Abstract
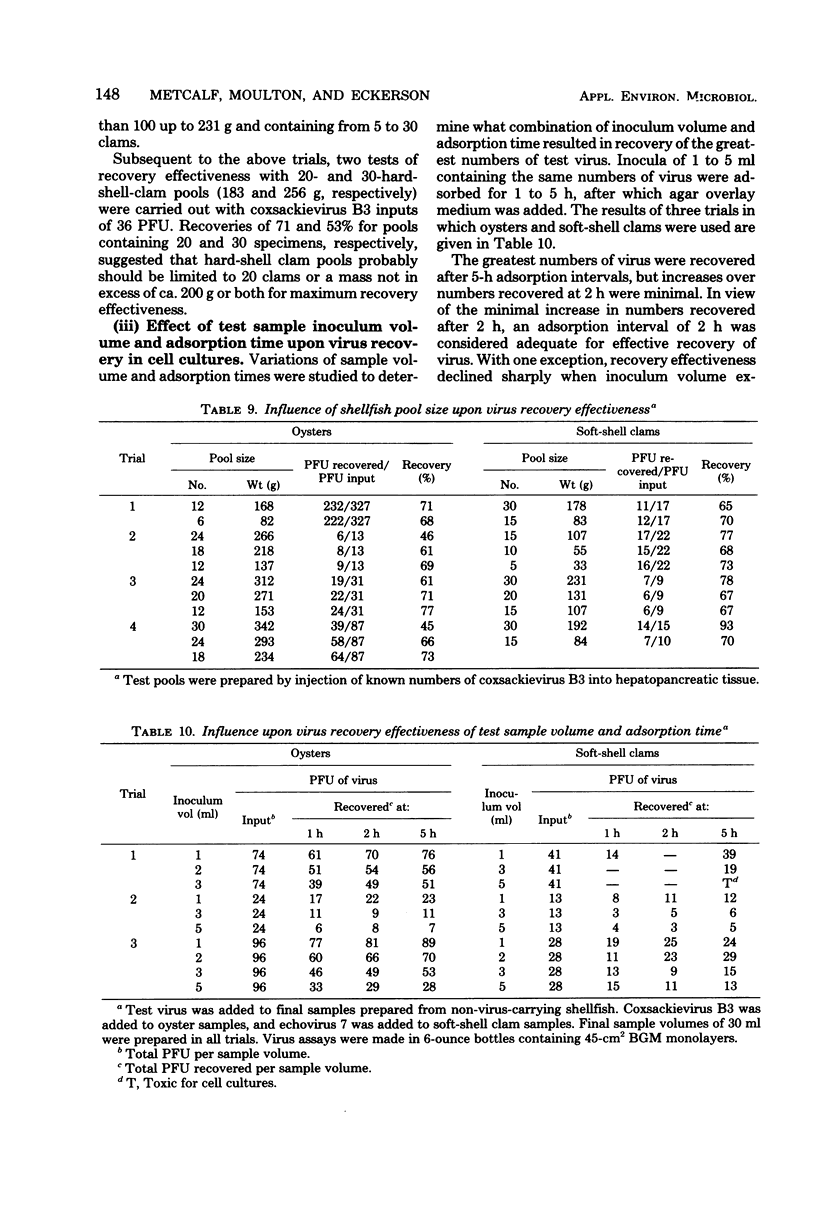
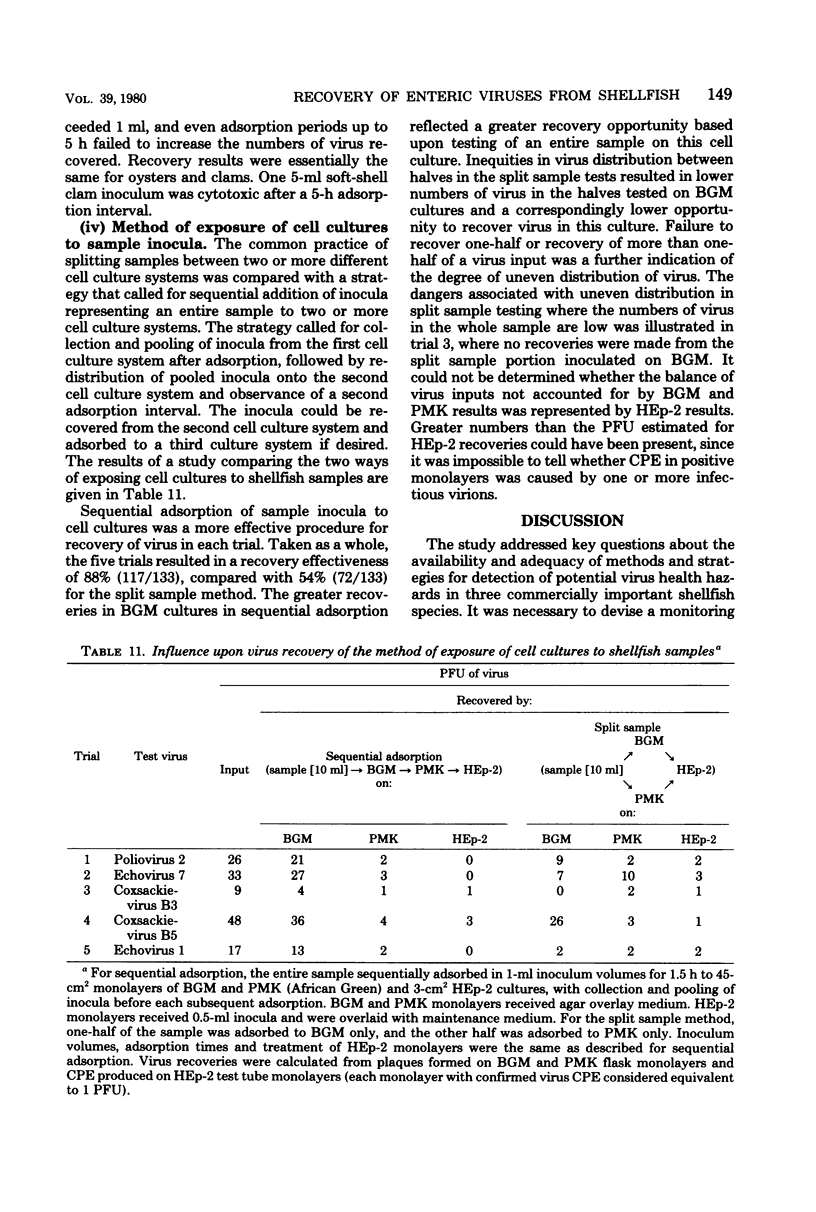
An improved recovery method and testing strategy were devised for recovery of low numbers of enteric viruses from each of three commercially important shellfish species. Effective recovery of virus depended as much upon details of the test strategy adopted for use of the improved method with each species as on the method itself. The most important test details involved sample composition, pool size, and method of use of cell cultures. Recovery sensitivity measured permitted detection of 25 to 3 plaque-forming units of enteroviruses and 100 to 27 plaque-forming units of reovirus through their recovery in cell culture, with effectivenesses averaging 64 and 46%, respectively. Test samples prepared by the improved recovery method were virtually cytotoxicity free. Optimal recovery of virus on 45-cm2 cell culture monolayers was obtained with 1-ml inocula adsorbed for 2 h. The most effective recovery of virus from shellfish samples was made by a sequential adsorption procedure which allowed equal exposure of an entire sample to each of two or more cell cultures. Removal of nonviral contaminants from test samples by antibiotic treatment was preferable to the use of ether or membrane filtration procedures.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- Di Girolamo R., Liston J., Matches J. Ionic bonding, the mechanism of viral uptake by shellfish mucus. Appl Environ Microbiol. 1977 Jan;33(1):19–25. doi: 10.1128/aem.33.1.19-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Morphologic characteristics of plaques produced on monkey kidney monolayer cultures by enteric viruses (poliomyelitis, Coxsackie, and echo groups. J Immunol. 1957 Feb;78(2):128–135. [PubMed] [Google Scholar]
- Herrmann J. E., Cliver D. O. Methods for detecting food-borne enteroviruses. Appl Microbiol. 1968 Oct;16(10):1564–1569. doi: 10.1128/am.16.10.1564-1569.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenbader K. D., Jr, Cliver D. O. Polyelectrolyte flocculation as an aid to recovery of enteroviruses from oysters. Appl Microbiol. 1972 Oct;24(4):540–543. doi: 10.1128/am.24.4.540-543.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu O. C., Seraichekas H. R., Murphy B. L. Fate of poliovirus in northern quahaugs. Proc Soc Exp Biol Med. 1966 Feb;121(2):601–607. doi: 10.3181/00379727-121-30841. [DOI] [PubMed] [Google Scholar]
- METCALF T. G., STILES W. C. THE ACCUMULATION OF ENTERIC VIRUSES BY THE OYSTER, CRASSOSTREA VIRGINICA. J Infect Dis. 1965 Feb;115:68–76. doi: 10.1093/infdis/115.1.68. [DOI] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Ho H. H., Riggs J. L., Lennette E. H. Comparative sensitivity of various cell culture systems for isolation of viruses from wastewater and fecal samples. Appl Environ Microbiol. 1978 Sep;36(3):480–486. doi: 10.1128/aem.36.3.480-486.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J. T., Sullivan R., Larkin E. P., Peeler J. T. Comparison of methods for the recovery of virus inoculated into ground beef. Appl Microbiol. 1973 Oct;26(4):497–501. doi: 10.1128/am.26.4.497-501.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ver B. A., Melnick J. L., Wallis C. Efficient filtration and sizing of viruses with membrane filters. J Virol. 1968 Jan;2(1):21–25. doi: 10.1128/jvi.2.1.21-25.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W. Demonstration of solids-associated virus in wastewater and sludge. Appl Environ Microbiol. 1976 Mar;31(3):354–358. doi: 10.1128/aem.31.3.354-358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W., Pierce L. V. Demonstration of virus in groundwater after effluent discharge onto soil. Appl Microbiol. 1975 Jun;29(6):751–757. doi: 10.1128/am.29.6.751-757.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



