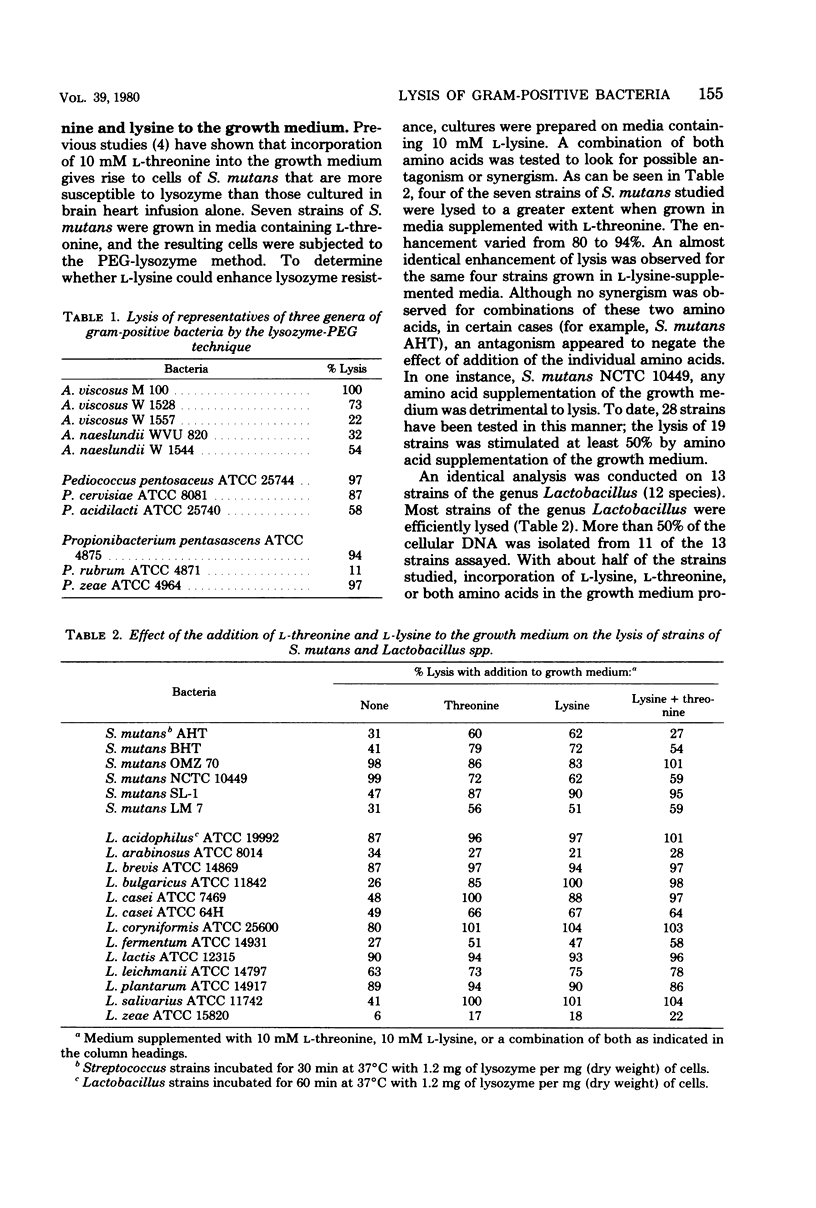
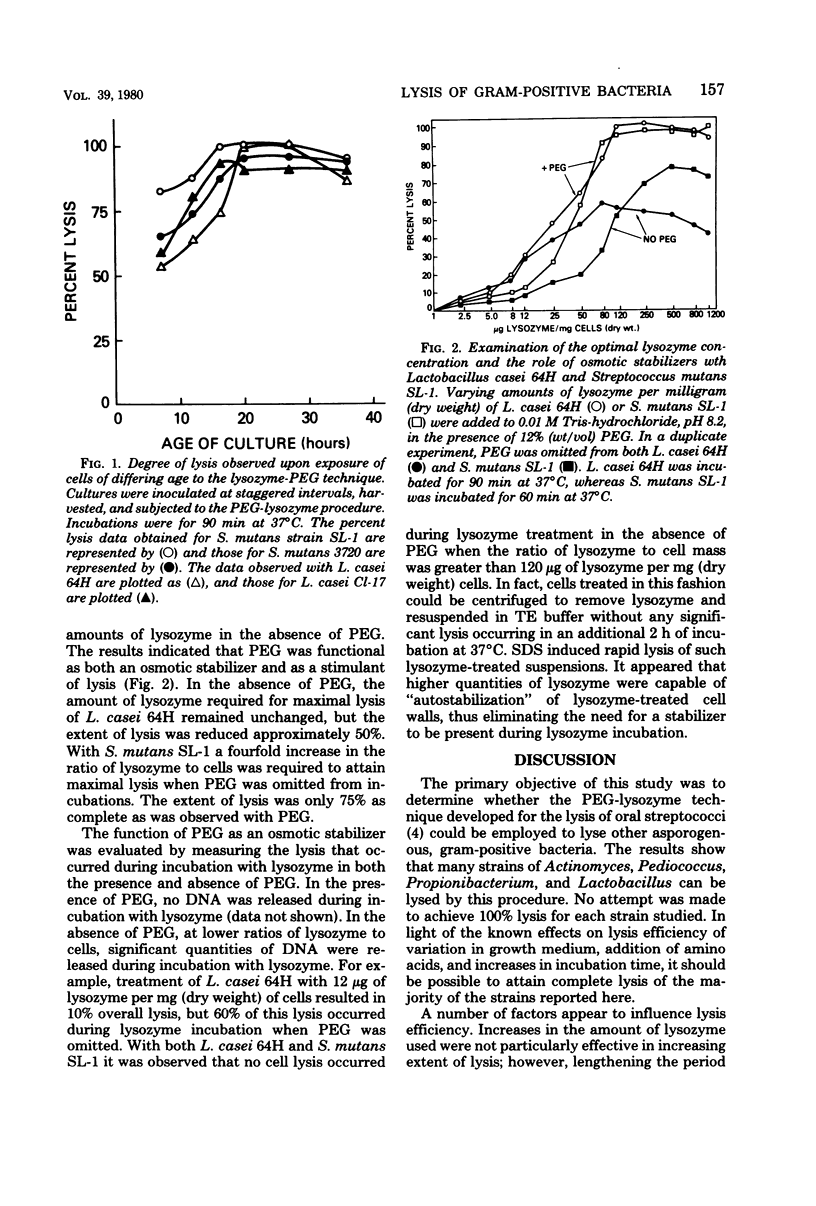
Abstract
A method developed for the lysis of oral streptococci that employed the action of lysozyme suspended in dilute tris(hydroxymethyl)aminomethane-hydrochloride buffer containing polyethylene glycol has been adapted for use with lactobacilli, actinomycetes, propionibacteria, and pediococci. Most of the cellular deoxyribonucleic acid was liberated from many strains of bacteria usually thought to be lysozyme resistant. The major observations were as follows: (i) supplementation of the growth medium with L-threonine, L-lysine, or both frequently produced cells that were more susceptible to lysis by lysozyme; (ii) glucose-containing media produced cells that were more easily lysed than those from cultures grown on other substrates; (iii) polyethylene glycol not only served as an osmotic stabilizer, it also enhanced the extent of lysis; and (iv) dilute tris(hydroxymethyl)aminomethane buffer was superior to the buffer systems most commonly employed in published muramidase-based lysis techniques. Stationary-phase cells of Lactobacillus casei and Streptococcus mutans were more easily lysed than those isolated from log-phase cultures. The method as detailed in this report should be generally applicable for the lysis of gram-positive, asporogenous bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Barker D. C., Thorne K. J. Spheroplasts of Lactobacillus casei and the cellular distribution of bactoprenol. J Cell Sci. 1970 Nov;7(3):755–785. doi: 10.1242/jcs.7.3.755. [DOI] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFTHYMIOU C., HANSEN P. A. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962 May-Jun;110:258–267. doi: 10.1093/infdis/110.3.258. [DOI] [PubMed] [Google Scholar]
- HOLDEN J. T., VANBALGOOY J. N. EFFECT OF NUTRITIONAL AND PHYSIOLOGICAL FACTORS ON THE REACTION BETWEEN LACTOBACILLUS PLANTARUM AND MURAMIDASE. Biochem Biophys Res Commun. 1965 May 3;19:401–406. doi: 10.1016/0006-291x(65)90136-1. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Araki Y., Ito E. Occurrence of glucosamine residues with free amino groups in cell wall peptidoglycan from bacilli as a factor responsible for resistance to lysozyme. J Bacteriol. 1973 Feb;113(2):592–598. doi: 10.1128/jb.113.2.592-598.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf R. H., Deibel R. H. Differential lytic response of enterococci associated with addition order of lysozyme and anions. J Bacteriol. 1969 Sep;99(3):674–680. doi: 10.1128/jb.99.3.674-680.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neujahr H. Y., Börstad B., Logardt I. M. Factors affecting the resistance of Lactobacillus fermenti to lysozyme. J Bacteriol. 1973 Nov;116(2):694–698. doi: 10.1128/jb.116.2.694-698.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



