Abstract
Production of connective tissue growth factor (CCN2, also known as CTGF) is a hallmark of hepatic fibrosis. This study examined early primary cultures of hepatic stellate cells (HSC) for (i) CCN2 regulation of its cognate receptor integrin subunits; and (ii) interactions between CCN2 and integrin α5β1, heparan sulphate proteoglycans (HSPG) or fibronectin (FN) in supporting cell adhesion. HSC were isolated from healthy male Balb/c mice. mRNA levels of CCN2 or α5, β1, αv or β3 integrin subunits were measured in days 1–7 primary culture HSC, and day 3 or day 7 cells treated with recombinant CCN2 or CCN2 small interfering RNA. Interactions between CCN2 and integrin α5β1, HSPG or FN were investigated using an in vitro cell adhesion assay. Co-incident with autonomous activation over the first 7 days, primary culture HSC increasingly expressed mRNA for CCN2 or integrin subunits. Addition of exogenous CCN2 or knockdown of endogenous CCN2 differentially regulated integrin gene expression in day 3 versus day 7 cells. Either full length CCN2 (‘CCN21–4’) or residues 247–349 containing module 4 alone (‘CCN24’) supported day 3 cell adhesion in an integrin α5β1- and HSPG-dependent fashion. Adhesion of day 3 cells to FN was promoted in an integrin α5β1-dependent manner by CCN21–4 or CCN24, whereas FN promoted HSPG-dependent HSC adhesion to CCN21–4 or CCN24. These findings suggest CCN2 regulates integrin expression in primary culture HSC and supports HSC adhesion via its binding of cell surface integrin α5β1, a novel CCN2 receptor in primary culture HSC which interacts co-operatively with HSPG or FN.
Keywords: connective tissue growth factor, CCN2, hepatic stellate cells, cell adhesion, integrin, fibronectin
Introduction
Liver fibrosis or cirrhosis is a common result of chronic liver disease such as that caused by excessive alcohol consumption or hepatitis virus infection. A main feature of liver fibrosis is overt deposition of scar tissue due to an increased expression/synthesis or impaired degradation of extracellular matrix (ECM) components such as collagen types I and III or fibronectin (FN), the latter of which is among first such proteins to be deposited in the space of Disse and around the central veins in experimental cirrhosis [1, 2]. Hepatic stellate cells (HSC) are a principal source of hepatic ECM, especially during fibrosing liver injury when they become transcriptionally activated and highly responsive to growth factors and chemokines that are released by injured hepatocytes or infiltrating macrophages [3, 4]. In their activated state, HSC are highly proliferative, chemotactic and pro-fibrogenic, the latter of which is due to the action transforming growth factor β1 (TGF-β1) which stimulates collagen production and is highly overexpressed in many fibrotic tissues [1–4]. Recently, connective tissue growth factor (CCN2, also known as CTGF), a mosaic matricellular protein containing four evolutionarily conserved structural modules, has emerged as an important player in the fibrotic response because it acts downstream of TGF-β and interacts co-operatively with TGF-β to enhance fibrotic pathways [5, 6], although the underlying mechanisms are complex and likely highly dependent on the cell type and environmental context [5, 7]. Nonetheless, CCN2 expression is increased in activated HSC in vitro as well as in fibrotic liver tissue and down-regulation of CCN2 gene expression by small interfering RNA (siRNA) or short hairpin RNA reverses, at least partially, HSC activation and the amount of collagen deposition in rodent models of hepatic fibrosis [5, 8–13].
The exact mechanisms by which CCN2 drives HSC activation and liver fibrosis are unclear even though it is well documented that CCN2 plays important roles in cell proliferation, cell survival and apoptosis, cell adhesion and migration [7, 14–17]. We and others have previously reported that integrins αvβ3 and α5β1 are CCN2 receptors on, respectively, rat HSC or rat pancreatic stellate cells [15, 17–19] and that the principal binding sites for these receptors reside in the C-terminal 103 residues of CCN2, a region that is also the location of one or more heparin-binding domains which function to regulate CCN2 activity via their engagement of cell-surface heparan sulphate proteoglycans (HSPG) [15]. To date, there are no published data regarding the regulation or role of CCN2 receptors in mouse HSC (mHSC) as a function of activation despite the increasing use of mouse models of CCN2 action in fibrosis. Therefore, in this study primary mHSC were used to determine CCN2 regulation of its cognate receptor integrin subunits and to analyse interactions between CCN2 and integrin α5β1, HSPG or FN in supporting HSC adhesion.
Materials and methods
Reagents
Heparinase I, bovine serum albumin (BSA) and anti-α smooth muscle actin (α-SMA) were purchased from Sigma-Aldrich (St. Louis, MO, USA); Dulbecco’s minimum essential medium (DMEM), DMEM/F-12, foetal bovine serum, penicillin/streptomycin, L-glutamine and trypsin/EDTA (ethylenediaminetetraacetic acid) were from Mediatech, Inc. (Manassas, VA, USA); OptiPrep™, Trizol®, DNA ladder, CyQuant NF Cell Proliferation Assay Kit, Alexa Fluor® 568 goat anti-chicken secondary antibody, SuperScript™ II reverse trascriptase and Platinum® Taq DNA polymerase were from Invitrogen (Carlsbad, CA, USA); collagenase type IV was from Worthington Biochemical Corporation (Lakewood, NJ, USA); protease inhibitor cocktail (EDTA-free), Pronase E and DNase I were from Roche Diagnostics GmbH (Mannheim, Germany); Silencer® Select siRNAs [scrambled, targeting glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or CCN2], siPORT™ NeoFX™ Transfection Agent, Oligo(dT)12–16 and SYBR® Green PCR Master Mix reagent were from Applied Biosystems (Foster City, CA, USA); FN was from EMD Chemicals, Inc. (Gibbstown, NJ, USA); anti-integrin antibodies were from Millipore Corporation (Billerica, MA, USA); anti-mouse IgG Texas Red conjugated secondary antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); recombinant full length human CCN2 containing all four structural modules (termed ‘CCN21–4’) and an isoform of CCN2 comprising a naturally occurring C-terminal fragment of 103 residues (amino acids 247–349) and which contains module 4 alone (termed ‘CCN24’) were produced as we have previously described [20, 21]; recombinant human TGF-β1 was from eBioscience, Inc. (San Diego, CA, USA). Anti-CCN2 IgY was produced by immunization of chickens with human CCN2 produced in this laboratory.
Isolation and primary culture of mHSC
HSC were isolated from healthy male Balb/c mice (Harlan Laboratories, Indianapolis, IN, USA), 6–8 weeks of age, by a modification of previously described methodology [22]. This protocol was approved by the Institutional Animal Care and Use Committee of The Research Institute at Nationwide Children’s Hospital (Columbus, OH, USA). mHSC were harvested by gradient centrifugation using OptiPrep™, resuspended in DMEM/F12 containing 20% foetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin, and plated into plastic tissue culture dishes or tissue culture plates (Corning Incorporated, Corning, NY, USA), and maintained at 37°C in a humidified atmosphere of 5% CO2/95% air. The next day, cells were replaced with fresh DMEM/F12 containing 10% foetal bovine serum, and medium replacement was then repeated every other day if applicable. By analysis through autofluorescence of vitamin A and trypan blue exclusion, the purity and vitality of the isolated HSC were more than 95% and 97%, respectively (data not shown).
RNA extraction and reverse transcription
Total cellular RNA was extracted using Trizol Reagent, and reverse transcribed to produce cDNA using Superscript II Reverse Transcriptase and Oligo(dT)12–16 primers according to the manufactures’ directions.
PCR and quantitative real-time PCR
The primer sequences were as follows: α-SMA: 5′-TTC GTT ACT ACT GCT GAG CGT GAG A-3′ (sense) and 5′-AAA GAT GGC TGG AAG AGG GTC-3′ (antisense) (200 bp); β-actin: 5′-TGT TAC CAA CTG GGA CGA CA-3′ (sense) and 5′-CTT TTC ACG GTT GGC CTT AG-3′ (antisense) (130 bp); CCN2: 5′-CCA GGA AGT AAG GGA CAC GA-3′ (sense) and 5′-GGT TCT CAC TTT GGT GGG AT-3′ (antisense) (148 bp); integrin α5: 5′-CTT AGA ATC CGA GAC TGG GC-3′ (sense) and 5′-TCA GGA CAA ATC CCA AAC AA-3′ (antisense) (126 bp); integrin αv: 5′-TCC CAG GAC TGT ATT GCT GA-3′ (sense) and 5′-CAG TGT GGG CGA AGT AAA TG-3′ (antisense) (119 bp); integrin β1: 5′-TCA CAA ATA CTG TAA GCT GTC C-3′ (sense) and 5′-AGC ACT GTC AAA ATG AAA AGG C-3′ (antisense) (200 bp); integrin β3: 5′-ACT GTG CCT GCC AGG CCT TT-3′ (sense) and 5′-TCC TTG GGG CTG CAC TCT TC-3′ (antisense) (169 bp). Primers were validated by PCR and products were confirmed by1.5% agrose gel electrophoresis using ethidium bromide staining. To measure gene expression, first strand cDNA products mixed with primers and SYBR® Green PCR Master Mix reagent were subjected to quantitative real-time PCR on an Applied Biosystems 7500 Real-Time PCR System, and the cycling parameters were: 50°C for 2 min., 95°C for 10 min., followed by 40 cycles of 95°C for 15 sec. and 60°C for 1 min. Samples were run in triplicate, and expression values were normalized to housekeeping gene β-actin primers using the ΔΔCt method.
Immunofluorescence
Cells grown on cover slips in 6- or 12-well tissue culture plates were fixed with 4% paraformaldehyde for 15 min. at 4°C. After blocking with 5% BSA in PBS, cells were incubated with primary antibody against α-SMA or CCN2 in blocking solution for 1 hr at 37°C, then secondary antibody conjugated with Texas red or Alexa Fluor® 568 for 45 min. at room temperature. The cover slips were mounted in 10% glycerol and photomicrographs were recorded with a Zeiss AxioCam HR attached to a Zeiss Axioskop (Carl Zeiss Vision GmbH, Germany).
Cell adhesion assay
Cell adhesion assays were performed as described [15, 17]. Briefly, cells were seeded into CCN2- or FN-coated 96-well tissue culture plates in the presence or absence of CCN2 or FN for 30 min. In some experiments, cells were pre-treated with anti-integrin antibody, NaClO3 and/or Na2SO4 for 30 min. or 24 hrs; specific details are provided in the figure legends. Wells were then rinsed with PBS to remove non-adherent cells and cell adhesion was subsequently measured by fluorescent emission from the wells at 520 nm following addition of 50 μl CyQuant reagent in lysis buffer.
siRNA transfection
Day 3 or day 7 cells were transfected with 50 nM siRNA targeting mouse CCN2 gene (siCCN2) (sense, 5′-UGA CAA UAC CUU CUG CAG Att-3′; antisense, 5′- UCU GCA GAA GGU AUU GUC Att-3′) or scrambled siRNA using siPORT™ NeoFX™ Transfection Agent according to the manufacturer’s directions, and total cellular RNA was harvested 48 hrs later. Preliminary siRNA transfection efficiency was determined using siRNA targeting GAPDH gene according to the manufacturer’s directions, with siRNA CCN2 transfection producing a knockdown in endogenous CCN2 mRNA expression of about 90%. Preliminary experiments with another siCCN2 (sense, 5′- AGA AUA UGA UGU UCA UCA Att-3′; antisense, 5′- UUG AUG AAC AUC AUA UUC Utt-3′) demonstrated similar results regarding CCN2 knockdown efficiency and regulation of integrin subunits gene expression (data not shown), showing that the effects observed were authentic rather than non-specific or off-target in nature.
Statistical analysis
Data are presented as mean ± S.D. and anova one-way test was performed with P < 0.05 as statistical significance.
Results
CCN2 mRNA expression in primary cultures of mHSC
As assessed by RT-PCR, immunostaining or quantitative real-time PCR, mHSC underwent autonomous activation over the first 7 days of culture as shown by increased expression of mRNA for α-SMA, a well-characterized HSC activation marker (Fig. 1). During the same time period, CCN2 gene expression increased as well (Fig. 2), consistent with previous studies which documented activation-associated expression of CCN2 in primary HSC from rats [10, 12].
fig 1.
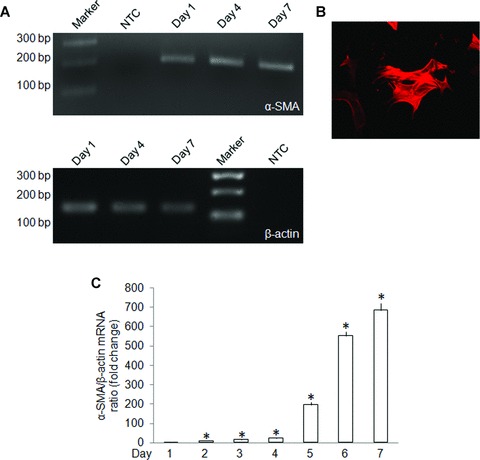
α-SMA production by primary culture mHSC. (A) RT-PCR of total cellular RNA extracted from primary mHSC for determination of α-SMA gene expression on day 1, day 4 or day 7 of primary culture. A DNA marker ladder and non-cDNA template (NTC) are also shown. (B) Immunofluorescent detection of α-SMA protein in day 3 mHSC (magnification ×100). This staining pattern was seen in about 40% of the cells on day 3. (C) Quantitative real-time PCR detection of α-SMA mRNA on days 1–7. Data are the means ± S.D. of triplicate determinations and are representative of three independent experiments. *P < 0.05 versus day 1.
fig 2.
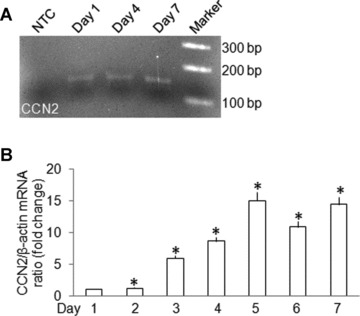
CCN2 expression by primary culture mHSC. (A) RT-PCR of total cellular RNA extracted from primary mHSC for determination of CCN2 gene expression on day 1, day 4 or day 7 of primary culture. A DNA marker ladder and non-cDNA template (NTC) are also shown. (B) Quantitative real-time PCR detection of CCN2 mRNA on days 1–7. Data are the means ± S.D. of triplicate determinations and are representative of three independent experiments. *P < 0.05 versus day 1.
mRNA expression of the αv, β3, α5 or β1 integrin subunits CCN2 in primary cultures of mHSC
During the first 7 days of culture, expression in mHSC of mRNA for the αv, β3, α5 or β1 integrin subunits increased gradually, with a variable expression pattern for each subunit (Fig. 3). Maximal expression levels were observed on day 5 for the α5 subunit, on days 5–6 for the β1 subunit and on day 7 for the αv and β3 subunits (Fig. 3).
fig 3.
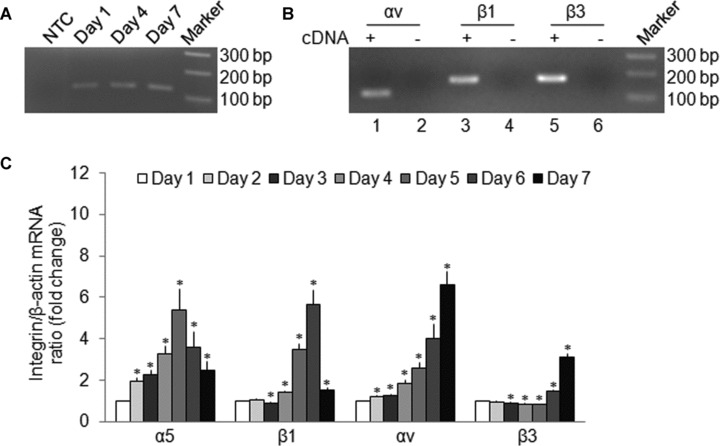
Integrin expression by primary culture mHSC. Total cellular RNA extracted from primary cultures of mHSC was subjected to reverse transcription, followed by PCR (A, B) or real-time PCR (C) to determine gene expression of the α5, αv, β1 or β3 intergin subunits. (A) RT-PCR for integrin α5 assessed on day 1, day 4 or day 7. A DNA marker ladder and non-cDNA template (NTC) are also shown. (B) RT-PCR for integrin αv (lane 1), β1 (lane 3) or β3 (lane 5) and their non-cDNA template controls (lanes 2, 4 and 6, respectively). A DNA marker ladder is also shown. (C) Quantitative real-time PCR detection of integrins α5, αv, β1 or β3 on days 1–7. Data are the means ± S.D. of triplicate determinations and are representative of three independent experiments. *P < 0.05 versus day 1.
Differential modulation of the α5, αv, β1 and β3 integrin subunits by CCN2 in primary cultures of mHSC
When added to transitional mHSC on day 3, recombinant human CCN21–4 (200 ng/ml) caused increased expression of the α5, β1 or αv integrin subunits by 4.19-, 1.36- or 1.14-fold, respectively, whereas expression of the integrin β3 subunit was reduced by 48% (Fig. 4A). On the other hand, in activated mHSC on day 7, CCN2 caused about an 11-fold increased expression of the β1, αv or β3 subunits whereas expression of the α5 subunit was reduced by 48% (Fig. 4B). These results thus show that CCN2 modulates gene expression of its cognate receptor integrin subunits – integrins αv, β3, α5 and β1– in primary cultures of mHSC isolated from normal mice. Meanwhile, CCN2 differentially regulates expression of the above individual integrin subunit gene expression in early and activated primary cultures of mHSC. A role for endogenous CCN2 in integrin expression in mHSC was demonstrated by treating the cells with 50 nM siCCN2. In day 3 transitional cells, this treatment down-regulated the α5 subunit by 95% but up-regulated the β3 or β1 subunits by 1.85- or 3.85-fold, respectively (Fig. 5A) whereas in day 7 activated cells it caused a down-regulation of the α5, αv or β3 subunits by 95%, 26% or 47%, respectively, but 6.73-fold up-regulation of the β1 subunit (Fig. 5B). The efficacy of the siCCN2 was confirmed by immunostaining of mHSC which showed decreased in CCN2 protein on day 3 or day 7 (Fig. 5C). These results thus confirm that CCN2 differentially modulates gene expression of the above integrin subunits in primary cultured mHSC.
fig 4.
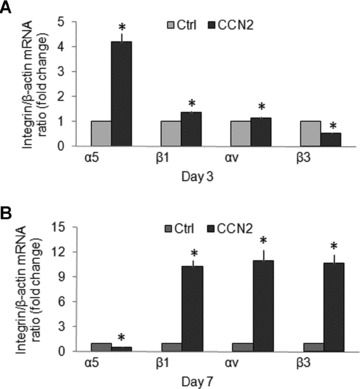
Regulation of integrin subunit expression by recombinant CCN2. mHSC on (A) day 3 or (B) day 7, both of which were pre-incubated in DMEM/F12 containing 0.5% foetal bovine serum for 24 hrs, were treated with or without CCN2 (200 ng/ml) for an additional 24 hrs prior to analysis of integrin gene expression by quantitative real-time PCR. The data are the means ± S.D. of triplicate determinations and are representative of three independent experiments. Ctrl: non-treated cells. *P < 0.05 versus Ctrl.
fig 5.
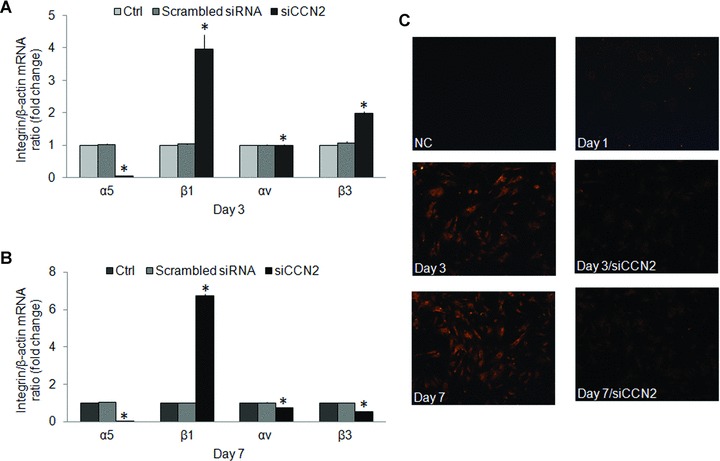
CCN2 siRNA-mediated down-regulation of integrin expression. mHSC on (A) day 3 or (B) day 7 were treated for 48 hrs with siCCN2 (50 nM) prior to analysis of mRNA expression of the integrin α5, αv, β1 or β3 subunits by quantitative real-time PCR. Data are the means ± S.D. of triplicate determinations and are representative of three independent experiments. *P < 0.05 versus Ctrl. (C) Immunofluorescent detection of CCN2 in day 1 (without siCCN2 treatment), day 3 or day 7 mHSC treated with or without siCCN2 (magnification ×100). NC: negative control.
Integrin or HSPG dependency of CCN2-mediated mHSC adhesion
As compared to plates coated with albumin alone, adhesion of day 3 mHSC was 2-, 2.5- or 2.3-fold higher in plates coated with, respectively, CCN21–4, CCN24 or FN (Fig. 6). Pre-treatment of the cells with 10 mM sodium chlorate to block sulphation of HSPG inhibited mHSC adhesion to CCN21–4 or CCN24 by 63% and 43%, respectively, but concurrent incubation with 10 mM sodium sulphate reversed this effect (Fig. 6). Pre-incubation of the cells with anti-integrin α5β1 antibody (10 μg/ml) decreased their adhesion to either CCN21–4 or CCN24 by 68% and 57%, respectively (Fig. 6). These data demonstrate that adhesion to CCN2 by transitional mHSC involves engagement by cell surface HSPG and/or integrin α5β1 with one or more binding sites in the C-terminal 103 residues of CCN2.
fig 6.
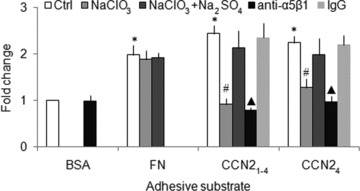
CCN2 or FN promotes mHSC adhesion. Day 3 mHSC were pre-incubated with 10 mM NaClO3 in the presence or absence of 10 mM Na2SO4, for 24 hrs or with 10 μg/ml anti-integrin α5β1 antibody for 30 min. and then plated for 30 min. into 96-well tissue culture plates (105 cells/well in 100 μl of medium) that had been pre-coated with 2 μg/ml CCN21–4, CCN24 or FN. Wells were then washed and the frequency of adherent cells was evaluated using a CyQuant NF Assay Kit. The data are the means ± S.D. of quadruplicate determinations and are representative of three independent experiments. *P < 0.05 versus BSA-coated group; #P < 0.05 versus Ctrl or NaClO3+ Na2SO4 group; ▴P < 0.05 versus Ctrl group.
Co-operative interaction between CCN2 and FN in promoting mHSC adhesion
CCN21–4 or CCN24 (200 ng/ml) promoted mHSC adhesion to FN by 2.2- and 1.5-fold, respectively, but their effects were decreased 62% or 50%, respectively, by anti-integrin α5β1 antibody (10 μg/ml) (Fig. 7). Similarly, FN (200 ng/ml) promoted mHSC adhesion to CCN21–4 by 1.9-fold, but pre-treatment of the cells with heparinase I (2 units/ml) decreased FN-enhanced cell adhesion by 53%, and prior occupancy of heparin-binding site by pre-incubation with exogenously added heparin (2 μg/ml) decreased the cell binding to FN by 59% (Fig. 8). On the other hand, FN promoted mHSC adhesion to CCN24 by 1.2-fold, an effect that was inhibited by 37% with heparin or by 29% after pre-treatment of the cells with heparinase I (Fig. 8). The effect of heparinase I was unaffected by exposure to protease inhibitor cocktail, showing that contaminating proteolytic enzymes did not account for the decreased cell adhesion (Fig. 8).
fig 7.
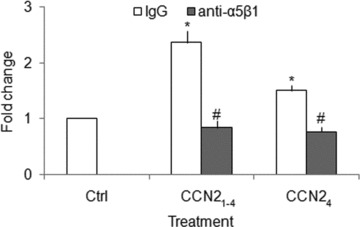
CCN2 promotes mHSC cell adhesion to FN. Day 3 primary mHSC were pre-incubated with or without 10 μg/ml anti-integrin α5β1 for 30 min. and then placed for 30 min. in 96-well tissue culture plates (105 cells/well in 100 μl of medium) pre-coated with FN (2 μg/ml) in the presence or absence of 200 ng/ml CCN21–4 or CCN24. Wells were washed and the frequency of adherent cells was evaluated using a CyQuant NF Assay Kit. Data are representative of three separate experiments. Values represent mean ± S.D. of quadruplicate determinations from three experiments. *P < 0.05 versus Ctrl group; #P < 0.05 versus IgG group.
fig 8.
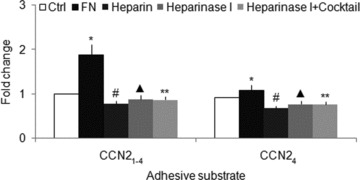
FN promotes mHSC adhesion to CCN2. Day 3 primary mHSC were pre-incubated with or without 2 μg/ml heparin or 2 units/ml heparinase I in the presence or absence of 1× Protease Inhibitor Cocktail for 30 min., and then placed for 30 min. in 96-well tissue culture plates plates (105 cells/well in 100 μl of medium) that had been pre-coated with 2 μg/ml CCN21–4 or CCN24, in the presence or absence of 200 ng/ml FN. Wells were washed and frequency of adherent cells was evaluated using a CyQuant NF Assay Kit. Data are representative of three separate experiments. Values represent mean ± S.D. of quadruplicate determinations from three experiments. Cocktail: protease inhibitor cocktail. *P < 0.05 versus Ctrl group; #P < 0.05 versus FN group; ▴P < 0.05 versus FN group; **P > 0.05 versus Heparinase I group.
Discussion
Detailed investigations over the last decade have firmly established that integrins are a principal class of cell-surface receptors for CCN2 and related molecules [23]. The binding interactions between these proteins and specific integrins are complex and involve engagement of unique non-Arg-Gly-Asp motifs in the ligands that in some cases are functionally regulated by HSPG co-receptors [15, 17, 19, 23–27]. Engagement of cell surface integrins and HSPG by CCN2 likely represents a major mechanism for modulating the responses of the target cell to a diverse variety of molecular stimuli and environmental cues.
We have previously shown that adhesion of primary rat HSC to CCN2 employs an integrin αvβ3 binding site located in residues 247–239 [15] and that adhesion of the rat HSC-T6 cell line to CCN2 involves an integrin α6β1 binding site in residues 199–243 [28]. In other studies we showed that integrin α5β1 supported CCN2-mediated adhesion of primary rat pancreatic stellate cells [17, 18]. In view of the emerging importance of mouse models in probing CCN2 action in the liver in vivo [29, 30], studies of integrin-CCN2 interactions in primary cultures of mHSC are important so that underlying mechanisms can be explored. Therefore, in this investigation, we examined the role of integrins in CCN2-dependent mHSC adhesion. Because quiescent HSC typically exhibit low expression of CCN2 and integrins, our study was focused on a relatively early stage of HSC culture to establish which integrins are functional as CCN2 receptors during the period of transitional activation. Using day 3 cells we showed that CCN2 is indeed an adhesive substrate and that this is attributable to engagement by CCN2 of integrin α5β1 and HSPG. Additionally, because the binding of CCN24 was both HSPG- and integrin α5β1 dependent, the data demonstrate the importance of binding sites for these moieties in the C-terminal 103 residues of CCN2. Although the precise location of the integrin α5β1 binding site for mHSC has yet to be mapped in CCN2, we have previously identified an integrin α5β1 binding domain in residues 285–291 of CCN2 that supports adhesion of rat pancreatic stellate cells [17]. Moreover, heparin-binding domains in CCN2 have been localized to stretches of highly basic residues in the C-terminal portion of the CCN2 molecule, including residues 247–260 and residues 274–286 [31].
Because blocking antibodies to the mouse form of integrin αvβ3 are not available, we cannot exclude the possibility that integrin αvβ3 also plays a role in CCN2-induced adhesion of mHSC, as we have shown previously for rat HSC [15]. Indeed incubation of day 3 mHSC with echistatin, a snake venom disintegrin with high specificity for integrin β1 and β3, was somewhat more effective than anti-integrin α5β1, anti-integrin β1 or anti-integrin β3 in blocking CCN2-induced cell adhesion (data not shown) suggesting that additional integrins likely function as CCN2 adhesion receptors in transitional mHSC.
In this study we showed that CCN2 expression increases as a function of culture-induced activation, consistent with previous data from other systems [10]. Although it has been documented that certain integrins are up-regulated in fibrotic liver disease or during HSC activation [32–36], previous studies have not addressed whether this phenomenon may be related to the action of CCN2 itself, leading us to investigate if integrin expression by mHSC was CCN2 dependent. Previously in human mesangial cells, CCN2 was shown to stimulate the production of integrin α5β1 and to mediate, at least partially, TGF-β1-induced gene expression of integrin α5β1[27]. The results from our study show that, when treated with recombinant CCN2, mHSC demonstrated enhanced levels of the α5, β1 or αv integrin subunits in day 3 cells and of the β1, αv or β3 subunits in day 7 cells. On the other hand, the β3 or α5 subunits were reduced by CCN2 treatment on, respectively, days 3 or 7, suggesting that integrin expression in response to CCN2 is highly dependent on the state of activation of the cells. This conclusion is supported by the siRNA data which showed that down-regulation of endogenous CCN2 expression led to variable changes in integrin expression depending on the subunit in question and the stage of culture. Therefore, while these data show clearly that integrin subunit expression in primary mHSC is CCN2 dependent, the precise role played by CCN2 is especially influenced by the particular microenvironment at a specific point in time. This contextual action is fully consistent with the emerging matricellular action of CCN2 [37]. We have further shown that the integrin α6 subunit, which also functions as a CCN2 receptor [28], is also produced by primary mHSC on day 3 and day 7 but its expression appears to be independent of CCN2 stimulation (data not shown).
In this study we demonstrated that FN, which is also an integrin α5β1 ligand in HSC [38], interacts co-operatively with CCN2 to promote mHSC. Similar interactions have been reported for fibroblasts [24], chondrocytes [25] and oval cells [19], and FN has been shown to regulate the cellular response to CCN2 [39]. In the mHSC system, incubation with anti-integrin α5β1 antibody or heparinase decreased CCN21–4- or CCN24-induced cell adhesion to FN, demonstrating that residues 247–349 were involved in this modulatory effect. Consistent with this observation are previous reports which showed that residues 249–349 of CCN2 were involved in a direct binding interaction with the FN protein which enhanced chondrocyte adhesion via integrin α5β1[25]. From studies of hepatic oval cells, an additional FN-binding site has been localized to residues 21–107 of CCN2 [19] and therefore a role for this site in co-operative binding interactions between CCN21–4 and FN in our mHSC studies cannot currently be excluded. Whatever the nature of the underlying molecular mechanisms, the data are important in the context of fibrotic liver disease in which the co-operative interactions between CCN2 and FN are likely manifested within the provisional matrix which contains enriched levels of each molecule.
In summary, the current study demonstrates that during primary culture, mHSC increasingly express CCN2, as they become autonomously activated in vitro, and that CCN2 differently modulates its cognate integrin receptor subunits (α5, αv, β1, β3) in transitional or activated mHSC. Moreover, CCN2 promotes heparin-dependent adhesion of early primary culture mHSC through its C-terminal domain and it interacts co-operatively with FN through a common cell surface receptor, integrin α5β1. Collectively these data demonstrate the presence of complex interacting pathways between CCN2, its intergin receptors and other binding moieties (e.g. HSPG, FN) in regulating HSC function.
Acknowledgments
This work was supported by NIH grant 5R01AA016003 awarded to D.R.B. We thank Sherri Kemper for generating recombinant CCN2.
Conflict of interest
None.
References
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iredale J. Defining therapeutic targets for liver fibrosis: exploiting the biology of inflammation and repair. Pharmacol Res. 2008;58:129–36. doi: 10.1016/j.phrs.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Stellate cells: a moving target in hepatic fibrogenesis. Hepatology. 2004;40:1041–3. doi: 10.1002/hep.20476. [DOI] [PubMed] [Google Scholar]
- 4.Reeves HL, Friedman SL. Activation of hepatic stellate cells–a key issue in liver fibrosis. Front Biosci. 2002;7:d808–26. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- 5.Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1–9. doi: 10.1016/s1386-6346(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 6.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–44. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors. 2009;35:200–8. doi: 10.1002/biof.30. [DOI] [PubMed] [Google Scholar]
- 8.George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther. 2007;14:790–803. doi: 10.1038/sj.gt.3302929. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Li D, Xie Q, et al. RNA interfering connective tissue growth factor prevents rat hepatic stellate cell activation and extracellular matrix production. J Gene Med. 2008;10:1039–47. doi: 10.1002/jgm.1223. [DOI] [PubMed] [Google Scholar]
- 10.Paradis V, Dargere D, Bonvoust F, et al. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82:767–74. doi: 10.1097/01.lab.0000017365.18894.d3. [DOI] [PubMed] [Google Scholar]
- 11.Uchio K, Graham M, Dean NM, et al. Down-regulation of connective tissue growth factor and type I collagen mRNA expression by connective tissue growth factor antisense oligonucleotide during experimental liver fibrosis. Wound Repair Regen. 2004;12:60–6. doi: 10.1111/j.1067-1927.2004.012112.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams EJ, Gaca MD, Brigstock DR, et al. Increased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cells. J Hepatol. 2000;32:754–61. doi: 10.1016/s0168-8278(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 13.Yuhua Z, Wanhua R, Chenggang S, et al. Disruption of connective tissue growth factor by short hairpin RNA inhibits collagen synthesis and extracellular matrix secretion in hepatic stellate cells. Liver Int. 2008;28:632–9. doi: 10.1111/j.1478-3231.2008.01730.x. [DOI] [PubMed] [Google Scholar]
- 14.Gao R, Ball DK, Perbal B, et al. Connective tissue growth factor induces c-fos gene activation and cell proliferation through p44/42 MAP kinase in primary rat hepatic stellate cells. J Hepatol. 2004;40:431–8. doi: 10.1016/j.jhep.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin αvβ3 and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–55. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- 16.Gao R, Brigstock DR. Activation of nuclear factor kappa B (NF-κB) by connective tissue growth factor (CCN2) is involved in sustaining the survival of primary rat hepatic stellate cells. Cell Commun Signal. 2005;3:14. doi: 10.1186/1478-811X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao R, Brigstock DR. A novel integrin α5β1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut. 2006;55:856–62. doi: 10.1136/gut.2005.079178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao R, Brigstock DR. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin α5β1 as a novel CCN2 receptor. Gastroenterology. 2005;129:1019–30. doi: 10.1053/j.gastro.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 19.Pi L, Ding X, Jorgensen M, et al. Connective tissue growth factor with a novel fibronectin binding site promotes cell adhesion and migration during rat oval cell activation. Hepatology. 2008;47:996–1004. doi: 10.1002/hep.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball DK, Moussad EE, Rageh MA, et al. Establishment of a recombinant expression system for connective tissue growth factor (CTGF) that models CTGF processing in utero. Reproduction. 2003;125:271–84. doi: 10.1530/rep.0.1250271. [DOI] [PubMed] [Google Scholar]
- 21.Ball DK, Rachfal AW, Kemper SA, et al. The heparin-binding 10 kDa fragment of connective tissue growth factor (CTGF) containing module 4 alone stimulates cell adhesion. J Endocrinol. 2003;176:R1–7. doi: 10.1677/joe.0.176r001. [DOI] [PubMed] [Google Scholar]
- 22.Leask A, Chen S, Pala D, et al. Regulation of CCN2 mRNA expression and promoter activity in activated hepatic stellate cells. J Cell Commun Signal. 2008;2:49–56. doi: 10.1007/s12079-008-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Abraham DJ, Shi-Wen X, et al. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635–46. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshijima M, Hattori T, Inoue M, et al. CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin α5β1. FEBS Lett. 2006;580:1376–82. doi: 10.1016/j.febslet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 26.Schober JM, Chen N, Grzeszkiewicz TM, et al. Identification of integrin αMβ2 as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–65. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- 27.Weston BS, Wahab NA, Mason RM. CTGF mediates TGF-β-induced fibronectin matrix deposition by upregulating active α5β1 integrin in human mesangial cells. J Am Soc Nephrol. 2003;14:601–10. doi: 10.1097/01.asn.0000051600.53134.b9. [DOI] [PubMed] [Google Scholar]
- 28.Tong ZY, Brigstock DR. Intrinsic biological activity of the thrombospondin structural homology repeat in connective tissue growth factor. J Endocrinol. 2006;188:R1–8. doi: 10.1677/joe.1.06719. [DOI] [PubMed] [Google Scholar]
- 29.Brigstock DR. Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): from pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J Cell Commun Signal. 2009;3:5–18. doi: 10.1007/s12079-009-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong Z, Chen R, Alt DS, et al. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–47. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brigstock DR, Steffen CL, Kim GY, et al. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272:20275–82. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- 32.Dodig M, Ogunwale B, Dasarathy S, et al. Differences in regulation of type I collagen synthesis in primary and passaged hepatic stellate cell cultures: the role of alpha5beta1-integrin. Am J Physiol Gastrointest Liver Physiol. 2007;293:G154–64. doi: 10.1152/ajpgi.00432.2006. [DOI] [PubMed] [Google Scholar]
- 33.Levine D, Rockey DC, Milner TA, et al. Expression of the integrin α8β1 during pulmonary and hepatic fibrosis. Am J Pathol. 2000;156:1927–35. doi: 10.1016/s0002-9440(10)65066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quondamatteo F, Kempkensteffen C, Miosge N, et al. Ultrastructural localization of integrin subunits α3 and α6 in capillarized sinusoids of the human cirrhotic liver. Histol Histopathol. 2004;19:799–806. doi: 10.14670/HH-19.799. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Murphy FR, Gehdu N, et al. Engagement of αvβ3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–4006. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Zhang Y, Zhang J, et al. Expression of fibronectin receptor, integrin α5β1 of hepatic stellate cells in rat liver fibrosis. Chin Med J. 2000;113:272–76. [PubMed] [Google Scholar]
- 37.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–83. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milliano MT, Luxon BA. Initial signaling of the fibronectin receptor (α5β1 integrin) in hepatic stellate cells is independent of tyrosine phosphorylation. J Hepatol. 2003;39:32–7. doi: 10.1016/s0168-8278(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 39.Droppelmann CA, Gutierrez J, Vial C, et al. Matrix metalloproteinase-2-deficient fibroblasts exhibit an alteration in the fibrotic response to connective tissue growth factor/CCN2 because of an increase in the levels of endogenous fibronectin. J Biol Chem. 2009;284:13551–61. doi: 10.1074/jbc.M807352200. [DOI] [PMC free article] [PubMed] [Google Scholar]


