Abstract
Purpose
To study whether long-term treatment with estrogen, selective estrogen receptor modulators, or growth hormone can prevent the development of abnormal voiding patterns during filling cystometry in a postpartum, ovariectomized female rat.
Materials and Methods
Immediately after spontaneous delivery, 60 primiparous Sprague-Dawley rats were randomly divided into six equal groups. One group served as uninjured sham controls and five groups underwent intravaginal balloon dilation. On day seven, previously dilated animals underwent bilateral ovariectomy and implantation of a subcutaneous hormone delivery pump. The five treatment groups received normal saline (control), estrogen, raloxifene, levormeloxifene, or growth hormone for 7 weeks. Conscious cystometry was performed 7 weeks after ovariectomy. Urethral sphincter tissue was harvested for elastin immunohistochemistry and real-time polymerase chain reaction of Alpha 1a adrenergic receptor mRNA.
Results
No abnormal voiding patterns were detected in the group treated with growth hormone. The estrogen, raloxifene and levormeloxifene groups had greater detrusor overactivity and urethral relaxation incontinence than control animals. The raloxifene group had a significantly lower baseline bladder pressure and opening pressure. Growth hormone treated animals had higher elastin content in the urethra. Urethral Alpha 1a adrenergic receptor mRNA concentration was significantly lower in the selective estrogen receptor modulator treated animals compared to controls.
Conclusions
Growth hormone prevents the development of abnormal voiding patterns during filling cystometry in an animal model of parturition induced incontinence; estrogen and selective estrogen receptor modulators may worsen voiding patterns.
Keywords: Animal model, Estrogen, Growth hormone, Incontinence, Selective estrogen receptor modulators
Introduction
Lower urinary tract symptoms (LUTS) (such as urgency and stress urinary incontinence), are common in post-menopausal women [1]. It was long believed that the hormonal changes of menopause were to blame for the high incidence of LUTS in this population. Ergo, until recently hormone replacement therapy was a mainstay of medical therapy for postmenopausal with LUTS [2]. However, several recent studies have concluded that hormone replacement therapy may actually increase the incidence of urinary incontinence in post-menopausal women[3, 4].
Selective Estrogen Receptor Modulators (SERMs) are a class of molecules often utilized in the treatment of osteoporosis and breast cancer. SERMs have been associated with urinary incontinence in postmenopausal women[5]. Growth Hormone (GH) is a trophic hormone that has been shown to reduce age-related loss of muscle function and ligament damage[6]. Its secretion diminishes with age and to our knowledge the role of GH in bladder function after menopause has not been investigated[6].
To better characterize the relationship between voiding and hormones, we examined the effects of long term estrogen, two SERMs (levormeloxifene and raloxifene), and GH therapy on the development of abnormal voiding patterns during filling cystometry in a postpartum, ovariectomized female rat model. Estrogen and GH were selected because of the variations in secretion after menopause in humans. Levormeloxifene and raloxifene were studied because these SERMs were previously used in human trials. Our hypothesis is that estrogen and SERMs will increase abnormal patterns of voiding and supplemental GH will improve them.
Materials and methods
Study design
All animal experiments were approved by our Institutional Animal Care and Use Committee. After spontaneous vaginal delivery, 60 three-month-old Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were randomly divided into six equal groups. Group 1 served as a postpartum uninjured control (sham). The remaining five groups underwent intravaginal balloon dilation to simulate prolonged second-stage labor followed one week later by bilateral ovariectomy to induce surgical menopause. Group 2 served as the injured controls and was treated with saline, while the remaining groups were treated with estrogen (group 3), levormeloxifene (group 4), raloxifene (group 5), or GH (group 6). All 6 groups went on to undergo filling cystometry and be harvested urethral histology.
Birth trauma and vaginal dilation
After delivery, groups 2–6 were anesthetized with ketamine (90 mg/kg intraperitoneal) and xylazine (10mg/kg intraperitoneal). The balloon of a transurethral catheter (18F; Bard, Covington, GA) with the tip cut off was placed intravaginally and was filled with 3 ml water. A 130 g weight was placed on the catheter providing a constant pull directing pressure to the pelvic floor for 4 hours.
Ovariectomy and hormone pump placement
One week after birth trauma, bilateral ovariectomy was performed. Simultaneously, experimental agents were dissolved in subcutaneous pumps with DMSO 20% (Alzet Osmotic Pumps, Cupertino, CA)[7]. The pumps were implanted subcutaneously in the dorsum of the neck and replaced 4 weeks after initial insertion. The pumps contained normal saline (injured controls), 17b-estradiol (0.1 mg/kg/day; Sigma-Aldrich, St. Louis, MO), levormeloxifene (1 mg/kg/day; Novo Nordisk, Bagsværd, Denmark), raloxifene (1 mg/kg/day; Sigma-Aldrich, St. Louis, MO), or rat recombinant GH (0.3 mg/kg/day; (NIDDK-Rat-GH-B-12/courtesy of NIDDK-NIH and Dr. A. F. Parlow)).
Conscious cystometry
Seven weeks after pump implantation conscious 4-channel filling cystometry was performed in all rats. The tip of a polyethylene-90 (PE-90) tube (Clay-Adams, Parsippany, NJ) was heated to create a collar and implanted at the bladder dome 24 h prior to filling cystometry. A second probe (PE-90 tubing fitted with a small balloon[8]) was placed in the intra-abdominal space to measure the abdominal component of the vesical pressure. The tubing was tunneled through the abdominal wall and exited the dorsal neck skin.
Conscious animals were placed in a tunnel attached to a metabolic cage grid (Braintree Scientific, Braintree, MA). Artificial bladder filling was done at a non-physiological filling rate of 0.1 ml/min using an infusion pump (KD Scientific, Holliston, MA). Intravesical and abdominal pressures, along with voided volume, were recorded using LabView 6.0 software (National Instruments, Austin, TX) at a rate of 10 samples/sec.
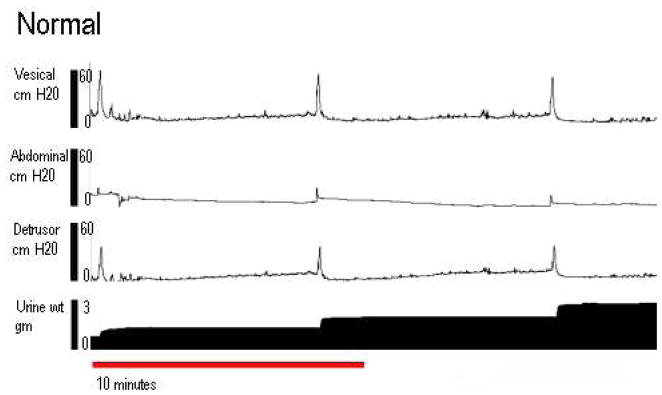
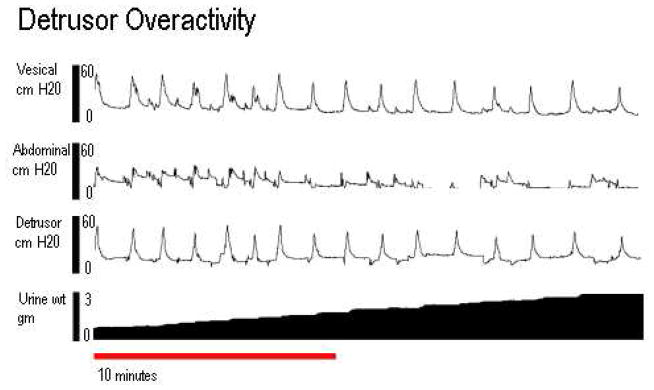
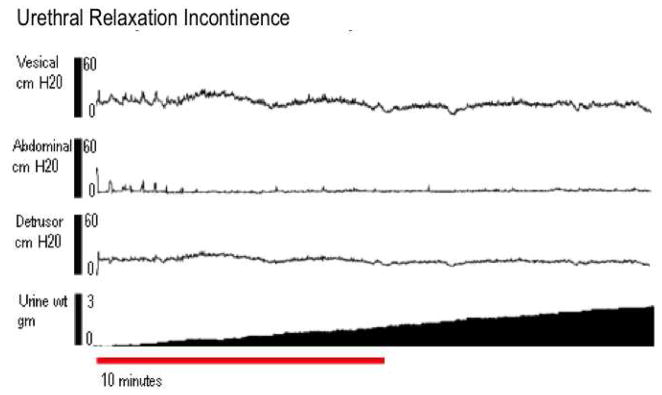
Cystometric variables were recorded by obtaining mean values of 4 voiding cycles as previously described [9]. Baseline pressure was defined as the lowest bladder pressure between voiding intervals. Opening pressure was defined as the pressure at the onset of urine flow. Maximum pressure was defined as the highest pressure during voiding. Bladder capacity was defined as the maximal voided volume plus residual urine. The voiding function of each rat was classified as normal, detrusor overactivety (DO), urethral relaxation incontinence (URI), or DO with URI. Normal micturition pattern was defined as bladder contractions resulting in a voiding frequency of ≥ 4 contractions in 10 min (Figure 1a) as previously described[9]. DO was defined as contraction frequencies > 4 times in 10 min (Figure 1b). URI was defined as bladder pressures below 15 cm H2O with continuous urine leakage and zero post-void residual urine (Figure 1c). The filling cystometries were classified by a urologist and a gynecologist who were not blinded. Nevertheless, a senior urologist who made the final determination was blinded. Residual urine was measured by aspirating the bladder after the final void of the cystometry. Methods, definitions and units conform to the standards recommended by the International Continence Society, except where specifically noted [10] [11].
Figure 1.
Four channel conscious cystometry. The top tracing is vesical pressure; the second is abdominal pressure, the third is detrusor pressure, and the bottom tracing is the cumulative amount of voided urine. A: An example of normal tracing. B: An example of DO. C: An example of URI.
Histochemistry
Freshly dissected tissue was fixed with cold 2% formaldehyde and 0.2% picric acid in 0.1M phosphate buffer followed by immersion in buffer containing 30% sucrose. The fixed tissues were then frozen in OCT compound. Sections were cut at 6 microns.
Picrosirius red (0.1%; Electronic Microscopy Sciences, Hatfield, PA) was used to incubate tissue sections at room temperature for 1 hour. The staining solution was removed, and the sections were washed three times with 0.1% acetic acid. The sections were dehydrated through a graded series of alcohols, cleared in histo-clear and cover-slipped, using histo-mount as a sealant. Under polarized light, Picrosirius stains type I collagen red or yellow and type III collagen green.
Trichrome staining was performed by immersing sections in warm (58°C) Bouin’s solution for 15 min, rinsing, staining with Weigert’s Hematoxylin for 10 min, and rinsing until only nuclei remained stained. Sections were then stained for smooth muscle with Biebrich Scarlet-Acid Fuchsin for 3 min, rinsed, and immersed in phosphomolybdic acid for 45 min. Next, collagen was stained blue with Aniline Blue for 3 min, and the tissues were rinsed in distilled water for 2 min, immersed in 1% acetic acid for 2 min, and rinsed in distilled water for 2 min twice. Finally, the tissues were rehydrated through increasing concentrations of ethanol, left to air dry, and mounted.
Total ribonucleic acid isolation and reverse transcription to cDNA were conducted as previously described[12]. All reagents for SYBR Green real-time PCR were purchased from Applied Biosystems (Foster City, CA). Primers for rat alpha 1A and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Integrated DNA Technologies (Coralville, IA).
The pathologist was blinded to the treatment assignment of each specimen which had a unique number identifier.
Statistical analysis
Univariate analysis was performed on the cystometric variables comparing sham and treatment groups to the control group. Univariate linear regression was used to estimate and test differences of each treatment group from control for numeric measures. Chi-square test was used for abnormal voiding pattern differences among the groups. Throughout the text, figures, and tables, the mean value ± SD was used to describe the results, unless otherwise indicated. Statistics were calculated using SAS, version 9.1(SAS, Cary, NC).
Results
Classification of voiding pattern
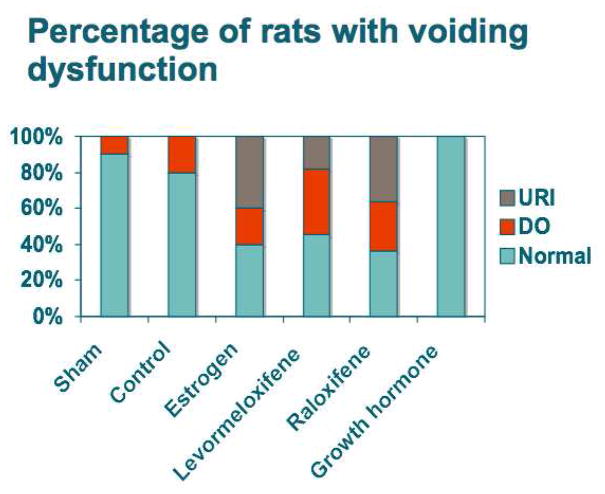
There were significant differences in the incidence of abnormal voiding patterns between groups (Chi square, p-value = 0.017). All GH animals had normal voiding patterns with flat pressure plateaus between voiding complexes, similar to control animals (Figure 2). Conversely, the estrogen, levormeloxifene, raloxifene groups had considerably more DO and URI.
Figure 2.
The percentage of rats with abnormal voiding patterns in various groups (10 animals per group). URI was seen in the estrogen, raloxifene and levormeloxifene groups, (Chi square, p-value = 0.017).
Bladder pressure profiles
Table 1 contains recorded bladder capacity and bladder pressures for each group. The raloxifene group had significantly lower bladder capacity and maximum pressure relative to control animals (p= 0.02 for each). The levormeloxifene group had significantly lower baseline bladder pressure and opening pressure relative to the control group (p=0.01 and p=0.04, respectively). No groups had significant residual urine (results not shown).
Table 1.
Univariate linear regression analysis of conscious cystometric outcomes comparing control group to sham and treatment groups for bladder capacity, baseline pressure, maximum pressure and opening threshold.
| Bladder capacity | ||||
| Group | (ml) | Effect | CI | P-Value |
| Control | 0.4+/−0.1 | ref | ||
| Sham | 0.3+/−0.1 | −0.02 | (−0.23 to 0.2) | 0.88 |
| Estrogen | 0.5+/−0.3 | 0.04 | (−0.17 to 0.24) | 0.73 |
| Levormeloxifene | 0.3+/−0.2 | −0.17 | (−0.37 to 0.04) | 0.11 |
| Raloxifene | 0.2+/−0.2 | −0.26 | (−0.47 TO 0.05) | 0.02 |
| GH | 0.6+/−0.2 | 0.08 | (−0.13 to 0.29) | 0.44 |
| Baseline pressure | ||||
| Group | (cm H2O) | Effect | CI | P-Value |
| Control | 9.5+/−6.1 | ref | ||
| Sham | 8.2+/−6.0 | −1.16 | (−6.0 to 3.67) | 0.63 |
| Estrogen | 7.8+/−5.3 | −1.7 | (−6.29 to 2.89) | 0.46 |
| Levormeloxifene | 2.9+/−2.3 | −6.65 | (−11.24 TO −2.06 | 0.01 |
| Raloxifene | 5.4+/−4.4 | −4.14 | (−8.73 to 0.45) | 0.08 |
| GH | 5.3+/−4.5 | −4.22 | (−8.81 to 0.37) | 0.07 |
| Maximum pressure | ||||
| Group | (cm H2O) | Effect | CI | P-Value |
| Control | 50.4+/−12.1 | ref | ||
| Sham | 55.4+/−24.4 | 1.85 | (−14.85 to 18.54 | 0.83 |
| Estrogen | 39.4+/−19.8 | −12.07 | (−27.9 to 3.77) | 0.13 |
| Levormeloxifene | 39.2+/−14.3 | −9.28 | (−25.12 to 6.56) | 0.24 |
| Raloxifene | 33.0+/−17.2 | −18.49 | (−34.33 TO −2.65) | 0.02 |
| GH | 48.5+/−14.1 | −2.96 | (−18.80 to 12.88) | 0.71 |
| Opening pressure | ||||
| Group | (cm H2O) | Effect | CI | P-Value |
| Control | 19.3+/−6.9 | ref | ||
| Sham | 27.5+/−8.5 | −0.99 | (−8.62 to 6.63) | 0.80 |
| Estrogen | 17.3+/−7.6 | −2.16 | (−9.39 to 5.08) | 0.55 |
| Levormeloxifene | 11.9+/−4.5 | −7.59 | (−14.82 TO −0.36) | 0.04 |
| Raloxifene | 13.7+/−7.3 | −5.84 | (−13.07 to 1.4) | 0.11 |
| GH | 20.2+/−7.6 | −4.31 | (−11.54 to 2.92) | 0.24 |
Histochemistry
Trichrome staining of the urethra revealed a striking decrease in the intercellular matrix staining in the control, estrogen and SERMs treated groups compared to sham and GH treated rats (Figure 3 & 4). The expression of collagen type I was decreased while collagen type III was increased in the control, estrogen and SERM groups relative to sham and GH rats. The difference in elastin expression was also remarkable (Figure 5). In the urethra of sham and GH treated animals, elastic fibers were long, well-organized, and near the muscle bundles. The control and estrogen-treated groups showed fragmentation and disorganization of the elastic fibers.
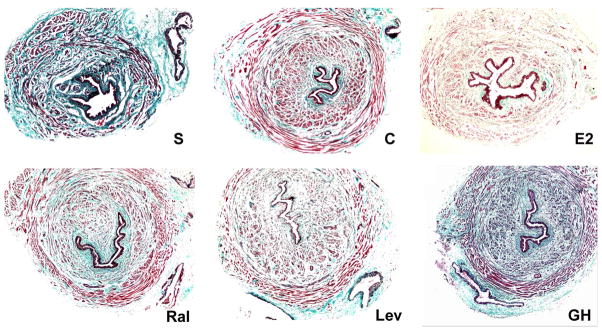
Figure 3.
Representative photos of cross section of mid-urethra with trichome staining from the following groups: S-Sham; C-Control; E2-Estrogen; Ral-Raloxifene; Lev-Levormeloxifene; GH-growth hormone. After delivery, balloon dilation and ovariectomy, the matrix content in urethral wall was significantly decreased in all groups except the sham and the GH group.
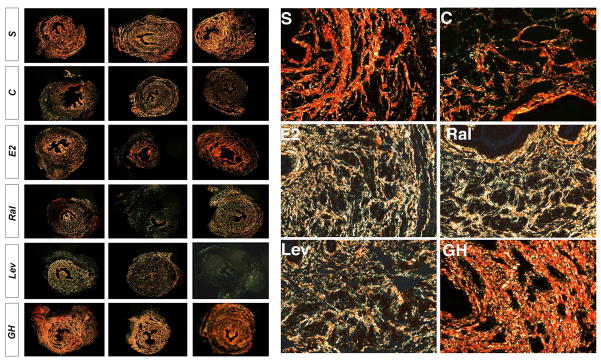
Figure 4.
Representative photos of cross-section of mid-urethra depicting collagen I and collagen III staining with Picrosirius red. Red color staining represents collagen I which is more abundant in urethral wall of the sham (S) and GH group as well as the control (C) group, though in lesser degree. Contrarily, more collagen III stainings (green color) are noted in control (C), estrogen (E2), raloxifene (Ral) and levormeloxifene (Lev) groups. Figures on the left are from 3 different rats (10x). Figures on the right are higher magnification (40x).
Figure 5.
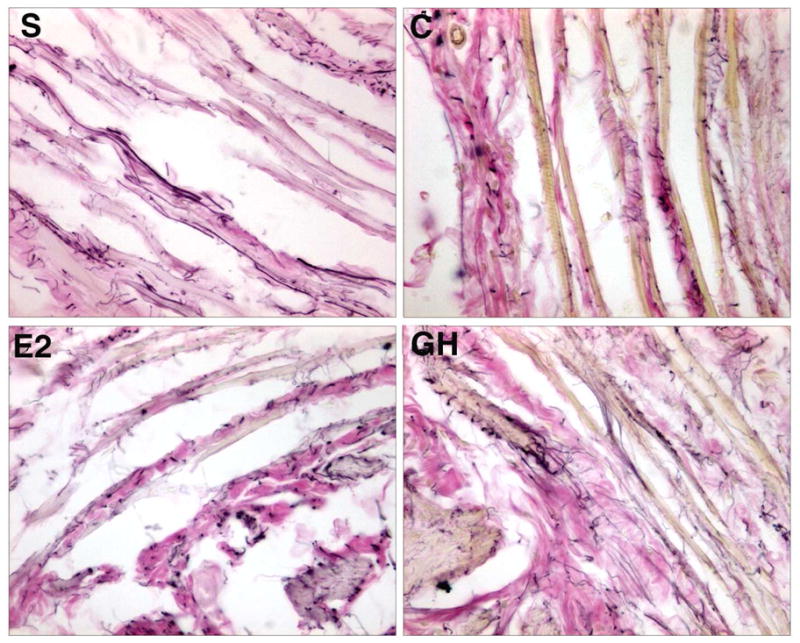
High power view of representative photos of cross-section of mid-urethra stained for elastin fibers (arrows). Elastin fibers appear well-organized and long in the sham (S) and growth hormone (d) group. In the control (b) and Estrogen (c) rats, the elastin fibers are fragmented and disorganized.
Alpha 1a adrenergic receptor mRNA concentrations
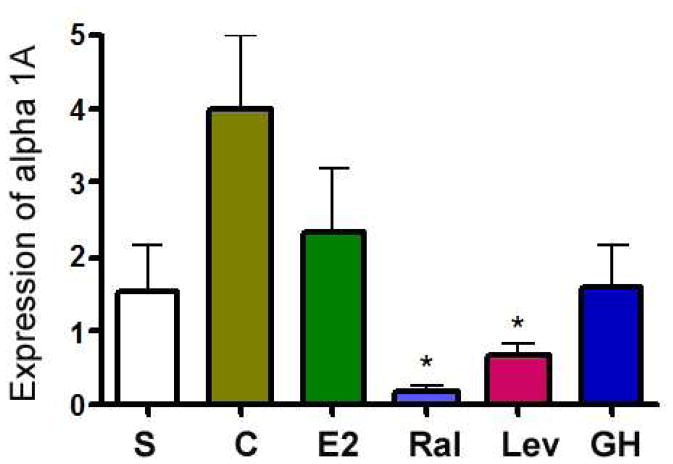
SERMs treated animals had significantly lower levels of alpha 1a adrenergic receptor mRNA in the urethra compared to the controls (both p-values =0.01). No other significant differences were detected (Figure 6).
Figure 6.
Alpha 1a receptor mRNA by real time PCR expressed as a ratio of alpha 1A to internal control (GADPH). The SERM groups had significantly (p=0.01) less expression of alpha 1a receptor mRNA in the urethral tissue compared to control.
Discussion
Previous studies have concluded that injury to periurethral nerves, urethral smooth and striated muscle were the primary mechanism to elicit voiding dysfunction in humans and animal models[13–15]. The role of estrogen in voiding abnormalities has been investigated by other authors. Hayashi et al. demonstrated that extended-term estrogen resulted in a higher incontinence rate in postpartum ovariectomized rats. Upregulation of neural nitric oxide synthase and down-regulation of alpha 1a and 1d adrenoreceptors was found in the urethras of the estrogen treated animals[16]. We observed a similar down-regulation of alpha 1a receptors in our estrogen treated animals. Both systemic and vaginal estrogens have been utilized in the management of urinary incontinence because several short-term clinical studies suggested that estrogen increases urethral closure pressure, urethral blood flow, and improves alpha-adrenergic receptor sensitivity[17]. However, two large, long-term, double-blinded, placebo-controlled studies[Heart Estrogen and Progestin Replacement Study and the Women’s Health Infitiative], showed increased incidence of incontinence in women on hormone therapy. Our study results are congruous with these clinical data.
Raloxifene is marketed for use in post-menopausal women to prevent osteoporosis and in clinical trials has not been shown to increase the risk of voiding abnormalities in humans[18–20]. Although there is no human data on voiding abnormalities from raloxifene, this drug promoted DO in our rat model. Raloxifene treated animals had significantly lower baseline bladder pressures and lower maximum pressures than controls.
Levormeloxifene has potent estrogenic effects on the endometrium and has been shown to cause prolapse and incontinence in humans[5]. Its phase III clinical trial was aborted due to the high incidence of adverse events, including an increased rate of urinary incontinence and endometrial hyperstimulation[5]. In our animal model, levormeloxifene produced an increase in abnormal voiding patterns, although not as severe as raloxifene.
The results of SERMs treatment in this study are similar to previously published 3 week results where 44% of the levormeloxifene and 66% of raloxifene treated animals had abnormal voiding patterns [9].
GH and its mediator, IGF-1 have diverse trophic, anabolic and lipolytic effects. GH and IGF-1 concentrations are known to diminish by more than 50% in healthy older adults. This decrease is known to cause a reduction of protein synthesis, a decrease in lean body and bone mass and potentially a decline in immune function[21].
In our study, the GH treated group had no evidence of abnormal voiding patterns. Interestingly, the opening pressure for this group was similar to that of the control but less than the sham group, indicating partial but not complete repair of birth trauma. The present rat model has been shown to induce DO and URI, likely through damage to muscle, extracellular matrix, ligaments and the pudendal nerve [22]. Given the diminished DO and efficient voiding in GH treated rats, nerve injury repair by GH is likely. While the role of GH in the development and treatment of voiding dysfunction requires more study, it does offer clinical and translational promise.
GH works in large part by inducing production of insulin like growth factor 1 (IGF-1). Kerns and colleagues demonstrated that IGF-I gene therapy improved the distal recovery of nerve structure and function after pudendal nerve crush injury[23]. IGF-1 enhanced the structural and functional recovery of leg muscles as well as counteracting motor neuron loss after sciatic nerve axotomy in newborn rats[24]. In a rat model of injury, IGF-I improved maximum force and ultimate stress strength of ligamentous tissue[6]. IGF-I resulted in the upregulation of fibroblast production and of cellular matrix proteins such as proteoglycans and type 1 collagen expression[6]. IGF-1 also upregulates aortic elastogenesis[25].
The smooth and striated muscles of the urethra play a key role in urinary continence[26]. Elastin fibers, by distension in response to urine flow with subsequent recoil, may also play a role in passive occlusion of the vesicourethral junction during storage and urethral length change during urination[27]. Vaginal tissues from women with stress urinary incontinence manifest increased collagen breakdown as well as increased elastolytic activity[28] [[29].
Injured control and estrogen/SERMs treated animals in our study manifested a marked decrease in collagen I, an increase in collagen III, and fragmentation/disorganization of elastin fibers. We theorize that the altered ratio of the extracellular matrix may have important ramifications in urinary incontinence. This hypothesis is supported by the normalization of collagen elastin morphology and urethral function in GH treated rats.
Our animal model has been validated in our lab and others[13, 30]. Furthermore, our technique of awake 4-channel cystometry accounts for abdominal pressure changes and avoids artifacts which may occur from anesthetized cystometry in rats[31]. A deficiency of animal models compared to human subjects is that the subject cannot indicate whether their voiding is involuntary. In the present model, animals who voided >4 times per voiding cycle could have either had a small bladder capacity or detrusor overactivity incontinence.
Study limitations include small sample size and the use of an animal model. In the future, studies should be undertaken to determine the mechanism of urethral repair produced by exogenous GH hormones. GH may have utility in the management of disease of the lower urinary tract in humans in the future. Additional pre-clinical and clinical studies are warranted.
Conclusion
In a rat model, long-term therapy GH may prevent the development of birth-trauma associated lower urinary tract disease. Treatment with estrogen and SERMs may exacerbate birth-trauma abnormal voiding patterns. These effects may in part be mediated by hormonal regulation of urethral alpha 1a adrenergic receptor and elastin expression.
Acknowledgments
Source of funding: RO1 DK069655-01, NIDDK/NIH
We wish to thank National Hormone and Peptide Program, directed by Dr. A. F. Parlow at Harbor UCLA Med Center for providing the rat GH. Statistical support was made possible by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
References
- 1.Huang AJ, Brown JS, Kanaya AM, et al. Quality-of-life impact and treatment of urinary incontinence in ethnically diverse older women. Arch Intern Med. 2006 Oct 9;166:2000–6. doi: 10.1001/archinte.166.18.2000. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. Drug therapy for urinary incontinence. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000 Apr;14:291–313. doi: 10.1053/beog.1999.0075. [DOI] [PubMed] [Google Scholar]
- 3.Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RE, Posner SF. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999 Jul;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 4.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. Jama. 2005 Feb 23;293:935–48. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002 Sep;187:521–7. doi: 10.1067/mob.2002.123938. [DOI] [PubMed] [Google Scholar]
- 6.Provenzano PP, Alejandro-Osorio AL, Grorud KW, et al. Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: evaluation of loaded and unloaded ligaments. BMC Physiol. 2007;7:2. doi: 10.1186/1472-6793-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuiling GA, Moes H, Koiter TR. Prolonged combined in vivo pre-treatment with luteinizing hormone-releasing hormone (LRH) and oestradiol benzoate causes long-lasting suppression of the autonomous and the LRH-stimulated secretion of luteinizing hormone and follicle stimulating hormone. An in vitro study. Acta Endocrinol (Copenh) 1984 Apr;105:468–73. doi: 10.1530/acta.0.1050468. [DOI] [PubMed] [Google Scholar]
- 8.Lee T, Andersson KE, Streng T, Hedlund P. Simultaneous registration of intraabdominal and intravesical pressures during cystometry in conscious rats--effects of bladder outlet obstruction and intravesical PGE2. Neurourol Urodyn. 2008;27:88–95. doi: 10.1002/nau.20460. [DOI] [PubMed] [Google Scholar]
- 9.Tantiwongse K, Fandel TM, Wang G, et al. The potential of hormones and selective oestrogen receptor modulators in preventing voiding dysfunction in rats. BJU Int. 2008 Mar 11; doi: 10.1111/j.1464-410X.2008.07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–74. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 11.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 12.Lin G, Shindel AW, Banie L, et al. Molecular Mechanisms Related to Parturition-Induced Stress Urinary Incontinence. Eur Urol. 2008 Mar 18; doi: 10.1016/j.eururo.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sievert KD, Emre Bakircioglu M, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol. 2001 Jul;166:311–7. [PubMed] [Google Scholar]
- 14.Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol. 2007 Apr;292:R1738–44. doi: 10.1152/ajpregu.00784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003 Sep;170:1027–31. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi N, Bella AJ, Wang G, et al. Effect of extended-term estrogen on voiding in a postpartum ovariectomized rat model. Can Urol Assoc J. 2007 Sep;1:256–63. doi: 10.5489/cuaj.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waetjen LE, Dwyer PL. Estrogen therapy and urinary incontinence: what is the evidence and what do we tell our patients? Int Urogynecol J Pelvic Floor Dysfunct. 2006 Sep;17:541–5. doi: 10.1007/s00192-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein SR, Johnson S, Watts NB, Ciaccia AV, Elmerick D, Muram D. Incidence of urinary incontinence in postmenopausal women treated with raloxifene or estrogen. Menopause. 2005 Mar;12:160–4. doi: 10.1097/00042192-200512020-00010. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Albertazzi P, Bottazzi M. The long-term effect of raloxifene on the genitourinary tract. Climacteric. 2007 Jun;10:244–8. doi: 10.1080/13697130701379311. [DOI] [PubMed] [Google Scholar]
- 20.Waetjen LE, Brown JS, Modelska K, Blackwell T, Vittinghoff E, Cummings SR. Effect of raloxifene on urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2004 Feb;103:261–6. doi: 10.1097/01.AOG.0000109429.67671.d1. [DOI] [PubMed] [Google Scholar]
- 21.Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007 Jan;211:173–80. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 22.Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25:388–96. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- 23.Kerns JM, Shott S, Brubaker L, et al. Effects of IGF-I gene therapy on the injured rat pudendal nerve. Int Urogynecol J Pelvic Floor Dysfunct. 2003 Feb;14:2–7. doi: 10.1007/s00192-002-0995-2. discussion 8. [DOI] [PubMed] [Google Scholar]
- 24.Vergani L, Di Giulio AM, Losa M, Rossoni G, Muller EE, Gorio A. Systemic administration of insulin-like growth factor decreases motor neuron cell death and promotes muscle reinnervation. J Neurosci Res. 1998 Dec 15;54:840–7. doi: 10.1002/(SICI)1097-4547(19981215)54:6<840::AID-JNR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe BL, Rich CB, Goud HD, et al. Insulin-like growth factor-I regulates transcription of the elastin gene. J Biol Chem. 1993 Jun 15;268:12418–26. [PubMed] [Google Scholar]
- 26.Brading AF. The physiology of the mammalian urinary outflow tract. Exp Physiol. 1999 Jan;84:215–21. doi: 10.1111/j.1469-445x.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 27.Dass N, McMurray G, Brading AF. Elastic fibres in the vesicourethral junction and urethra of the guinea pig: quantification with computerised image analysis. J Anat. 1999 Oct;195( Pt 3):447–53. doi: 10.1046/j.1469-7580.1999.19530447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen B, Wen Y, Yu X, Polan ML. The role of neutrophil elastase in elastin metabolism of pelvic tissues from women with stress urinary incontinence. Neurourol Urodyn. 2007;26:274–9. doi: 10.1002/nau.20347. [DOI] [PubMed] [Google Scholar]
- 29.Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:80–7. doi: 10.1007/s001920200020. discussion 7. [DOI] [PubMed] [Google Scholar]
- 30.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002 Sep;90:403–7. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 31.Kiddoo DA, Valentino RJ, Zderic S, et al. Impact of state of arousal and stress neuropeptides on urodynamic function in freely moving rats. Am J Physiol Regul Integr Comp Physiol. 2006 Jun;290:R1697–706. doi: 10.1152/ajpregu.00742.2005. [DOI] [PubMed] [Google Scholar]