inhibitor edrophonium. A) Examples of experimental traces that were analyzed with eq. 2 to obtain values of kE +I. The substrate S was acetylthiocholine ([S]0 = 5 µM), the inhibitor I was thioflavin T ([I] as indicated), and [E]tot was 0.31 nM (for [I] ≤ 5 uM) or 1.6 nM (for [I] ≥ 5 uM, not shown). Traces are slightly offset because of the absorbance of thioflavin T at 412 nm. B) Values of kE +I obtained from traces like those in Panel A were analyzed with eq. 3. For thioflavin T, KI = 1.24 ± 0.04 µM and α = 0.0017 ± 0.0033 (not significantly different from zero). For edrophonium, KI = 0.43 ± 0.02 µM and α = 0.0037 ± 0.0034 (not significantly different from zero).
The analyses in Fig. 3 were repeated in Fig. 4 with the substrate ATMA, a cationic acetanilide that is hydrolyzed much more slowly than acetylthiocholine (kE [I]=0 is 700-fold lower for ATMA than for acetylthiocholine, (17)). The absorbance difference for ATMA is smaller than that for acetylthiocholine in the Ellman assay, and a somewhat higher [S]0 of 10 µM (relative to the ATMA Kapp of 47 µM (17)) was necessary for precise fitting. Nevertheless, the hydrolysis curves (Fig. 4A) again showed a good exponential correspondence to eq. 2, and values of kE +I at various inhibitor concentrations could be analyzed with eq. 3 (Fig. 4B). Edrophonium inhibited ATMA hydrolysis with a KI value in good agreement with those reported in Figs. 2 and 3, and α for edrophonium was again zero. These values strongly support the conclusions that ATMA and edrophonium compete for binding to the A-site and that ATMA binding to the A-site is on the catalytic pathway for ATMA hydrolysis by AChE. However, in contrast to its inhibition of acetylthiocholine hydrolysis, thioflavin T failed to show any inhibition of ATMA hydrolysis and gave an α value of 1.0 (Fig. 4B). We previously reported an α of 0.47 for thioflavin T inhibition of ATMA hydrolysis (17), but this value was obtained from an analysis of reciprocal plot slopes with only two concentrations of thioflavin T. The greater number of thioflavin T concentrations examined in Fig. 4B make the 1.0 value for α more reliable.
Fig. 4.
Inhibition of ATMA hydrolysis by the A-site inhibitor edrophonium or the P-site inhibitor thioflavin T. A) Examples of experimental traces that were analyzed with eq. 2 to obtain values of kE +I. The substrate S was ATMA ([S]0 = 10 µM), the inhibitor I was edrophonium ([I] as indicated), and [E]tot was 190 nM (for [I] ≤ 3 uM) or 1900 nM (for [I] ≥10 uM, not shown). Times zero in the traces are offset to avoid overlap. B) Values of kE +I obtained from traces like those in Panel A were analyzed with eq. 3. Data for thioflavin T were averaged from two data sets, and errors were within the symbols. For edrophonium, KI = 0.54 ± 0.03 µM and α = 0.0003 ± 0.0016 (not significantly different from zero). No KI could be fitted for thioflavin T, so KI was fixed at 1.24 µM (Fig. 3) to give α = 0.98 ± 0.04.
To investigate thioflavin T inhibition with another slow substrate of AChE, we turned to carbachol (Fig. 1), an isosteric carbamate analog of acetylthiocholine. The kE for carbachol is 600,000-fold lower than that for acetylthiocholine, and the carbamoylation rate constant k12 can be separated from the decarbamoylation rate constant k21 by analysis of the approach to steady state carbamate turnover (25) (see eq. 5). This approach can be monitored by either thioflavin T fluorescence or simultaneous acetylthiocholine hydrolysis (24), and reactions monitored by acetylthiocholine hydrolysis are illustrated in Fig. 5A. Initial acetylthiocholine hydrolysis rates progressively decreased as AChE was carbamoylated until a final, relatively slow steady-state hydrolysis rate was attained. To achieve k12 rate constants that were second order and corresponded to eq. 6, carbachol was maintained at 0.5 mM (where Kapp ≡ KM was 5.7 mM (24)). Acetylthiocholine concentrations were 50 µM to minimize [S2]/Kapp2 in eq. 6. As with ATMA, thioflavin T failed to show any inhibition of carbamoylation by carbachol and gave an α value of 1.0 (Fig. 5B).
Fig. 5.
Thioflavin T effects on carbamoylation of AChE by carbachol monitored by acetylthiocholine hydrolysis. A) Reactions were initiated by stopped-flow mixing of AChE and thioflavin T in one syringe and carbachol, thioflavin T, acetylthiocholine, and DTNB in the second syringe (7). Final concentrations were 0.5 mM carbachol, 50 µM acetylthiocholine, 10 mM DTNB, thioflavin T as indicated, 0.5 nM AChE, 30 mM sodium phosphate, 60 mM NaCl, and 0.02% Triton X-100. Experimental traces were analyzed with eq. 5 to obtain values of k12. B) Values of k12 from traces like those in Panel A were obtained at levels of [S1] and [S2] that were low enough to correspond to eq. 6. [S1]/Kapp2 was assumed negligible (Kapp2 in this buffer was 90 µM), allowing k12 analysis with eq. 3. No KI could be fitted, so KI was fixed at 1.91 µM (24) to give α = 0.99 ± 0.02.
4. Discussion
4.1 With AChE substrates that reach equilibrium, bound P-site ligands do not lower the relative acylation rate constant a
The general expression for kE in the rate equation for Scheme 2 depends on whether substrate and inhibitor binding reach equilibrium with the enzyme. Classical kinetic analyses assume such equilibria, and with this assumption α in eq. 3 is given by ali, the ratio of the relative acylation rate constant a and the relative affinity i for the ternary complex involving substrate and inhibitor in Scheme 2. The substrates ATMA and carbachol do equilibrate with AChE, and Figs. 4 and 5 indicate that α is 1.0 when thioflavin T is bound at the P-site in ternary complexes with both substrates. Thermodynamic data indicate that i is close to 1.0 (0.9) for the ternary complex with carbachol at the A-site and thioflavin T at the P-site (7), so a also must also be close to 1.0 for the carbamoylation step k2 that arises from this complex. Less rigorous thermodynamic data for ATMA and thioflavin T suggested that i may be slightly greater than 1 (as much as 3) and a about 1.3 (17), but the more accurate value of α obtained here indicate that both are likely to be closer to 1.0. In no case has a ligand that binds specifically to the P-site been shown to inhibit the acylation reaction k2 by exhibiting an a < 1. However, an enhancement or acceleration of the acylation reaction (a > 1) has been observed in a few cases, but only when the ligand bound at the P-site is a close analog of acetylcholine. These cases include acceleration of acylation by a second molecule of substrate at the P-site when the first substrate molecule is already at the A-site, seen with ATMA and wild-type human AChE (17), acetylthiocholine with the human W86F mutant AChE (17), and the carbamate substrate M7C with wild-type eel and human AChE (25, 29). Neither substrate activation nor acceleration of acylation by another P-site ligand is seen with acetylthiocholine and wild-type [human] AChE, presumably because AChE has evolved to the extent where this acylation step is no longer rate-limiting with this highly specific substrate.
4.2 Values of the inhibition constant α provide important information about the mechanism of substrate hydrolysis
How then is the α value of zero for the ternary complex with acetylthiocholine at the A-site and thioflavin T at the P-site in Fig. 3 to be interpreted? It is extremely unlikely that a is zero for this complex for all of the reasons just cited. The answer lies in the nature of the rate-limiting step for kE with acetylthiocholine. As emphasized by Quinn (20), the rate constant kE monitors conversion of free enzyme and free substrate to a transition state(s) in the acylation stage of catalysis, and it incorporates all initial reversible steps in the pathway up to the first irreversible step. According to Scheme 2, the acylation reaction k2 for AChE is virtually irreversible, but it is not necessarily the first irreversible step, i.e., the step in which the forward reaction rate constant for an intermediate is considerably larger than the backward reaction rate constant for this intermediate. When k2 is larger than k−1 and k−S2 in Scheme 2, bound substrate does not reach equilibrium with AChE and k2 is no longer rate limiting. A general steady-state expression for kE from Scheme 2 may be derived 4, and it shows that eq. 3 involves a small approximation: The denominator of this equation includes additional [I] terms. However, the limiting value of kE [I]=0/kE +I at high [I] is still 1/α, as predicted in eq. 3, and the corresponding expression for α is given in eq. 7.
| (eq. 7) |
According to Scheme 2, when [I] is 0, kE ≡ kcat/Kapp = kSk1k2/(k−Sk−1 + k2k−S + k2k1) (15). When the substrate S is equilibrated with AChE (k2 ≪ k−1 and ak2 ≪ ik−S2), substitution into eq. 7 confirms that α = ali, as noted above. However, when S does not reach equilibrium (k2 ≫ k−1 and ak2 ≫ ik−S2), then α = (kS2/kS)(k1 + k−S)/k1. If S binding is rate limiting (k1 ≫ k−S), α = kS2/kS, the ratio of the association rate constant for S with EIP to the association rate constant for S with E. This ratio is expected to be close to zero, because bound P-site ligand blocks the entry of a second ligand to the A-site by a factor of close to 100, a process that we have called steric blockade (15, 23) . If S migration from the P-site to the A-site is rate limiting (k−S ≫ k1 but k2 ≫ k−1), then α = kS2(KS/k1). In this case α again would be expected to be close to zero because of the low relative value of kS2.
Therefore, eq. 7 reveals how a bound P-site ligand can inhibit second order substrate hydrolysis rate constants kE for rapidly hydrolyzed substrates like acetylthiocholine while showing no inhibition of slowly hydrolyzed substrates like ATMA or carbachol that reach equilibrium with the A-site. It remains to be determined whether substrate association or substrate migration from the P-site to the A-site is rate limiting for kE with substrates that show some inhibition by P-site ligands like thioflavin T.
Acknowledgments
This work was supported by grant NS-16577 from the National Institutes of Health, by contract HDTRA1-08-C-0015 from the Department of Defense, and by grants from the Muscular Dystrophy Association of America
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The manuscript is dedicated to Professor Pelayo Camps, from the Medicinal Chemistry Laboratory of the Faculty of Pharmacy of the University of Barcelone, on the occasion of his 65th anniversary.
Abbreviations: AChE, acetylcholinesterase; ATMA, 3-(acetamido)-N,N,Ntrimethylanilinium; DTNB, 5,5'-dithiobis-(2-nitrobenzoic acid); M7C, N-methyl(7-dimethylcarbamoxy)quinolinium iodide; TcAChE, AChE from Torpedo californica.
Throughout this paper we number amino acid residues according to the human AChE sequence unless otherwise noted.
T. L. Rosenberry, manuscript in preparation.
References
- 1.Rosenberry TL. Acetylcholinesterase. In: Meister A, editor. Advances in Enzymology. New York: John Wiley & Sons; 1975. pp. 103–218. [Google Scholar]
- 2.Giacobini E. Cholinesterase inhibitors: from the Calabar bean to Alzheimer therapy. In: Giacobini E, editor. Cholinesterases and Cholinesterase Inhibitors. London: Martin Dunitz; 2000. pp. 181–226. [Google Scholar]
- 3.Millard CB, Broomfield CA. Anticholinesterases: medical applications of neurochemical principles. J. Neurochem. 1995;64:1909–1918. doi: 10.1046/j.1471-4159.1995.64051909.x. [DOI] [PubMed] [Google Scholar]
- 4.Changeux J-P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol. Pharmacol. 1966;2:369–392. [PubMed] [Google Scholar]
- 5.Taylor P, Lappi S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry. 1975;14:1989–1997. doi: 10.1021/bi00680a029. [DOI] [PubMed] [Google Scholar]
- 6.De Ferrari GV, Mallender WD, Inestrosa NC, Rosenberry TL. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biol. Chem. 2001;276:23282–23287. doi: 10.1074/jbc.M009596200. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberry TL, Sonoda LK, Dekat SE, Cusack B, Johnson JL. Analysis of the reaction of carbachol with acetylcholinesterase using thioflavin T as a coupled fluorescence reporter. Biochemistry. 2008;47:13056–13063. doi: 10.1021/bi8015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 9.Harel M, Quinn DM, Nair HK, Silman I, Sussman JL. The X-ray structure of a transition state analog complex reveals the molecular origins of the catalytic power and substrate specificity of acetylcholinesterase. J. Am. Chem. Soc. 1996;118:2340–2346. [Google Scholar]
- 10.Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. The crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J. Am. Chem. Soc. 2008;130:7856–7861. doi: 10.1021/ja7109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourne Y, Taylor P, Radic Z, Marchot P. Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J. 2003;22:1–12. doi: 10.1093/emboj/cdg005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harel M, Kleywegt GJ, Ravelli RBG, Silman I, Sussman JL. Crystal structure of an acetylcholinesterase-fasciculin complex: interaction of a three-fingered toxin from snake venom with its target. Structure. 1995;3:1355–1366. doi: 10.1016/s0969-2126(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 13.Bourne Y, Taylor P, Marchot P. Acetylcholinesterase inhibition by fasciculin: Crystal structure of the complex. Cell. 1995;83:503–512. doi: 10.1016/0092-8674(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 14.Tara S, Elcock AH, Kirchhoff PD, Briggs JM, Radic Z, Taylor P, McCammon JA. Rapid binding of a cationic active site inhibitor to wild type and mutant mouse acetylcholinesterase: Brownian dynamics simulation including diffusion in the active site gorge. Biopolymers. 1998;46:465–474. doi: 10.1002/(SICI)1097-0282(199812)46:7<465::AID-BIP4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Szegletes T, Mallender WD, Thomas PJ, Rosenberry TL. Substrate binding to the peripheral site of acetylcholinesterase initiates enzymatic catalysis. Substrate inhibition arises as a secondary effect. Biochemistry. 1999;38:122–133. doi: 10.1021/bi9813577. [DOI] [PubMed] [Google Scholar]
- 16.Mallender WD, Szegletes T, Rosenberry TL. Acetylthiocholine binds to Asp74 at the peripheral site of human acetylcholinesterase as the first step in the catalytic pathway. Biochemistry. 2000;39:7753–7763. doi: 10.1021/bi000210o. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JL, Cusack B, Davies MP, Fauq A, Rosenberry TL. Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate. Biochemistry. 2003;42:5438–5452. doi: 10.1021/bi027065u. [DOI] [PubMed] [Google Scholar]
- 18.Stojan J, Golicnik M, Fournier D. Rational polynomial equation as an unbiased approach for the kinetic studies of Drosophila melanogaster acetylcholinesterase reaction mechanism. Biochim. Biophys. Acta. 2004;1703:53–61. doi: 10.1016/j.bbapap.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberry TL. Strategies to resolve the catalytic mechanism of acetylcholinesterase. J. Mol. Neurosci. 2010;40:32–39. doi: 10.1007/s12031-009-9250-3. [DOI] [PubMed] [Google Scholar]
- 20.Quinn DM. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987;87:955–979. [Google Scholar]
- 21.Mallender WD, Szegletes T, Rosenberry TL. Organophosphorylation of acetylcholinesterase in the presence of peripheral site ligands: Distinct effects of propidium and fasciculin. J. Biol. Chem. 1999;274:8491–8499. doi: 10.1074/jbc.274.13.8491. [DOI] [PubMed] [Google Scholar]
- 22.Eastman J, Wilson EJ, Cervenansky C, Rosenberry TL. Fasciculin 2 binds to a peripheral site on acetylcholinesterase and inhibits substrate hydrolysis by slowing a step involving proton transfer during enzyme acylation. J. Biol. Chem. 1995;270:19694–19701. doi: 10.1074/jbc.270.34.19694. [DOI] [PubMed] [Google Scholar]
- 23.Szegletes T, Mallender WD, Rosenberry TL. Nonequilibrium analysis alters the mechanistic interpretation of inhibition of acetylcholinesterase by peripheral site ligands. Biochemistry. 1998;37:4206–4216. doi: 10.1021/bi972158a. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberry TL, Sonoda LK, Dekat SE, Cusack B, Johnson JL. Monitoring the reaction of carbachol with acetylcholinesterase by thioflavin T fluorescence and acetylthiocholine hydrolysis. Chem.-Biol. Interact. 2008;175:235–241. doi: 10.1016/j.cbi.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberry TL, Johnson JL, Cusack B, Thomas J, Emani S, Venkatasubban KS. Interactions between the peripheral site and the acylation site in acetylcholinesterase. Chem. Biol. Interact. 2005;157–158:181–189. doi: 10.1016/j.cbi.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA. 1993;90:9031–9035. doi: 10.1073/pnas.90.19.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge AS, Humphrey DR, Rosenberry TL. Ambenonium is a rapidly reversible noncovalent inhibitor of acetylcholinesterase with one of the highest known affinities. Mol. Pharmacol. 1992;41:937–942. [PubMed] [Google Scholar]
- 28.Camps P, Cusack B, Mallender WD, El Achab R, Morral J, Muñoz-Torrero D, Rosenberry TL. Huprine X is a novel high affinity inhibitor of acetylcholinesterase that is of interest for the treatment of Alzheimer's disease. Mol. Pharmacol. 2000;57:409–417. [PubMed] [Google Scholar]
- 29.Tomlinson G, Mutus B, McLennan I. Modulation of acetylcholinesterase activity by peripheral site ligands. Mol. Pharmacol. 1980;18:33–39. [PubMed] [Google Scholar]