Abstract
β-Secondary deuterium isotope effects have been measured for equine serum butyrylcholinesterase-catalyzed hydrolysis of acetyl-L3-thiocholine (L = H or 2H). The dependencies of initial rates on isotopic substrate concentrations show close adherence to Michaelis-Menten kinetics, and yield the following isotope effects: D3kcat/Km = 0.98 ± 0.02 and D3kcat = 1.10 ± 0.02. The modestly inverse isotope effect on kcat/Km is consistent with partial rate limitation by a step that converts the sp2-hybridized ester carbonyl of the E + A reactant state into a quasi-tetrahedral transition state in the acylation stage of catalysis. On the other hand, the markedly normal isotope effect on kcat indicates that the Michaelis complex that accumulates at substrate saturation of the active site during catalytic turnover is a tetrahedral intermediate, whose decomposition is the rate-limiting step. These results compliment a previous report [J. R. Tormos et al., J. Am. Chem. Soc. 127 (2005) 14538–14539] that showed that substrate-activated hydrolysis of acetylthiocholine, catalyzed by recombinant human butyrylcholinesterase, is also rate limited by decomposition of an accumulating tetrahedral intermediate.
Keywords: butyrylcholinesterase, catalytic mechanism, transition state structure, isotope effects
1. Introduction
The cholinesterases comprise a family of enzymes that are noted for their catalytic power [1,2]. Acetylcholinesterase (AChE) catalyzes the hydrolysis of acetylcholine and acetylthiocholine (ATCh) with rate constants that approach diffusion control at low substrate concentrations, and accelerates the hydrolysis of acetylcholine by 1013-fold. Though an order of magnitude slower than AChE, BuChE nonetheless effects catalytic turnover of ATCh and butyrylthiocholine (BuTCh) with admirable efficiency [3]. Elucidating the molecular origins of the high catalytic activity of cholinesterases is therefore an endeavor of fundamental importance. However, due to the very high catalytic activities of cholinesterases (kcat ~ 104 s−1 for AChE-catalyzed hydrolysis of ATCh [1,2]; kcat ~ 103 s−1 for BuChE-catalyzed hydrolysis of BuTCh [3]), characterization of the structural changes in the substrate that accompany catalytic turnover can be challenging.
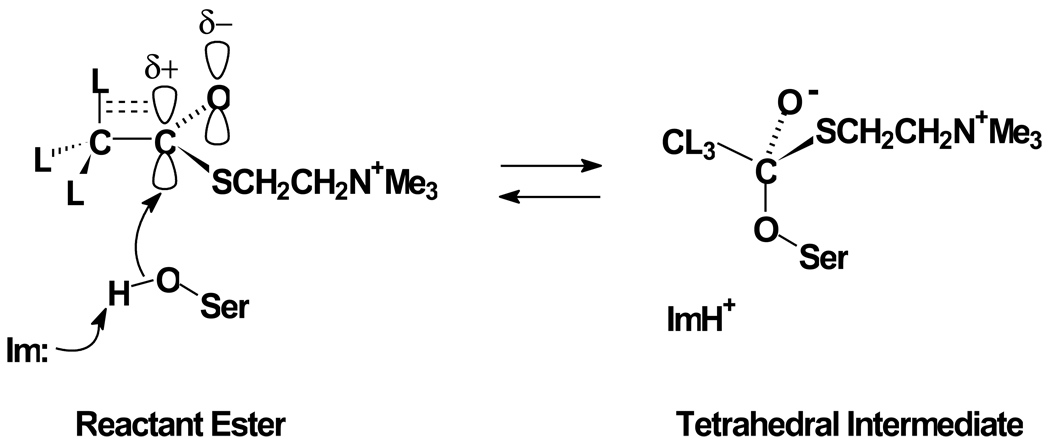
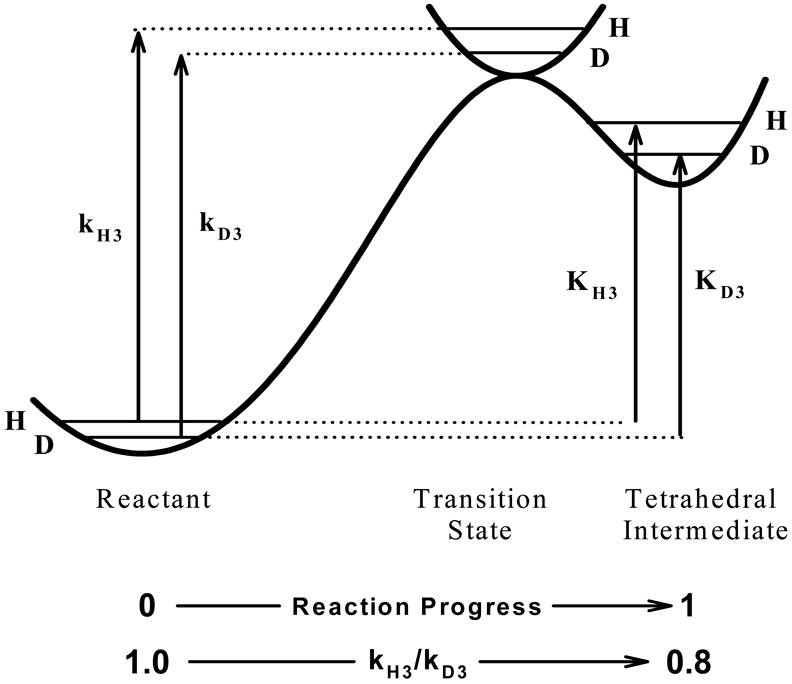
Kinetic secondary isotope effects provide probes of chemical reaction mechanisms, including those that occur in the active sites of enzymes, that report on structural changes as the substrate is converted from the reactant state to the transition state. Consider, for example, the conversion of acetylthiocholine to a tetrahedral intermediate (one of the several steps in acyl transfer reactions [4]) in the acylation stage of catalysis, as outlined in Figure 1. Nucleophilic attack of the active site serine on the substrate converts the carbonyl carbon from sp2 hybridization in the reactant state to sp3 hybridization in the tetrahedral intermediate. In the reactant state, the carbonyl C=O has a marked bond dipole that is δ+ at the carbonyl carbon. This and the π-system of C=O allow for resonance delocalization of β-CL (L = H or 2H) σ-electron density into the carbonyl system by hyperconjugation [5]. This electron delocalization decreases electron density in the β-CL bonds. In the tetrahedral intermediate the π-system is no longer available, hyperconjugation is not possible, and electron density is returned to β-CL bonds, which increases the strength (stiffness) of the bonds. The increased stiffness of β-CL bonds in the tetrahedral intermediate increases the curvature of the vibrational potential in the tetrahedral intermediate versus the reactant state, as shown in Figure 2. This renders the isotopic difference in β-CL zero point energies larger in the tetrahedral intermediate than in the reactant state, which in turn necessitates that ΔEH3 > ΔED3, and therefore D3K ≡ KH3/KD3 = 0.8. Correspondingly, the kinetic β-secondary deuterium isotope effect D3k ≡ kH3/kD3 will be increasingly inverse (i.e. will more closely approach the equilibrium isotope effect D3K = 0.8) the more the transition state resembles the tetrahedral intermediate. Therefore, inverse kinetic β-secondary deuterium isotope effects for acyl transfer reactions not only indicate that the sp2-hybridized reactant state is being converted to a sp3-hybridized tetrahedral intermediate, but also provide a measure of how far the reaction assembly has progressed from sp2 to sp3 hybridization in the transition state [5,6].
Fig. 1.
Structural origins of β-secondary deuterium isotope effects for the formation of the tetrahedral intermediate in the acylation stage BuChE-catalyzed hydrolysis of ATCh. The γ-oxygen of the active site serine covalently adds to the carbonyl carbon of the thioester function of the substrate, with general-base assistance by the imidazole sidechain (Im) of the active site histidine. This nucleophilic addition is marked by change of the hybridization of the carbonyl carbon from sp2 in the reactant state to sp3 in the tetrahedral intermediate.
Fig. 2.
Energetic origins of β-secondary deuterium isotope effects for the formation of the tetrahedral intermediate in the acylation stage of BuChE-catalyzed hydrolysis of ATCh. Verticle arrows denote values of ΔEa, and are labeled with the corresponding isotopic reaction rate or equilibrium constant. Interpretation of isotope effects in terms of structural changes in the substrate is discussed in the text.
When the substrate in the reactant state is sp3 hybridized and hybridization is changing toward sp2 in the transition state, the situation is the reverse of that depicted in Figure 2. In this case the β-CL vibrational potential will be shallower in the transition state than in the reactant state, and the kinetic β-secondary deuterium isotope effect will be normal, i.e. D3k > 1.0. Moreover, the upper limit for D3k, i.e. that for equilibrium conversion of the sp3 reactant state to the sp2 product should be 1/0.8, or about 1.25.
What are the expectations for kinetic β-secondary deuterium isotope effects for acyl transfer reactions, of which the elementary step shown is Figure 1 is a component? Most acyl transfer reactions proceed through addition-decomposition mechanisms, in which the nucleophile first attacks the susceptible carbonyl carbon to generate a tetrahedral intermediate, which breaks down with the expulsion of the leaving group to produce the products [4]. Tetrahedral intermediates in nonenzymic acyl transfer reactions are high-energy, steady-state intermediates, less stable than reactants or products by 40–100 kJ mol−1[4]. Therefore, according to the Hammond postulate [7] the transition states for formation and breakdown of the tetrahedral intermediate should resemble the intermediate both in energy and structure. In this situation, kinetic β-secondary deuterium isotope effects will always be inverse, no matter whether formation or breakdown of the intermediate is rate limiting, as illustrated in Figures 1 and 2.
Enzymes achieve their impressive catalytic power, as measured by the degree to which they accelerate erstwhile sluggish nonenzymic reactions, by lowering the energy of the transition state(s), which in turn lowers the free energy of activation [8]. Understanding how enzymes do this is the crux of understanding their catalytic function. A way in which cholinesterases, and other enzymes that catalyze acyl transfer reactions, may realize a substantial portion of their catalytic accelerations is to stabilize high-energy tetrahedral intermediates, which in turn will stabilize the transition states for their formation and decomposition. In this paper, results of isotope effect experiments are described that show that equine serum BuChE stabilizes a tetrahedral intermediate to such an extent that the intermediate is an accumulating Michaelis complex (i.e. reactant state) during catalytic turnover. Results for equine serum BuChE are compared to those in the literature for human BuChE catalysis [9], and are discussed in light of observations of tetrahedral intermediates in x-ray structures of the enzyme [10].
2. Materials and Methods
Equine serum BuChE was obtained gratis from Dr. B. P. Doctor of the Department of Biochemistry of Walter Reed Army Institute of Research. Acetyl-2H-thiocholine (deuterated ATCh) was synthesized as previously described [6]. ATCh, DTNB and buffer salts, Na2HPO4 and NaH2PO4, were obtained from Sigma Chemical Co. (St. Louis, MO, USA). SigmaPlot 8.0 (Systat, Chicago, IL, USA) was used for graphical presentation and quantitative analysis of experimental data.
Initial rates of equine serum BuChE-catalyzed hydrolysis of ATCh were measured by the coupled colorimetric assay described by Ellman et al. [11]. Reactions were followed at 27 °C and pH = 7.25 in 0.1 M NaH2PO4/Na2HPO4 buffer that contained 0.5 mM DTNB as increases in absorbance at 412 nm on a 96-well SPECTRAmax Plus 384 microplate reading UV-visible spectrophotometer. Initial rates were measured in the presence and absence of BuChE, and the nonenzymic rate was substracted from the total rate measured in the presence of enzyme to give the net enzyme reaction rate. The kinetic parameters Vmax and Km were calculated by fitting data to the Michaelis-Menten equation:
| (1) |
When [A] ≪ Km, equation 1 reduces to Vi = (Vmax/Km)[A], and first-order kinetics are observed. In this situation reactions were followed for at least three half lives, and pseudo first-order rate constants were calculated by fitting absorbance versus time data to the following equation:
| (2) |
In equation 2 A, A0 and A∞ are absorbances at times t, 0 and infinity, respectively, and k = Vmax/Km.
Because Vmax = kcat[E]T and the same enzyme concentration was used to measure initial rates for both isotopic ATCh substrates, isotope effects on Vmax are isotope effects on kcat. The nomenclature espoused by Northrop [12] and Cleland [13] for tabulating isotope effects is used herein. Thus, D3kcat = kcatH3/kcatD3 is the kinetic β-secondary deuterium isotope effect on kcat, which is measured as the ratio of the Vmax values of the isotopic substrates. The leading superscript denotes the heavy isotope utilized in the isotope effect determination. Similarly, D3kcat/Km = (kcat/Km)H3/(kcat/Km)D3, and is measured as the ratio of the Vmax/Km values of the isotopic substrates.
3. Results
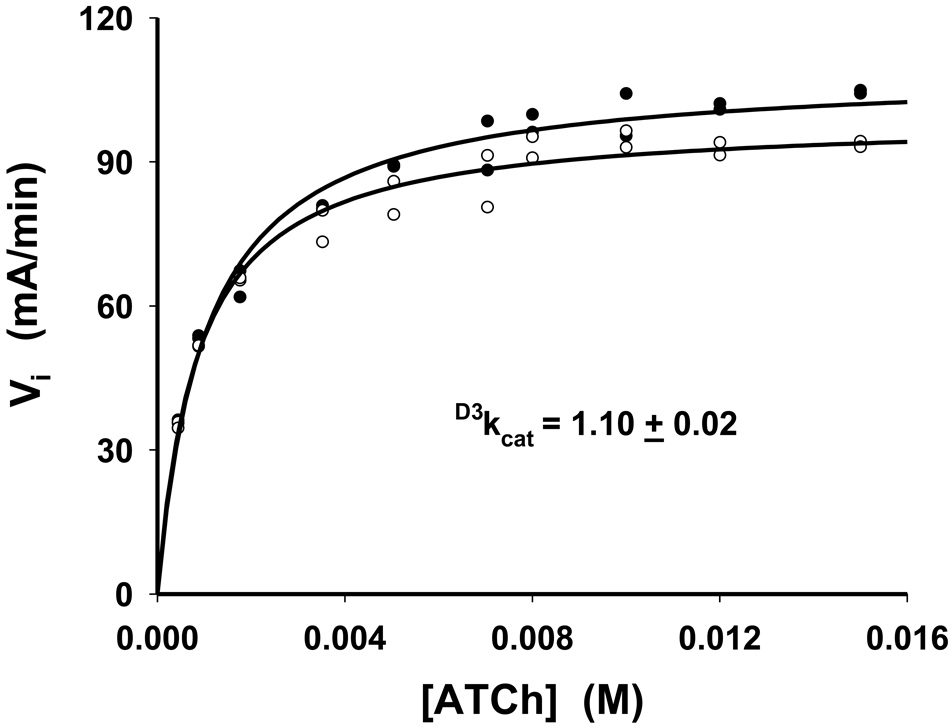
Initial rates were measured for equine serum BuChE-catalyzed hydrolysis of acetyl-H3-thiocholine and acetyl-2H3-thiocholine as a function of substrate concentration, and the data are plotted in Figure 3. For each of the isotopic substrates, close adherence to Michaelis-Menten kinetics is observed, as demonstrated by the nonlinear fits to the Michaelis-Menten equation (equation 1 above) in the figure. These fits provide the following results: acetyl-1H-thiocholine, VmaxH3 = 1.24 ± 0.03 × 10−7 M s−1, acetyl-2H-thiocholine, VmaxD3 = 1.14 ± 0.01 × 10−7 M s−1, . Consequently, these fits provide a measure of the isotope effect on kcat of reasonable precision, D3kcat = 1.10 ± 0.02. Additional isotope effects calculated from these data are and D3kcat/Km = 0.84 ± 0.10. The sizeable uncertainty in D3kcat/Km suggests that one should not view the value of the isotope effect as reliable. To measure this isotope effect with much greater precision, first-order time courses for turnover of 0.05 mM (~ Km/20) acetyl-1H3-thiocholine and acetyl-2H3-thiocholine were acquired over greater than three half-lives (> 88% of hydrolysis of the initial substrate concentration; data not shown). The isotope effect was determined in triplicate, and gave D3kcat/Km = 0.98 ± 0.02.
Fig. 3.
Dependencies of initial rates on substrate concentrations for equine serum BuChE-catalyzed hydrolysis of acetyl-L3-thiocholine (L = H or 2H). Solid lines were generated by fitting data to equation 1. Open circles, acetyl-2H3-thiocholine; closed circles, acetyl-H3-thiocholine.
4. Discussion
As discussed in the Introduction, the expectation for acyl transfer reactions is that transition states will resemble high-energy tetrahedral intermediates, and thus will be characterized by inverse kinetic β-secondary deuterium isotope effects. Indeed, the isotope effect D3kcat/Km = 0.98 ± 0.02 is inverse, though modestly so. The isotope effect on kcat/Km monitors the conversion of the E + A reactant state to the rate-limiting transition state that precedes or is concomitant with the first irreversible step in the mechanism. For cholinesterase reactions that are monitored under initial rate conditions, the first irreversible event is release of the alcohol product that completes the acylation stage of catalysis. This feature is illustrated by considering the following kinetic mechanism for BuChE catalysis:
The various species in the mechanism are E = free enzyme; A = free substrate; EA = initial noncovalent Michaelis complex; FP = complex of acylenzyme intermediate and alcohol product P; F = acylenzyme intermediate; EQ = complex of enzyme and acetate product Q. Note that k7 is the pseudo first-order rate constant for hydrolysis of the acylenzyme intermediate. The observed rate constants that are obtained from the Michaelis-Menten parameters for this mechanism are given by equations 3:
| (3) |
Hence, as alluded to above, kcat/Km monitors events up to the release of the alcohol product, with rate constant k5. Note that kcat/Km may be rate limited by substrate binding (k1), by chemical steps that produce the acylenzyme intermediate (k3), or by product release (k5). On the other hand, kcat contains the overall rate constants that convert the EA complex to the acylenzyme intermediate F (ka) and the acylenzyme intermediate to E + Q (kd). Hence, kcat may be rate limited by either the acylation (ka) or deacylation (kd) stage of catalysis. The overall acylation rate constant ka = k3k5/(k3 + k4 + k5), and hence the acylation stage of catalysis could be rate limited by chemical catalysis (k3) or by alcohol product release (k5). Similarly, the overall deacylation rate constant is kd = k7k9/(k7 + k8 + k9), and the deacylation stage of catalysis could be rate limited by chemical catalysis (k7) or by acetate product release (k9).
The modest isotope effect D3kcat/Km = 0.98 ± 0.02, which is just at the level of significance, suggests that conversion of the E + A reactant state to the acylenzyme intermediate is rate limited by more than one step. Since in the reactant state the susceptible carbonyl function of the substrate is sp2 hybridized and in the transition state of the chemical step(s) the carbonyl should be tending toward sp3 hybridization, one can expect a palpably inverse isotope effect, as outlined in Figures 1 and 2. That the isotope effect is inverse but very near unity suggests that kcat/Km for horse serum BuChE-catalyzed hydrolysis of ATCh is rate limited by more than one step. A reasonable model is one in which the chemical step, which is susceptible to the isotope effect, is partially rate limiting, and that substrate binding or product release (neither of which will produce an isotope effect), or both, also contribute to rate limitation.
Interpretation of the isotope effect on kcat is more straightforward, though the implications are unusual. The marked normal isotope effect on kcat requires that the rate limiting step is one in which an sp3-hybridized carbonyl carbon in the reactant state is being converted to an sp2-like transition state. This in turn requires that the accumulating Michaelis complex (reactant state) under conditions of substrate saturation is a tetrahedral intermediate, and that decomposition of this intermediate to an sp2-hybridized product is the rate-limiting step for kcat. The normal isotope effect on kcat could arise either from rate-limiting acylation or deacylation (see equation 3 and accompanying discussion), or from partial rate limitation by both stages of catalysis. That is, the isotope effect is consistent with rate limiting decomposition of the tetrahedral intermediate in either acylation or deacylation of equine BuChE.
These unusual results are in substantial agreement with x-ray crystallographic studies of human BuChE [10], which show a covalent tetrahedral adduct of the active site serine and a butyrate moiety. This moiety is capable of turnover in the solid state, since soaking crystals with the isosteric 3-bromopropionate resulted in replacement of butyrate in the covalent tetrahedral adduct by 3-bromopropionate. Observations of tetrahedral BuChE adducts in the solid state are in agreement with results herein that show that kcat is rate limited by decomposition of an accumulating tetrahedral intermediate, and also suggest that isotope effects on kcat are monitoring, at least in part, rate-limiting events in the deacylation stage of catalysis.
A previous study of human BuChE catalysis [9] gave results that are consistent with those describe herein. Human BuChE-catalyzed hydrolysis of ATCh showed marked deviation from the relatively simple Michaelis-Menten kinetics for the equine serum enzyme. The close adherence to Michaelis-Menten kinetics of the data for both isotopic substrates in Figure 3 shows that, at high concentrations, neither substrate activation nor inhibition is observed for equine BuChE. However, substrate activation at high substrate concentrations was observed for the human BuChE reaction, and sizeable normal kinetic β-secondary deuterium isotope effects were observed on substrate-activated kcat, but not on kcat itself [9]. These isotope effects were interpreted in terms of allosteric modulation of the active site when a second molecule of substrate binds to the peripheral site of the enzyme. The second molecule of substrate leads to activation via stabilization of the tetrahedral intermediate in the deacylation stage of catalysis, whose breakdown has become the rate-limiting step [9]. Note that the normal isotope effects for human BuChE in reference 9 and those reported herein for equine BuChE do not in themselves establish whether acylation or deacylation is rate limiting for kcat. Indeed, the normal isotope effects for either enzyme could arise from rate limiting decomposition of the tetrahedral intermediate in the acylation stage of catalysis, or in the deacylation stage of catalysis, or from a combination of the two.
Acknowledgements
KLW was supported by the Predoctoral Training Program in Biotechnology, NIH grant T32GM008365.
Abbreviations
- AChE
acetylcholinesterase
- BuChE
butytylcholinesterase
- ATCh
acetylthiocholine
- BuTCh
butyrylthiocholine
- D
2H
- L
the isotopes of hydrogen, H or 2H
- DTNB
5,5’-dithiobis(2-nitrobenzoic acid)
- D3kcat/Km
β-secondary deuterium isotope effect on kcat/Km
- D3kcat
β-secondary deuterium isotope effect on kcat.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quinn DM. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987;87:955–979. [Google Scholar]
- 2.Rosenberry TL. Acetylcholinesterase. Adv. Enzymol. Relat. Areas Mol. Biol. 1975;43:103–218. doi: 10.1002/9780470122884.ch3. [DOI] [PubMed] [Google Scholar]
- 3.Masson P, Legrand P, Bartels CF, Froment M-T, Schopfer LM, Lockridge O. Role of aspartate 70 and tryptophan 82 in binding of succinyldithiocholine to human butyrylcholinesterase. Biochemistry. 1997;36:2266–2277. doi: 10.1021/bi962484a. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs N. Physical Organic Chemistry. Harlow, England: Addison Wesley Longman Ltd.; Substitution Reactions at Carbon; pp. 418–536. [Google Scholar]
- 5.Hogg JL. Secondary Hydrogen Isotope Effects. In: Gandour RD, Schowen RL, editors. Transition State of Biochemical Processes. New York and London: Plenum Press; 1978. pp. 201–224. [Google Scholar]
- 6.Malany S, Sawai M, Sikorski RS, Seravalli J, Quinn DM, Radić Z, Taylor P, Kronman C, Velan B, Shafferman A. Transition state structure and rate determination for the acylation stage of acetylcholinesterase catalyzed hydrolysis of (acetylthio)choline. J. Am. Chem. Soc. 2000;122:2981–2987. [Google Scholar]
- 7.Hammond GS. A correlation of reaction rates. J. Am. Chem. Soc. 1955;77:334–338. [Google Scholar]
- 8.Schowen RL. Catalytic Power and Transition State Stabilization. In: Gandour RD, Schowen RL, editors. Transition State of Biochemical Processes. New York and London: Plenum Press; 1978. pp. 77–114. [Google Scholar]
- 9.Tormos JR, Wiley KL, Seravalli J, Nachon F, Masson P, Nicolet Y, Quinn DM. The reactant state for substrate-activated turnover of acetylthiocholine by butyrylcholinesterase is a tetrahedral intermediate. J. Am. Chem. Soc. 2005;127:14538–14539. doi: 10.1021/ja052401q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 11.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 12.Northrop DB. Determining the Absolute Magnitude of Hydrogen Isotope Effects. In: Cleland WW, O’Leary MH, Northrop DB, editors. Isotope Effects on Enzyme-Catalyzed Reactions. Baltimore, MD, USA: University Park Press; 1977. pp. 122–150. [Google Scholar]
- 13.Cleland WW. Secondary Isotope Effects on Enzymatic Reactions. In: Buncel E, Lee CC, editors. Isotopes in Organic Chemistry. Vol. 7. Amsterdam: Elsevier; 1987. pp. 61–113. [Google Scholar]