Abstract
Mouse models serve as relatively new yet powerful research tools to study intimal hyperplasia and wall remodeling of vein bypass graft failure. Several model variations have been reported in the past decade. However, the approach demands thoughtful preparation, selected sophisticated equipment, microsurgical technical expertise, advanced tissue processing and data acquisition. This review compares several described models, and aims (building on our personal experiences) to practically aid the investigators who want to utilize mouse models of vein graft failure.
INTRODUCTION
Surgical bypass via autologous vein stands as an evidence-based treatment of choice for selected patients with infra-inguinal lower extremity or coronary occlusive disease, 1 thus over half a million vein grafts are implanted annually in the United States. However, contemporary data shows that almost 40% of lower extremity vein bypass grafts develop occlusive lesions or fail within a year, 2 and almost half of cardiac bypass patients will lose (≥ 75% stenosis) a vein graft within a year. 3 These failures result in recurrent end organ ischemia, complex (and expensive) redo-revascularizations, and not infrequently loss of human limb or life.
Intimal hyperplasia and negative (inward) vein graft wall remodeling are the main culprits of vein bypass graft failure. The former has been intensively studied for decades, 4–6 while the latter has only recently triggered research interest. 7, 8 Since arterialized vein conduit tissue is rarely retrieved from patients, and due to the inability of contemporary cell and tissue culture approaches to mimic the multi-factorial events of early vein graft failure, animal models serve as an essential tool to explore the mechanisms of vein graft adaptations.
There are many animal models that simulate arterialized venous bypass graft failure, e.g. murine, 9–15 rattus, 16 rabbit, 17, 18 canine, 19 porcine, 20 monkey, 21, 22 and baboon. 23 Since the development of knockout and transgenic techniques, the use of mice in vascular research has boomed over the last two decades. With these approaches, researchers have tools to study the in vivo function of a specific gene product, while avoiding the difficulties associated with the use of antibodies or receptor agonists/antagonists (such as non-specificity, immunoreactivity, dosing and tachyphylaxis). Additionally, the vein bypass graft model in mice also serves as a platform to test the influences of factors such as diseases, inflammatory states, and hemodynamic changes on vein graft wall adaptations.
This review will summarize the advantages and drawbacks to current mouse models of vein graft adaptations (Table I), compare the technical variations in mouse vein graft constructions, and provide a practical guide for utilization of this species for surgical vein bypass investigations. Investigators interested in exploring the use of animals for research should familiarize themselves with federal laws (the Animal Welfare Act of 1966 and its amendments), the Guide for the Care and Use of Laboratory Animals (National Research Council), NIH Animal Research Advisory Committee Guidelines, 24 and appropriate local laboratory animal welfare guidelines.
Table I.
Mouse Vein Graft Model Advantages/Disadvantages
| Advantages | Disadvantages |
|---|---|
|
|
|
|
|
|
|
|
|
|
GENERAL TECHNICAL CONSIDERATIONS
Like most mammalian models of vascular diseases, in vivo mouse studies hold many confounding factors that contribute to observed variations in the vein graft adaptations (e.g. amount of intimal hyperplasia), though most of these are not well documented for mice in the formal research literature. In our experiences these include the surgeon’s technical skills, the level of sterility within the operative field and subsequent local/systemic infection and inflammation, procedural time, ischemia time of the vein segment, vein desiccation, physical trauma (careless dissection or over-stretching of the vein or recipient artery, etc.), artery clamp times and end organ ischemia (especially for aorta and common femoral artery models), total blood/fluid loss and replacement, usage of heparin, etc. Among these, the surgeon’s technique (especially minimization of physical vein endothelial trauma and the variation of hand-sewn anastomotic diameter) and ischemia/desiccation of the vein segment appear to be major contributors to the variability in early vein graft wall adaptations. For the novice microsurgeon attempting to master these complex models, it is recommended that practice time be completed to reach the plateau phase of the learning curve prior to engaging in any formal studies. The actual individual investigator’s time to approach the learning curve plateau is quite variable (estimated 20–60 surgeries), and depends largely on the training environment and learner’s original level of microsurgical technique.
Equipment
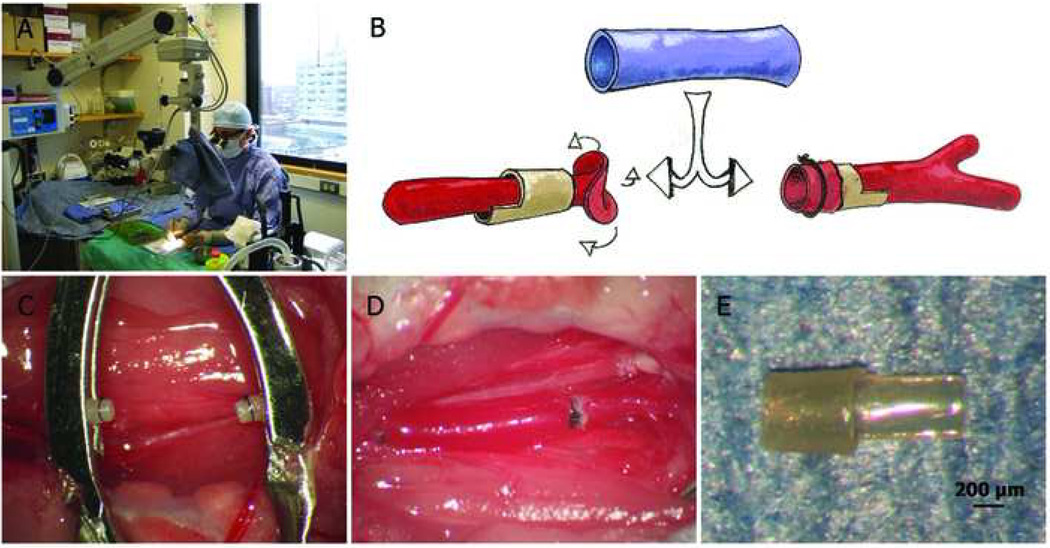
Basic microsurgical equipment is required for successful completions of these models. This include an environment controlled laboratory facility with a stable table and chair, autoclave/dry sterilizer, a set of high quality, well maintained microsurgical instruments, 5-0 to 12-0 sutures, and an operating microscope with high intensity operating lights. The high quality microsurgical operating microscope stands as a central component to the equipment. We utilize a Zeiss binocular, dual-headed OPMI-MD Surgical Microscope on S-5 Floor Stand, with T2 digital SLR camera/camcorder system (Fig 1A). The magnification can be altered from 4-fold up to 24-fold. X-Y-Z adjustable foot control aids in precision and sterility, and a large depth of field (which is absent on some dissecting microscopes) is useful.
Fig 1.
Cuff-technique mouse vein graft model. A. An example rodent survival operating suite with operating microscope and table-top small animal anesthesia system. B. Schematic representation of the cuff-technique vein bypass graft model. C. Two everted and secured carotid artery ends. D. Completed mouse carotid interposition vein graft. E. Hand-made PEEK anastomotic cuff with handle.
Intra-operative Strategies
The surgical procedure should be performed under aseptic conditions. Mouse skin preparation (hair removal and scrubbing with povidone-iodine or other disinfectants) and sterile draping (Fig 1A) are suggested.
Utilization of heparin is variable in published reports. We utilize heparinized saline (100 IU/ ml) as flush solution only. Fixed positioning of the inflow and outflow artery ends can be achieved by microsurgical approximator clamps, or simply securing two Micro Clips (Roboz Inc., Gaithersburg, MD) via a double looped (easily adjustable) 4-0 suture around the clip handles.
Immediately after vein graft construction, patency can be determined by the pulsation of vein graft and distal artery, blood flow observation through the thin vein graft wall, gentle empty-and-refill (strip) test on the inflow or outflow artery (not recommended on the vein graft), and intra-operative flowmetry (we utilize a Transonic TS420 flowmeter with 1PRB flowprobe, Transonic Systems Inc., Ithaca, NY) or ultrasonography 11. Simple judgment by turgidity of the vein graft is not reliable.
Peri-operative Strategies for Anesthesia and Analgesia
Since vein graft placement is a major survival surgery with prolonged operative time, use of a continuous inhalant anesthesia system (e.g. isoflurane via a calibrated vaporizer, with scavenging devices) is recommended rather than injectable approaches. Some researchers employ an “open”, non-calibrated method (bell-jar or nose cone), which presents a challenge when attempting to control the agent concentration and anesthetic depth. Frequent monitoring of physiological parameters such as respiratory rate and pain reflex is crucial. Prevention of hypothermia (via 37 °C heating pad and lamp) and peri-operative systemic fluid therapy minimizes anesthesia related complications and shortens recovery time. An anticholinergic drug (such as glycopyrrolate or atropine) to reduce secretions in the respiratory tract is recommended, and we administer an analgesic agent (buprenorphine) at the time of surgical recovery and via schedule for forty eight hours post operatively.
MOUSE VEIN BYPASS GRAFT MODELS
Cuff-Technique for Interposition Graft
Zou et al 9 first described a mouse vein graft model completed by the cuff technique, which has been adopted or modified by several research groups (Fig 1). 25–30 In brief, the right common carotid artery was gently mobilized free from surrounding connective tissues as far distally and proximally as possible. It was then ligated with sutures at the midpoint and divided. After controlling the inflow and outflow arteries via clamps placed at the pre-made polyethylene cuffs’ handles, the ligatures were removed and the proximal and distal artery ends were everted over the tubular body portion of the cuffs, which had a 0.65 mm outside diameter (OD) and a 0.5 mm inside diameter (ID). The vein segment (from separate donor supradiaphragmatic inferior vena cava (IVC) or autologous external jugular vein) was then sleeved over the artery cuffs and secured into position with fine ties (as shown in Fig 1B–1D). Mortality rate has been reported as 5.3%; patency rate at harvest time achieved 100%. Mean intima & media thickness (day 28, male C57BL/6 mice) was ~215 µm though our group has not observed as thick of a vein graft wall with this approach (we observe typically about 50 µm average intimal thickness and 100 µm average wall thickness).
Cuff selection may be an important factor in this model. Zou chose polyethylene tubing (OD 0.65 mm, ID 0.5 mm) for three month old mice. We find this material to be relatively soft and easy to distort if uneven force is exerted during artery eversion. The size is also relatively large for our 8–10 week old (20–24 grams) mice. Diao et al reported carotid ID at 0.35 ± 0.02 mm (8–10 week old, male C57BL/6J mice); 14 we also reported a similar result. 31 For the cuff material we prefer an autoclavable polyetheretherketone (PEEK) extruded tubing (OD 0.51mm, ID 0.41mm; from Zeus Inc., Orangeburg, SC) (Fig 1E).
Finally, 9-0 monofilament nylon suture performs well for ties, and 6-0 Vicryl (Ethicon Inc., Somerville, NJ) for incision closure.
Hand-Sewn Techniques for Interposition Graft
Using an end-to-side anastomotic method, Zhang et al 11 transplanted a 10 mm long donor’s IVC segment to a recipient’s common carotid (both from 17–20 grams male C57BL/6J mice), and secured each anastomosis with 8 to 12 continuous stitches of 11-0 nylon suture. The carotid segment between the IVC anastomoses was then ligated at both ends and cut. Operative time averaged around 20 minutes for IVC harvest and 90 minutes for carotid interposition grafting. Mortality rate was ≤ 10%, with a reported patency rate of > 95%. Mean wall (medial + neointimal) thickness (day 28) was 91 µm.
Cooley 12, 32 described a more delicate auto-graft model. Under 60X magnification, a branch of the external jugular vein (2 mm in length) was dissected and transplanted into the femoral artery using 6 to 10 interrupted stitches of 11-0 or 12-0 nylon suture per end-to-end anastomosis. Mortality rate was 15.9%, with patency rate at 81% on day 30. Mean wall thicknesses were 103 µm (proximal), 49 µm (middle) and 58 µm (distal).
Grafts to the abdominal aorta have also been reported. Diao et al 14 transplanted a 5 mm long external jugular vein segment into autologous infrarenal aorta. Six or seven interrupted 11-0 nylon stitches at each end-to-end anastomotic site were employed. With this model, average aorta clamping time for male C57BL/6J mice was 68 minutes. Mortality rate was 11.4%; patency rate on day 28 was 100%, and mean wall thickness (day 28) appeared to be ≤ 60 µm (calculated from their representative images and graph).
Finally, Salzberg et al 15 utilized a similar end-to-end model, but from the donor’s IVC to the recipient’s infrarenal abdominal aorta. Average aorta clamping time for male C57BL/KsJ mice was 30 min. Mean neointimal thickness (1 month post-operation) for this strain appeared to be ≤ 60 µm based on their data and representative images.
Hand-Sewn Technique for Vein Patch
In an attempt to mimic the events of the early vein conduit or an endarterectomy reconstruction 33–36 and building on a method employed in larger animals, 37 Shi and colleagues 10 described a vein patch as a surrogate for vein graft tissue. They harvested a ~5 mm long external jugular vein (from 7–12 week old male C57 BL/6 mice), cut open the conduit and trimmed it into an oval shape, and then used the patch to repair a surgically created defect in the ipsilateral carotid artery by a hand-sewn technique with 11-0 continuous suturing. The average time from venous patch harvest to common carotid artery reparation took about 40 minutes. The overall success rate was 92%. From the reported representative images, this model could induce obvious neointimal formation and wall thickening (day 20). Sakaguchi and associates 13 have reported an application of this technique to create a model that used a jugular patch to repair an abdominal aorta defect.
Table II summarizes the comparison of these mouse vein graft models, drawing upon the currently published literature and our personal microsurgical experiences (including over 400 mouse vein grafts).
Table II.
Comparison of Common Mouse Vein Graft Models
|
Zou’s 9 (Cuff- technique, IVC or jugular to carotid, end to end) |
Zhang’s 11 (Sewn, IVC to carotid, end to side) |
Cooley’s 12 (Sewn, jugular to femoral artery, end to end) |
Diao’s 14 & Salzberg’s 15 (Sewn, jugular or IVC to aorta, end to end) |
Shi’s 10 & Sakaguchi’s 13 (Vein patch technique, jugular to carotid or aorta) |
|
|---|---|---|---|---|---|
| Technical Challenge | ++ | +++ | ++++ | +++ | +++ |
| Relative Procedure Duration |
++ | ++++ | ++++ | ++++ | +++ |
| Extensive Bleeding Risk | + | +++ | ++ | ++++ | +++ |
| Risk of Thrombosis | + | ++ | ++++ | ++ | +++ |
| Relative Mortality | + | ++ | +++ | +++ | ++ |
| Reproducibility | +++ | ++ | + | ++ | ++ |
| Clinical Relevance | ++ | +++ | ++++ | +++ | ++ |
| Other Considerations | Foreign body (cuff) response at ends |
Short Vein graft (2mm) |
Low neointimal volume |
Not true vein graft |
IVC, inferior vena cava
MODEL VARIATIONS/MODIFICATIONS
Selection of a Mouse Strain and Genetic Background
Mouse strain has a significant impact on the vascular response to injury, and this should be taken into consideration when selecting a particular strain of mice for study. While there exists limited information on differences in susceptibilities to intimal hyperplasia 38 or negative remodeling in mouse vein graft models, data obtained from arterial studies may serve as a useful baseline. 39, 40
One of the advantages of working with mice is the vast array of transgenic and knockout animals available that simulate and facilitate understanding of human diseases. For example, ApoE deficient mice were utilized to study hypercholesterolemia, 41 while leprdb/db mice served as a model for type 2 diabetes mellitus. 15 These genetic backgrounds can be combined with the mouse vein graft models to provide a wealth of information about the mechanisms of vein graft failure in different pathological settings.
Adjunctive Hemodynamic Manipulations
In addition, hemodynamic manipulations can also be utilized to study the influence of pressure and flow perturbations on vein graft adaptation. Wall tension is known to impact vein graft wall adaptations, 42, 43 but largely due to their small size, invasive pressure measurements along mouse vein graft constructions have not proved reliable to date.
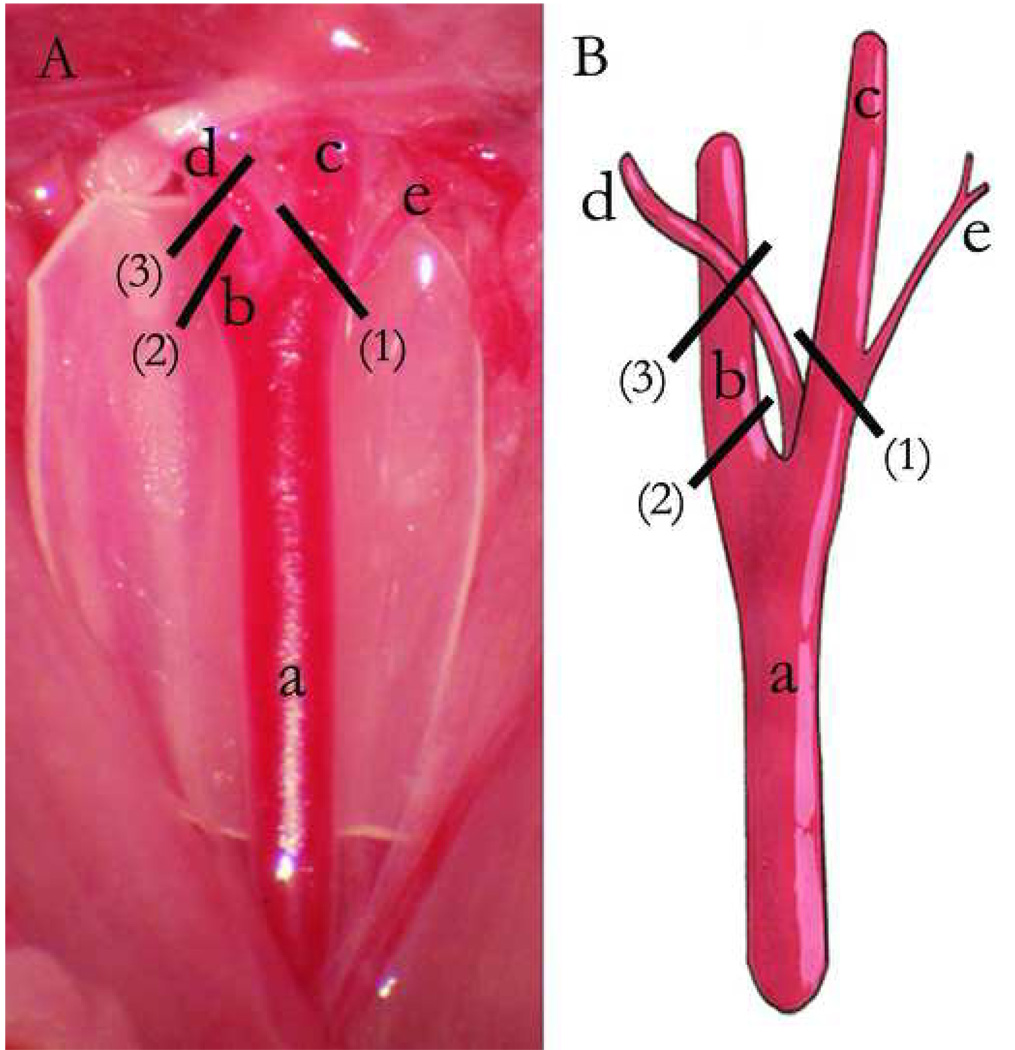
Conversely, flow manipulations in the mouse vein graft model have been reported. The mouse distal common carotid artery has two major branches, the internal carotid and external carotid. In addition to the presence of branches, they may be distinguished by size, with the internal carotid artery having a slightly larger diameter than the external carotid artery. The occipital artery, a small branch originating from the external carotid or the bifurcation, travels with the internal carotid. The superior thyroid artery is very small branch arising from the external carotid artery (Fig 2). 44
Fig 2.
Mouse carotid artery anatomy and partial ligation methods (A, Photographed right carotid anatomy (cut surgical glove background). B, Schematic representation). a, Right common carotid artery. b, Internal carotid artery. c, External carotid artery. d, Occipital artery. e, Superior thyroid artery. (1), Ligation of external carotid artery, including superior thyroid artery, but not occipital artery. (2), Ligation of internal carotid artery. (3), Ligation of internal carotid artery plus occipital artery.
Osterberg and colleagues 45 employed high-flow and low-flow vein graft models (cuff-technique) with 26–36 grams male C57BL/6 mice. By ligating the contralateral common carotid artery, they were able to induce a 20% higher vein graft flow rate (average baseline blood flow at 1.0 ml/min) immediately after ligation, and a 1.1 ml/min average value at day 42 in the high flow group. With ligation of the ipsilateral external carotid artery in the low flow group, a 50% immediate decrease in flow rate was achieved, and it became 0.4 ml/min at day 42. The neointimal area in the low flow grafts was 74% larger than that of high flow mice at day 42, with mean intimal thickness of ~ 55 µm vs ~ 33 µm respectively (calculated from graphs in their report).
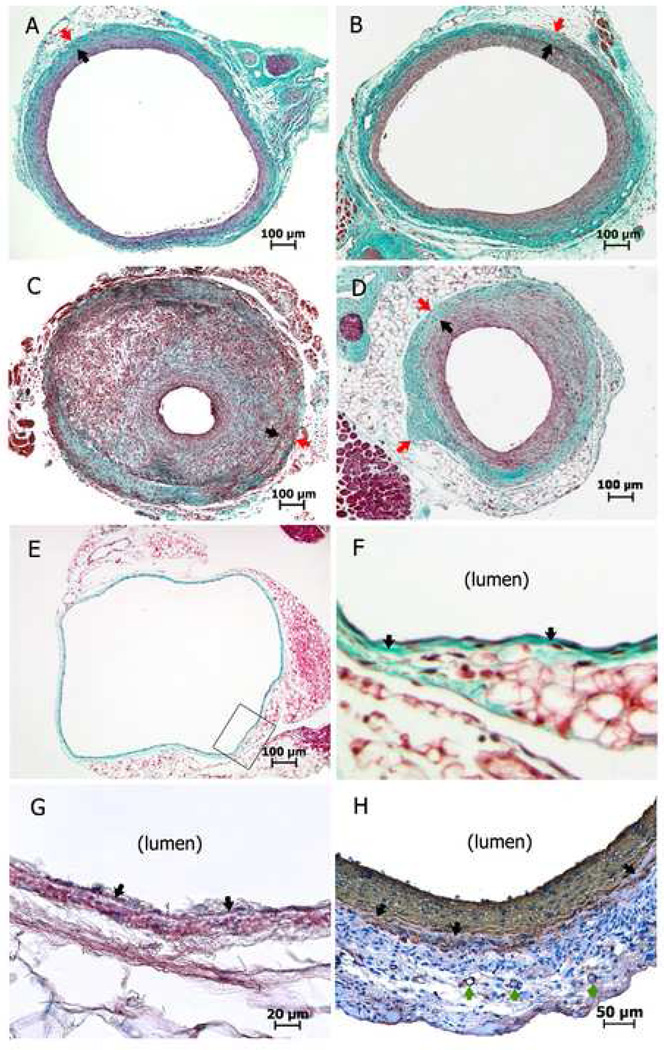
Manipulation of vein graft wall shear stress changes vein graft neointimal hyperplasia. 46, 47 We have completed various ligations of carotid branches to alter the flow rate. Ligation of the internal carotid artery plus occipital artery yielded an immediate 52% decrease in flow rate (1.18 ± 0.14 ml/min vs 0.57 ± 0.08 ml/min, control vs ligation, respectively), and a 73% decrease (1.31 ± 0.06 ml/min vs 0.36 ± 0.04 ml/min) at harvest (day 28). This method increased intimal thickness by 33% (50.2 ± 3.3 µm vs 66.9 ± 4.9 µm, Fig 3A vs 3B, respectively). Ligation of both the external and internal carotid artery (leaving only the occipital artery patent) resulted in an immediate mean flow rate ≤ 0.1 ml/min (n = 6). However, this led to a high rate of graft occlusion, as only one of three grafts were patent at day 14, and three of three were occluded at day 28. The patent vein graft did show tremendous intimal hyperplasia (Fig 3C).
Fig 3.
Masson trichrome staining (A–F) and α-actin immunohistochemistry staining (G, H) of cross-sections of mouse vein grafts/vein. Black arrows depict the internal elastic lamina (A–D, F–H); red arrows show the tunica adventitia/peri-vascular tissue boundary (A–D); and green arrows show the vasa vasorum (H). A, Normal flow vein graft at day 28. B, Vein graft with distal arterial partial (internal carotid + occipital artery) ligation at day 28. C, Vein graft with distal arterial partial (external carotid + internal carotid) ligation (leaving the occipital artery patent) at day 14. D, Negative wall remodeling in a mouse vein graft at day 28. E, Normal IVC. F, Enlarged area defined by the black box of Fig 3E. G (normal IVC) and H (mouse vein graft at day 28), note some rose-red or brownish-red positive expression of α-actin in the tunica media (beyond the internal elastic lamina).
The above results demonstrate the importance of various hemodynamic altering techniques for the wall adaptation of vein grafts. Attempts to ligate only the external carotid, only the internal carotid, or a combination of the internal carotid plus occipital artery (Fig 2) produced limited flow decreases, and consequently only modest intimal hyperplasia was observed. Conversely, combining external carotid and internal carotid artery ligation (with only occipital outflow) too severely restricted flow, with exorbitant intimal hyperplasia, making this manipulation unpractical.
SPECIMEN PROCESSING AND ANALYSIS CONSIDERATIONS
Financial and labor investments in complex mouse surgical models are high, thus thoughtful and meticulous specimen harvest and processing are paramount. Tissue segments intended for frozen section histology or molecular analyses should be harvested fresh, though the frozen method for optimal morphologic analysis of a vein graft is not recommended because of the compromised anatomic preservation. We prefer whole animal perfusion fixation (by 10% formalin or 4% paraformaldehyde) under physiological mean arterial pressure (~100 mmHg) with intravascular access via the left ventricle or aortic arch. The right atrium or supradiaphragmatic IVC can be cut as the outflow tract of fixative fluid.
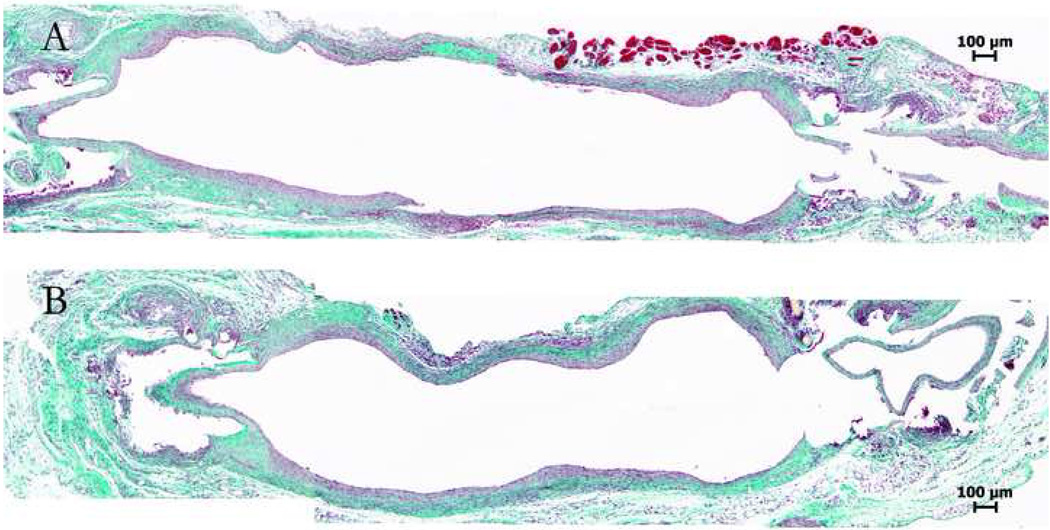
The intimal hyperplasia formation along the vein graft is not uniform: it is usually the thickest at the proximal, thinnest in the middle, and intermediate at the distal segment of the graft.11, 12, 14 This observation is also confirmed by our morphological analyses on serial cross-sections (data not shown) and longitudinal sections (Fig 4) of the mouse vein grafts. Because of this variation, meticulous localized sectioning and comparison of specific segments of the vein graft is important. Using a landmark (such as cuff edges in cuff-technique model, or anastomotic sutures in hand-sewn models), paraffin sections are obtained at 50 to 200 µm intervals, and computer image processing is employed to translate these into quantified morphologic endpoints such as intimal thickness, wall area and lumen narrowing.
Fig 4.
Masson trichrome staining of two longitudinally sectioned mouse vein grafts (cuff-technique, cuffs were removed at ends, leading to artifact). Note variations in intimal thickness along the length of the graft.
The microscopic anatomic definitions of mouse vein graft wall and artery wall do not match very well. At least for the common mouse strains that we have worked with (C57BL/6J and B6129SF2/J), the only obvious elastic lamina of the vein graft is recognized by us as the internal elastic lamina because only a single (endothelial) cell layer covers it (Fig 3F) and α-actin positive cells appear beyond it (Fig 3G) in the wall of mouse IVC. The tunica media of mouse vein graft could be obscure because of the early smooth muscle cell loss, 45 but may still present in some cases (Fig 3H). The mouse vein graft wall does not have a well defined external elastic lamina, thus the evaluation of the tunica media and tunica adventitia requires a different method. Some authors utilize the vasa vasorum as a landmark to discern the interface between the media and adventitia. 9, 11 We find this is difficult since the vasa vasorum may not always be present or associated with the true media/adventitia interface (Fig 3H). While the tunica adventitia has a relatively clear boundary with the surrounding, looser connective tissue; it is not always round (as shown in Fig 3D) and should not be confused with the external elastic lamina. To circumvent these issues, our practice is to combine the “media + adventitia” layers for morphological analysis.
Finally, similar to the clinical setting, 8 positive or negative (Fig 3D) vein graft wall remodeling can occur. A single parameter, such as intimal area or thickness, can not adequately describe total wall adaptation. For example, the grafts in Fig 3B and Fig 3D have similar intimal areas, but there are obvious differences in intimal thickness. Thus histopathological analysis of the vein graft tissue should not be confined to just the neointimal thickness or area, but also incorporate other parameters such as calculated ideal lumen area, total wall thickness or area, length of the internal elastic lamina in the perfusion fixed section, and ratios among these parameters. 48
MOUSE VEIN GRAFT LIMITATIONS
Current mouse vein graft models hold disadvantages. In most wild type mice there is limited neointimal formation, which hampers efforts to identify therapies to attenuate intimal hyperplasia. Several possible reasons contribute to this limitation. Our six-month mouse vein grafts did not show exorbitant neointimal formation (data not shown), and this suggests that simply extending the observation period does not resolve this limitation.
Normal mouse IVC has a very thin wall (average wall thickness is 12 µm, distended with arterial pressure, from male C57Bl/6J mice at age 9 weeks): one cell layer thick tunica intima, one elastic lamina, just one or two cell layers in the media + adventitia (Fig 3E–3G). This renders early histological evaluation extremely challenging.
Also, in mouse vein graft intimal hyperplasia, participation of graft extrinsic cells appears to be significant. A certain portion of neointimal cells and repopulated endothelial cells originate from non-wall origin (such as bone marrow), 49–52 and we do not know if such processes occur in the human scenario. Cells of various origins may respond differently to therapeutic agents.
Comprehensive hemodynamic and biomechanical assessments of the various mouse vein graft constructions have not been reported. Thus correlation with actual human physical parameters may be limited.
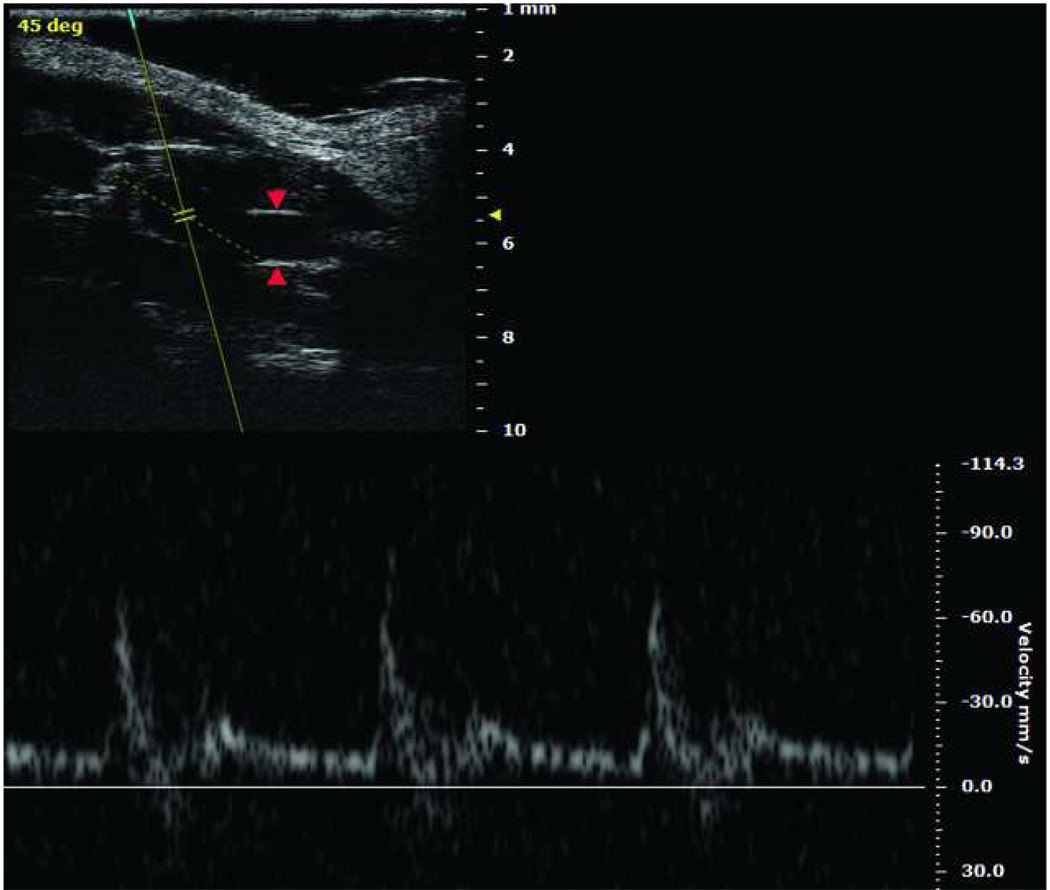
Finally, imaging also remains a challenge, though an overall heightened interest in small animal imaging will probably soon provide tools to gain in vivo anatomic and biologic information in the mouse setting. For instance, commercially available high frequency ultrasound duplex can provide good resolution of mouse vein graft dimensions and blood velocities (Fig 5). Duplex potentially offers a technically easy, non-invasive method for longitudinal anatomic mouse vein graft imaging. However, published reports that employ vascular imaging modalities for mouse vein grafts are rare.
Fig 5.
Upper left Duplex ultrasound scanning of mouse right carotid vein graft model (cuff-technique), taken by Vevo 2100 Imaging System (VisualSonics Inc., Toronto, Canada). Red arrows show vein graft wall. Lower: Velocity of vein graft blood flow.
CONCLUSION
Despite the anatomical and physiological differences between mice and men, and the inherent technical challenges with small animal surgery, the mouse continues to serve as an important stepping stone from laboratory bench work to bedside practice. Knowledge of key advantages and limitations of currently available mouse vein graft models will help tailor experiments to systematically unlock mechanisms that drive vein graft failure, and challenge all to improve on mouse based investigative strategies to understand vascular diseases.
ACKNOWLEDGEMENTS
The authors thank Dr. Zhihua Jiang (University of Florida, College of Medicine) for critical manuscript review.
The study was funded by the National Heart, Lung, and Blood Institute R01HL079135, T32HL007734, and the Carl and Ruth Shapiro Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 51. [DOI] [PubMed] [Google Scholar]
- 3.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 4.Carrel A, Guthrie CC. Uniterminal and biterminal venous transplantations. Surg Gynecol Obstet. 1906;2:266–277. [Google Scholar]
- 5.Grondin CM, Meere C, Castonguay Y, Lepage G, Grondin P. Progressive and late obstruction of an aorto-coronary venous bypass graft. Circulation. 1971;43:698–702. doi: 10.1161/01.cir.43.5.698. [DOI] [PubMed] [Google Scholar]
- 6.Wallitt EJ, Jevon M, Hornick PI. Therapeutics of vein graft intimal hyperplasia: 100 years on. Ann Thorac Surg. 2007;84:317–323. doi: 10.1016/j.athoracsur.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Lau GT, Ridley LJ, Bannon PG, Wong LA, Trieu J, Brieger DB, et al. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006;114:I435–I440. doi: 10.1161/CIRCULATIONAHA.105.001008. [DOI] [PubMed] [Google Scholar]
- 8.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: Clinical implications. J Vasc Surg. 2010;51:736–746. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C, Patel A, Zhang D, Wang H, Carmeliet P, Reed GL, et al. Plasminogen is not required for neointima formation in a mouse model of vein graft stenosis. Circ Res. 1999;84:883–890. doi: 10.1161/01.res.84.8.883. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Hagen PO, Kisslo J, Peppel K, Freedman NJ. Neointimal hyperplasia rapidly reaches steady state in a novel murine vein graft model. J Vasc Surg. 2002;36:824–832. [PubMed] [Google Scholar]
- 12.Cooley BC. Murine model of neointimal formation and stenosis in vein grafts. Arterioscler Thromb Vasc Biol. 2004;24:1180–1185. doi: 10.1161/01.ATV.0000129330.19057.9f. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi T, Asai T, Belov D, Okada M, Pinsky DJ, Schmidt AM, et al. Influence of ischemic injury on vein graft remodeling: role of cyclic adenosine monophosphate second messenger pathway in enhanced vein graft preservation. J Thorac Cardiovasc Surg. 2005;129:129–137. doi: 10.1016/j.jtcvs.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Diao Y, Xue J, Segal MS. A novel mouse model of autologous venous graft intimal hyperplasia. J Surg Res. 2005;126:106–113. doi: 10.1016/j.jss.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Salzberg SP, Filsoufi F, Anyanwu A, von Harbou K, Karlof E, Carpentier A, et al. Increased neointimal formation after surgical vein grafting in a murine model of type 2 diabetes. Circulation. 2006;114:I302–I307. doi: 10.1161/CIRCULATIONAHA.105.001339. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch GM, Karnovsky MJ. Inhibition of vein graft intimal proliferative lesions in the rat by heparin. Am J Pathol. 1991;139:581–587. [PMC free article] [PubMed] [Google Scholar]
- 17.Klyachkin ML, Davies MG, Svendsen E, Kim JH, Massey MF, Barber L, et al. Hypercholesterolemia and experimental vein grafts: accelerated development of intimal hyperplasia and an increase in abnormal vasomotor function. J Surg Res. 1993;54:451–468. doi: 10.1006/jsre.1993.1071. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS, et al. A novel vein graft model: adaptation to differential flow environments. Am J Physiol Heart Circ Physiol. 2004;286:H240–H245. doi: 10.1152/ajpheart.00760.2003. [DOI] [PubMed] [Google Scholar]
- 19.Morinaga K, Eguchi H, Miyazaki T, Okadome K, Sugimachi K. Development and regression of intimal thickening of arterially transplanted autologous vein grafts in dogs. J Vasc Surg. 1987;5:719–730. doi: 10.1067/mva.1987.avs0050719. [DOI] [PubMed] [Google Scholar]
- 20.Chen SJ, Wilson JM, Muller DW. Adenovirus-mediated gene transfer of soluble vascular cell adhesion molecule to porcine interposition vein grafts. Circulation. 1994;89:1922–1928. doi: 10.1161/01.cir.89.5.1922. [DOI] [PubMed] [Google Scholar]
- 21.McCann RL, Larson RM, Mitchener JS, 3rd, Fuchs JC, Hagen PO. Intimal thickening and hyperlipidemia in experimental primate vascular autografts. Ann Surg. 1979;189:62–67. doi: 10.1097/00000658-197901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonchek LI, Boerboom LE, Olinger GN, Pepper JR, Munns J, Hutchinson L, et al. Prevention of lipid accumulation in experimental vein bypass grafts by antiplatelet therapy. Circulation. 1982;66:338–341. doi: 10.1161/01.cir.66.2.338. [DOI] [PubMed] [Google Scholar]
- 23.Zilla P, Human P, Wolf M, Lichtenberg W, Rafiee N, Bezuidenhout D, et al. Constrictive external nitinol meshes inhibit vein graft intimal hyperplasia in nonhuman primates. J Thorac Cardiovasc Surg. 2008;136:717–725. doi: 10.1016/j.jtcvs.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 24.Guidelines for Survival Rodent Surgery. Bethesda, MD: National Institutes of Health; Office of Animal Care and Use (OACU) 2007
- 25.Lardenoye JH, de Vries MR, Lowik CW, Xu Q, Dhore CR, Cleutjens JP, et al. Accelerated atherosclerosis and calcification in vein grafts: a study in APOE*3 Leiden transgenic mice. Circ Res. 2002;91:577–584. doi: 10.1161/01.res.0000036901.58329.d7. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Chyu KY, Faria Neto JR, Yano J, Nathwani N, Ferreira C, et al. Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;110:1701–1705. doi: 10.1161/01.CIR.0000142857.79401.69. [DOI] [PubMed] [Google Scholar]
- 27.Fogelstrand P, Osterberg K, Mattsson E. Reduced neointima in vein grafts following a blockage of cell recruitment from the vein and the surrounding tissue. Cardiovasc Res. 2005;67:326–332. doi: 10.1016/j.cardiores.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, et al. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–642. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- 29.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Z, Yu P, Tao M, Ifantides C, Ozaki CK, Berceli SA. Interplay of CCR2 signaling and local shear force determines vein graft neointimal hyperplasia in vivo. FEBS Lett. 2009;583:3536–3540. doi: 10.1016/j.febslet.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Z, Berceli SA, Pfahnl CL, Wu L, Killingsworth CD, Vieira FG, et al. Impact of IL-1beta on flow-induced outward arterial remodeling. Surgery. 2004;136:478–482. doi: 10.1016/j.surg.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 32.Cooley BC. Model of murine interpositional vein grafting. Microsurgery. 2005;25:209–212. doi: 10.1002/micr.20106. [DOI] [PubMed] [Google Scholar]
- 33.Khodadad G, Lougheed WM. Repair of Small Arteries with Contact Cement and Teflon Graft. J Neurosurg. 1964;21:552–560. doi: 10.3171/jns.1964.21.7.0552. [DOI] [PubMed] [Google Scholar]
- 34.Jones BM, Mayou BJ. An onlay vein patch technique for the repair of small vessels. Br J Plast Surg. 1981;34:454–457. doi: 10.1016/0007-1226(81)90057-6. [DOI] [PubMed] [Google Scholar]
- 35.Archie JP., Jr The geometry and mechanics of saphenous vein patch angioplasty after carotid endarterectomy. Tex Heart Inst J. 1987;14:395–400. [PMC free article] [PubMed] [Google Scholar]
- 36.Lord RS, Raj TB, Stary DL, Nash PA, Graham AR, Goh KH. Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectomy. Part I. Perioperative results. J Vasc Surg. 1989;9:521–529. [PubMed] [Google Scholar]
- 37.Margovsky AI, Meek AC, Lord RS. Acute platelet deposition after carotid endarterectomy in sheep: vein patch compared with gelatin-sealed Dacron and polytetrafluoroethylene patch closure. J Vasc Surg. 1996;24:200–206. doi: 10.1016/s0741-5214(96)70094-0. [DOI] [PubMed] [Google Scholar]
- 38.Cooley BC. Mouse strain differential neointimal response in vein grafts and wire-injured arteries. Circ J. 2007;71:1649–1652. doi: 10.1253/circj.71.1649. [DOI] [PubMed] [Google Scholar]
- 39.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the "Glagov phenomenon" is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 41.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz LB, O'Donohoe MK, Purut CM, Mikat EM, Hagen PO, McCann RL. Myointimal thickening in experimental vein grafts is dependent on wall tension. J Vasc Surg. 1992;15:176–186. doi: 10.1067/mva.1992.33805. [DOI] [PubMed] [Google Scholar]
- 43.Huynh TT, Davies MG, Trovato MJ, Svendsen E, Hagen PO. Alterations in wall tension and shear stress modulate tyrosine kinase signaling and wall remodeling in experimental vein grafts. J Vasc Surg. 1999;29:334–344. doi: 10.1016/s0741-5214(99)70386-1. [DOI] [PubMed] [Google Scholar]
- 44.Korshunov VA, Berk BC. Flow-Induced Vascular Remodeling in the Mouse: A Model for Carotid Intima-Media Thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 45.Osterberg K, Mattsson E. Intimal hyperplasia in mouse vein grafts is regulated by flow. J Vasc Res. 2005;42:13–20. doi: 10.1159/000082802. [DOI] [PubMed] [Google Scholar]
- 46.Morinaga K, Okadome K, Kuroki M, Miyazaki T, Muto Y, Inokuchi K. Effect of wall shear stress on intimal thickening of arterially transplanted autogenous veins in dogs. J Vasc Surg. 1985;2:430–433. [PubMed] [Google Scholar]
- 47.Jiang Z, Berceli SA, Pfahnl CL, Wu L, Goldman D, Tao M, et al. Wall shear modulation of cytokines in early vein grafts. J Vasc Surg. 2004;40:345–350. doi: 10.1016/j.jvs.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 48.Ozaki CK. Cytokines and the early vein graft: strategies to enhance durability. J Vasc Surg. 2007;45 Suppl A:A92–A98. doi: 10.1016/j.jvs.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q. Both donor and recipient origins of smooth muscle cells in vein graft atherosclerotic lesions. Circ Res. 2002;91:e13–e20. doi: 10.1161/01.res.0000037090.34760.ee. [DOI] [PubMed] [Google Scholar]
- 50.Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res. 2003;93:e76–e86. doi: 10.1161/01.RES.0000097864.24725.60. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004;24:470–476. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 52.Diao Y, Guthrie S, Xia SL, Ouyang X, Zhang L, Xue J, et al. Long-term engraftment of bone marrow-derived cells in the intimal hyperplasia lesion of autologous vein grafts. Am J Pathol. 2008;172:839–848. doi: 10.2353/ajpath.2008.070840. [DOI] [PMC free article] [PubMed] [Google Scholar]