Summary
Nucleolar dominance is an epigenetic phenomenon that occurs in genetic hybrids and describes the expression of 45S rRNA genes inherited from one progenitor due to the silencing of the other progenitor’s rRNA genes. Nucleolar dominance is a manifestation of rRNA gene dosage control, which also occurs in non-hybrids, regulating the number of active rRNA genes according to the cellular demand for ribosomes and protein synthesis. Ribosomal RNA gene silencing involves changes in DNA methylation and histone modifications, but the molecular basis for choosing which genes to silence remains unclear. Recent studies indicate a role for short interfering RNAs (siRNAs) or structured regulatory RNAs in rRNA gene silencing in plants or mammals, respectively, suggesting that RNA may impart specificity to the choice mechanism.
Introduction
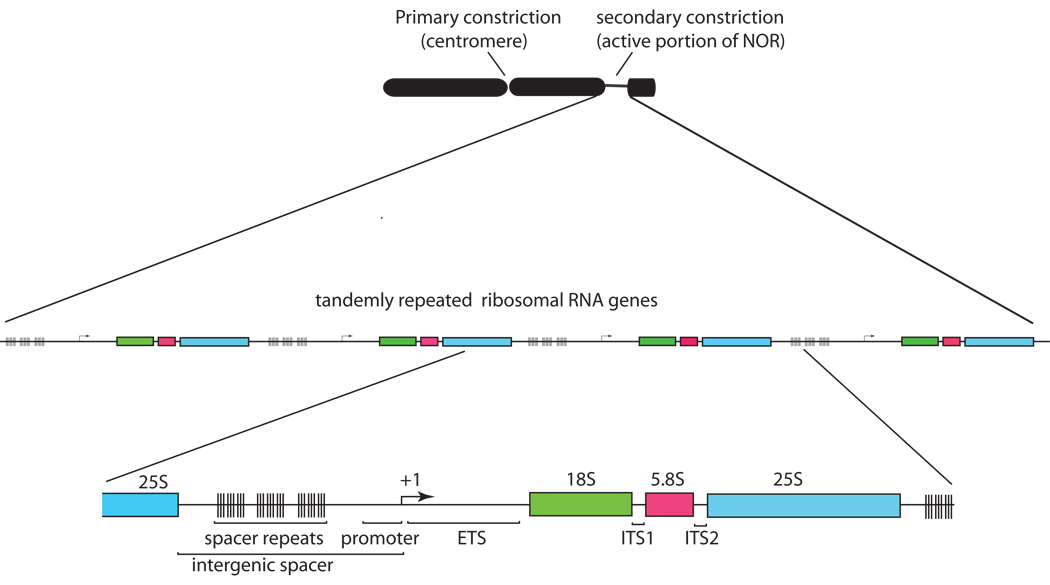
In eukaryotes, 45S rRNA genes are tandemly arrayed by the hundreds, and sometimes by the thousands, at chromosomal loci spanning millions of basepairs. RNA polymerase I (Pol I) transcribes the 45S rRNA genes, producing primary transcripts that are then processed extensively, yielding one molecule each of 18S, 5.8S and 25–28S rRNA, with the exact size being species-dependent (Figure 1). These RNAs, together with 5S rRNA transcribed by Pol III, comprise the structural and catalytic centers of ribosomes, the protein synthesizing machines of the cell [1,2] [3].
Figure 1. Ribosomal RNA genes are arrayed in long tandem repeats at NORs.
Secondary constrictions (NORs) observed on metaphase chromosomes correspond to decondensed chromatin that is indicative of rRNA gene repeats that had been active in the preceding interphase. The rRNA gene repeats are arranged in long tandem arrays of 45S rRNA genes, each including the coding regions for the 18S, 5.8S and 25S rRNAs, and each separated from the adjacent rRNA gene by an intergenic spacer. Intergenic spacers include repetitive elements and the gene promoter. The transcription start site is indicated by +1. Sequences removed during processing of 45S transcripts include the external transcribed spacer (ETS) and the internal transcribed spacers (ITS) separating the 18S, 5.8S and 25S rRNAs.
The chromosomal loci where rRNA genes are clustered are known as Nucleolus Organizer Regions (NORs) [4] and the nucleolus, the site of ribosome assembly, only forms at NORs if the rRNA genes are active [1]. NORs that are active during interphase remains relatively decondensed at metaphase, forming so-called secondary constrictions - the primary constrictions being the centromeres (Figure 1). The basis for secondary constriction formation is the persistent binding of Pol I transcription factors that happen to stain intensively with silver. In animals, the Pol I transcription factor, UBF (Upstream Binding Factor) is particularly important for secondary constriction formation, as demonstrated by the recent engineering of “pseudo-NORs” composed of repeated UBF binding sites [5,6]. These pseudo-NORs display the secondary constrictions and silver staining characteristics of true NORs [7]. Although plants do not appear to have an obvious UBF homolog, their secondary constrictions, like those of animals, stain intensively with silver [8].
Unlike regions of NORs that were transcriptionally active during interphase, and therefore remain relatively decondensed at metaphase, inactive NORs become fully condensed at metaphase. This difference in metaphase NOR condensation, which reflects the activity state of the rRNA genes during the preceding interphase, was the molecular basis for the initial discovery of nucleolar dominance as a phenomenon affecting chromosome morphology [9]. Specifically, Navashin noted that in multiple different interspecific hybrid combinations of species within the plant genus, secondary constrictions frequently formed on the chromosomes inherited from only one of the two progenitors, regardless of whether that progenitor served as the maternal or paternal parent. Decades later, it became clear that NORs are the loci where rRNA genes are tandemly arrayed and studies in Xenopus hybrids showed that the molecular basis for nucleolar dominance is the uniparental expression of rRNA genes [10].
Despite its ~80 year history and its occurrence in interspecific hybrids ranging from plants, invertebrates, frogs, flies, fish and mammals (for reviews see [11,12], the mechanisms responsible for nucleolar dominance are still not resolved. However, recent progress includes evidence that nucleolar dominance is a large –scale gene silencing phenomenon in which noncoding RNAs may be key to selective rRNA gene inactivation.
Chromatin modifications are involved in nucleolar dominance in plants
The hypothesis that dominant genes are preferentially activated and underdominant genes are off by default has been largely supplanted by evidence that the underdominant genes are preferentially silenced through changes in DNA methylation and repressive histone modifications. Initially, the chemical inhibitor of DNA methylation, aza-deoxycytosine (aza-dC) was shown to disrupt nucleolar dominance in wheat-rye hybrids [13] and in Brassica allotetraploid hybrids [14]. In the Brassica hybrids, chemical inhibition of histone deacetylation using Trichostatin A (TSA) was also sufficient to prevent nucleolar dominance. Importantly, simultaneous treatment with both aza-dC and TSA did not yield additive or synergistic effects, indicating that DNA methylation and histone deacetylation are partners in the same repression pathway [14]. Moreover, blocking DNA methylation was found to prevent the occurrence of repressive histone modifications, and blocking histone deacetylation disrupted cytosine hypermethylation, indicating that cytosine methylation and repressive histone modifications specify one another in a self-reinforcing loop at rRNA genes [15].
In Arabidopsis suecica, the allotetraploid hybrid of A. thaliana and A. arenosa, the A. thaliana-derived rRNA genes are silenced. Transgene-induced RNA interference (RNAi) has proven to be a useful approach for identifying specific chromatin modifying activities required for nucleolar dominance in A. suecica. Systematic knockdown of the sixteen predicted histone deacetylases identified HDT1 and HDA6 as activities required for rRNA gene silencing in nucleolar dominance [15,16]. Similar experiments targeting the predicted DNA methyltransferases showed that the de novo methyltransferase, DRM2, is required for the silencing of the A. thaliana-derived rRNA genes in the hybrid [17]. Likewise, knockdown of the twelve predicted methylcytosine binding domain proteins revealed the need for MBD6 and MBD10 [17]. Although MBD10 localizes throughout the nucleus, MBD6 colocalizes preferentially with rRNA gene loci. Immunolocalization and chromatin immunoprecipitation (ChIP) experiments indicate that MBD6-rRNA gene interactions are DRM2-dependent, suggesting that MBD6 recognizes cytosine methylation patterns established by DRM2 [17]. In mammals, methylcytosine binding domain proteins assist in the formation of heterochromatin by interacting with other chromatin modifying proteins, including histone deacetylases [18,19]. Whether MBD6 does likewise is currently unknown.
Nucleolar dominance as a manifestation of rRNA gene dosage control
What is the point of nucleolar dominance? The answer appears to lie in dosage control, a system that operates in non-hybrids, too, to regulate the number of active rRNA genes according to the physiological needs of the cell [20–22]. In proliferating cells, rRNA synthesis typically accounts for 50%, or more, of all nuclear transcription due to the demand for ribosomes and protein synthesis. By contrast, rRNA gene transcription drops precipitously in non-growing cells [2,23,24]. Whether in yeast or in humans, rRNA gene regulation occurs at two major levels. The first level of control regulates the number of rRNA genes in the on or off states [20]. Transcription among the active subset of rRNA genes is then fine-tuned by regulating the number of transcribing RNA polymerase I (Pol I) enzymes per gene [22,25] via post-translational modifications of Pol I transcription factors [2,24,26–32].
Several lines of evidence indicate that nucleolar dominance is a manifestation of dosage control. When silenced rRNA genes subjected to nucleolar dominance are derepressed with aza-dC or TSA, or by knockdown of required chromatin modifying activities, transcription from the dominant set of rRNA genes also increases, suggesting that the mechanisms responsible for the silencing of the underdominant genes are also silencing the subset of the dominant rRNA genes being dosage controlled [14]. In addition, chromatin immunoprecipitation (ChIP) experiments have revealed that the histone and DNA modifications controlling the proportion of active and inactive rRNA genes in non-hybrid A. thaliana are also responsible for nucleolar dominance in hybrid A. suecica [15].
An important point is that rRNA genes subjected to nucleolar dominance are not permanently inactivated but, instead, are silenced in a developmentally regulated manner [33,34]. In newly germinated A. suecica seedlings, the A. thaliana-derived and A. arenosa-derived rRNA genes are both highly expressed, but as true leaves emerge, the A. thaliana-derived rRNA genes become progressively inactivated [33]. These observations indicate that at one or more critical times per life cycle, the ribosomal RNA genes of both progenitors are needed in order to meet the physiological demands of the organism. During other periods of development, the ribosomal RNA genes are presumably in excess over the needs of the cells and the excess genes are silenced.
A role for small RNAs in nucleolar dominance
The finding that HDA6 and DRM2 are required for nucleolar dominance suggested a link to the RNA-directed DNA methylation (RdDM) pathway in Arabidopsis thaliana given that both activities have been identified in genetic screens for components of the RdDM pathway [35,36]. In this pathway, the plant-specific DNA-dependent RNA polymerase, Pol IV is thought to generate transcripts that are then used as templates by the RNA-dependent RNA polymerase, RDR2. Resulting double-stranded RNAs are then diced into 24 nt small RNAs by DICER-LIKE 3 (DCL3) and loaded into ARGONAUTE 4-containing effector complexes that somehow guide the de novo cytosine methyltransferase, DRM2 to DNA sequences homologous to the siRNAs. Exploration of the potential role of the RdDM pathway in nucleolar dominance led to the finding that RNAi-mediated knockdown of RDR2, DCL3 or DRM2 disrupts the silencing of the A. thaliana-derived rRNA genes in A. suecica [17]. In agreement with these results, small RNAs corresponding to the rRNA gene promoter and intergenic regions are eliminated in DCL3-RNAi lines and are depleted in RDR2-RNAi plants, suggesting a role for the siRNAs in the selective silencing of A. thaliana-derived rRNA genes [17] (summarized in Figure 2).
Figure 2. Comparison of models for rRNA gene silencing in nucleolar dominance (in plants) and in mammals, highlighting the roles of RNA.
A. Nucleolar dominance in the hybrid Arabidopsis suecica. Intergenic noncoding transcripts are produced and processed into 24 nucleotide (nt) small RNAs (siRNAs) by RDR2 and DCL3. The small RNAs guide de-novo methylation by DRM2, enabling MBD6 and MBD10 to bind methylated rRNA genes. HDA6 deacetylates the histones, facilitating further heterochromatin formation and rRNA gene silencing at the underdominant genes. B. Mammalian rRNA gene silencing. Intergenic noncoding transcripts are produced and processed into pRNAs. NoRC is targeted to the rRNA promoter through association with pRNA and silences a subclass of rRNA repeats in trans through recruitment of other chromatin modifiers. DNMT: DNA methyltransferase; HMT: histone methyltransferase; HDAC: histone deacetylase; MBD: methylcytosine binding domain protein.
RNA and chromatin-dependent rRNA gene silencing in mammals
The involvement of siRNA-directed DNA methylation in the establishment and/or maintenance of nucleolar dominance in plants is intriguing in light of evidence for RNA-mediated rRNA gene silencing in mammals. In mice, a subset of the rRNA genes is silenced via the action NoRC (nucleolar remodeling complex), a complex of TIP5 (TTFI-interaction protein #5), and SNF2h, an ATP-dependent chromatin remodeller [37–40]. Recent evidence indicates that NoRC recruitment is RNA dependent. The requisite RNA is not siRNA, but highly structured 200–300 nt RNAs whose transcription initiates within the intergenic spacers and extends through the promoter region [41,42]. These structured RNAs, dubbed pRNAs, interact with the TIP5 protein and facilitate NoRC recruitment to the rRNA gene promoter, thereby mediating heterochromatin formation and gene silencing. The pRNAs were recently shown to arise from a particular subclass of rRNA loci, indicating that they work in trans at distant rRNA gene repeats [43] (Figure 2).
A side-by-side consideration of RNA-mediated rRNA gene silencing in plants and mammals is enlightening. In mammals, NoRC inhibits rRNA gene transcription in an aza-dC and TSA-reversible manner [39], implicating DNA methylation and histone deacetylation in rRNA gene silencing, as in plants. NoRC physically interacts with the Sin3 co-repressor complex, which includes the histone deacetylases HDAC1 and HDAC2 [44], which are members of the same gene family as Arabidopsis HDA6 [16]. Moreover, NoRC interacts with the DNA methyltransferases DNMT1 and DNMT3 [39,45], the latter being a de novo DNA methyltransferase that is structurally related to Arabidopsis DRM2 [46], which is required for nucleolar dominance [17]. Moreover, the chromatin marks of active and silenced rRNA genes are similar in mouse and plants. In both cases, active gene promoters associate with histone H3 trimethylated on lysine 4 (H3K4me3) and with hyperacetylated histones H3 and H4. Likewise, silenced rRNA genes associate with methylated H3K9, deacetylated H3 and H4, and have cytosine-hypermethylated promoters. In both mammals and plants, RNA molecules generated from intergenic spacer sequence sequences appear to mediate silencing [17,41,42]. A difference, at present, is that plants appear to silence rRNA genes via siRNA-directed cytosine methylation and heterochromatin formation, which is a process that operates throughout the genome, whereas mammals appear to have evolved an rRNA-gene-specific system involving long noncoding pRNAs and NoRC. Nonetheless, the overall similarities of rRNA gene silencing in plants and mammals are striking [11,20].
Conclusions and Outlook
The big question in the study of nucleolar dominance concerns how one set of rRNA genes can be chosen for inactivation. Because nucleolar dominance occurs independent of maternal or paternal effects, and is developmentally regulated following embryogenesis, imprinting in the gametes does not appear to be involved. Unlike the random inactivation of one X-chromosome inactivation in somatic cells of female eutherian mammals [47,48], nucleolar dominance is not random. Instead, it is always the same parental set of rRNA genes that is silenced, implying a mechanism that can choose between the different sets of rRNA genes in the genome. This choice mechanism is not based simply on the number of rRNA genes at an NOR, as NORs with fewer genes can be dominant over NORs with more rRNA genes [34,49]. The hypothesis that inactivating a species-specific transcription factor might inactivate the matching set of rRNA genes [50,51] also fails to explain nucleolar dominance in plant hybrids whose progenitors' rRNA genes are fully functional when transfected into the other species [52,53]. Likewise, the appealing hypothesis that dominant genes have a higher binding affinity for one or more transcription factors, stemming from experiments involving large doses of rRNA minigenes injected into Xenopus oocytes [54–56], are not supported by transient expression or in vitro transcription experiments in plants [52,53]. Against the backdrop of these previous hypotheses, the new evidence that RNA is involved in nucleolar dominance is exciting in that base-pairing interactions, in principle, could impart the specificity needed to tell the rRNA genes apart.
In wheat-rye hybrids, it was recently demonstrated that nucleolar dominance involves the up-regulation of the dominant set of rRNA genes, concomitant with the repression of the underdominant set of genes [57]. Considered together with evidence that intergenic pRNA transcripts can lead to silencing of rRNA genes in trans in mice [43], a possibility is that transcription of the dominant class of rRNA genes results in the silencing of the repressed class. Moreover, nucleolar dominance is known to involve proteins of the RNA-directed DNA methylation pathway [17], utilizing siRNAs that can act in trans at homologous loci. However, it is not clear why siRNAs would act only in trans and not silence the loci from which they arise.
Other unexplained observations include evidence that it could be the NOR, and not the individual rRNA genes, that is the unit of regulation in nucleolar dominance [58]. For instance, in Arabidopsis thaliana × A. lyrata hybrids, rRNA transgenes integrated at ectopic loci escape the silencing imposed on rRNA genes within the A. thaliana NORs [59]. This result is unexpected if each individual rRNA gene is silenced independently.
Determining the origins of noncoding transcripts giving rise to intergenic regulatory RNAs, determining their regulation during development, identifying the basis for rRNA gene specificity and what prevents regulatory RNAs from silencing all rRNA genes in the genome are questions that will provide focus in the coming years.
Acknowledgements
Our work is supported by National Institutes of Health grant GM60380 and by the generous support of Monsanto Company, St. Louis, MO, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.McKeown PC, Shaw PJ. Chromatin: linking structure and function in the nucleolus. Chromosoma. 2009;118:11–23. doi: 10.1007/s00412-008-0184-2. [DOI] [PubMed] [Google Scholar]
- 2.Moss T, Langlois F, Gagnon-Kugler T, Stefanovsky V. A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cell Mol Life Sci. 2007;64:29–49. doi: 10.1007/s00018-006-6278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 4.McClintock B. The relationship of a particular chromosomal element to the development of the nucleoli in Zea mays. Zeit. Zellforsch. Mik. Anat. 1934;21:294–328. [Google Scholar]
- 5.Prieto JL, McStay B. Recruitment of factors linking transcription and processing of pre-rRNA to NOR chromatin is UBF-dependent and occurs independent of transcription in human cells. Genes Dev. 2007;21:2041–2054. doi: 10.1101/gad.436707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mais C, Wright JE, Prieto JL, Raggett SL, McStay B. UBF-binding site arrays form pseudo-NORs and sequester the RNA polymerase I transcription machinery. Genes Dev. 2005;19:50–64. doi: 10.1101/gad.310705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prieto JL, McStay B. Pseudo-NORs: a novel model for studying nucleoli. Biochim Biophys Acta. 2008;1783:2116–2123. doi: 10.1016/j.bbamcr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Caperta AD, Neves N, Viegas W, Pikaard CS, Preuss S. Relationships between transcription, silver staining, and chromatin organization of nucleolar organizers in Secale cereale. Protoplasma. 2007;232:55–59. doi: 10.1007/s00709-007-0277-4. [DOI] [PubMed] [Google Scholar]
- 9.Navashin M. Chromosomal alterations caused by hybridization and their bearing upon certain general genetic problems. Cytologia. 1934;5:169–203. [Google Scholar]
- 10.Honjo T, Reeder RH. Preferential transcription of Xenopus laevis ribosomal RNA in interspecies hybrids between Xenopus laevis and Xenopus mulleri. J. Mol. Biol. 1973;80:217–228. doi: 10.1016/0022-2836(73)90168-x. [DOI] [PubMed] [Google Scholar]
- 11.McStay B. Nucleolar dominance: a model for rRNA gene silencing. Genes Dev. 2006;20:1207–1214. doi: 10.1101/gad.1436906. [DOI] [PubMed] [Google Scholar]
- 12.Preuss S, Pikaard CS. rRNA gene silencing and nucleolar dominance: insights into a chromosome-scale epigenetic on/off switch. Biochim Biophys Acta. 2007;1769:383–392. doi: 10.1016/j.bbaexp.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neves N, Heslop-Harrison JS, Viegas W. rRNA gene activity and control of expression mediated by methylation and imprinting during embryo development in wheat x rye hybrids. Theor. Appl. Genet. 1995;91:529–533. doi: 10.1007/BF00222984. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZJ, Pikaard CS. Epigenetic silencing of RNA polymerase I transcription: a role for DNA methylation and histone modification in nucleolar dominance. Genes Dev. 1997;11:2124–2136. doi: 10.1101/gad.11.16.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence RJ, Earley K, Pontes O, Silva M, Chen ZJ, Neves N, Viegas W, Pikaard CS. A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell. 2004;13:599–609. doi: 10.1016/s1097-2765(04)00064-4. [DOI] [PubMed] [Google Scholar]
- 16.Earley K, Lawrence RJ, Pontes O, Reuther R, Enciso AJ, Silva M, Neves N, Gross M, Viegas W, Pikaard CS. Erasure of histone acetylation by Arabidopsis HDA6 mediates large-scale gene silencing in nucleolar dominance. Genes Dev. 2006;20:1283–1293. doi: 10.1101/gad.1417706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Preuss SB, Costa-Nunes P, Tucker S, Pontes O, Lawrence RJ, Mosher R, Kasschau KD, Carrington JC, Baulcombe DC, Viegas W, et al. Multimegabase silencing in nucleolar dominance involves siRNA-directed DNA methylation and specific methylcytosine-binding proteins. Mol Cell. 2008;32:673–684. doi: 10.1016/j.molcel.2008.11.009.. **The authors show that several RNA-directed DNA methylation pathway proteins are essential for the establishment and maintenance of nucleolar dominance in Arabidopsis.
- 18.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 19.Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 20.Grummt I, Pikaard CS. Epigenetic silencing of RNA polymerase I transcription. Nat Rev Mol Cell Biol. 2003;4:641–649. doi: 10.1038/nrm1171. [DOI] [PubMed] [Google Scholar]
- 21.Grummt I. Different epigenetic layers engage in complex crosstalk to define the epigenetic state of mammalian rRNA genes. Hum Mol Genet. 2007;16(Spec No 1):R21–R27. doi: 10.1093/hmg/ddm020. [DOI] [PubMed] [Google Scholar]
- 22.McStay B, Grummt I. The epigenetics of rRNA genes: from molecular to chromosome biology. Annu Rev Cell Dev Biol. 2008;24:131–157. doi: 10.1146/annurev.cellbio.24.110707.175259. [DOI] [PubMed] [Google Scholar]
- 23.Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci. 1999;24:437–440. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- 24.Grummt I. Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev. 2003;17:1691–1702. doi: 10.1101/gad.1098503R. [DOI] [PubMed] [Google Scholar]
- 25.French SL, Osheim YN, Cioci F, Nomura M, Beyer AL. In exponentially growing Saccharomyces cerevisiae cells, rRNA synthesis is determined by the summed RNA polymerase I loading rate rather than by the number of active genes. Mol Cell Biol. 2003;23:1558–1568. doi: 10.1128/MCB.23.5.1558-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss T. At the crossroads of growth control; making ribosomal RNA. Curr Opin Genet Dev. 2004;14:210–217. doi: 10.1016/j.gde.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Milkereit P, Tschochner H. A specialized form of RNA polymerase I, essential for initiation and growth-dependent regulation of rRNA synthesis, is disrupted during transcription. Embo J. 1998;17:3692–3703. doi: 10.1093/emboj/17.13.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peyroche G, Milkereit P, Bischler N, Tschochner H, Schultz P, Sentenac A, Carles C, Riva M. The recruitment of RNA polymerase I on rDNA is mediated by the interaction of the A43 subunit with Rrn3. Embo J. 2000;19:5473–5482. doi: 10.1093/emboj/19.20.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanovsky VY, Pelletier G, Hannan R, Gagnon-Kugler T, Rothblum LI, Moss T. An immediate response of ribosomal transcription to growth factor stimulation in mammals is mediated by ERK phosphorylation of UBF. Mol Cell. 2001;8:1063–1073. doi: 10.1016/s1097-2765(01)00384-7. [DOI] [PubMed] [Google Scholar]
- 30.Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23:8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanaugh AH, Hirschler-Laszkiewicz I, Hu Q, Dundr M, Smink T, Misteli T, Rothblum LI. Rrn3 phosphorylation is a regulatory checkpoint for ribosome biogenesis. J Biol Chem. 2002;277:27423–27432. doi: 10.1074/jbc.M201232200. [DOI] [PubMed] [Google Scholar]
- 32.Pelletier G, Stefanovsky VY, Faubladier M, Hirschler-Laszkiewicz I, Savard J, Rothblum LI, Cote J, Moss T. Competitive recruitment of CBP and Rb-HDAC regulates UBF acetylation and ribosomal transcription. Mol Cell. 2000;6:1059–1066. doi: 10.1016/s1097-2765(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 33.Pontes O, Lawrence RJ, Silva M, Preuss S, Costa-Nunes P, Earley K, Neves N, Viegas W, Pikaard CS. Postembryonic establishment of megabase-scale gene silencing in nucleolar dominance. PLoS One. 2007;2:e1157. doi: 10.1371/journal.pone.0001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen ZJ, Pikaard CS. Transcriptional analysis of nucleolar dominance in polyploid plants: biased expression/silencing of progenitor rRNA genes is developmentally regulated in Brassica. Proc. Natl. Acad. Sci. USA. 1997;94:3442–3447. doi: 10.1073/pnas.94.7.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ. RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol. 2009;21:367–376. doi: 10.1016/j.ceb.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strohner R, Nemeth A, Jansa P, Hofmann-Rohrer U, Santoro R, Langst G, Grummt I. NoRC--a novel member of mammalian ISWI-containing chromatin remodeling machines. Embo J. 2001;20:4892–4900. doi: 10.1093/emboj/20.17.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strohner R, Nemeth A, Nightingale KP, Grummt I, Becker PB, Langst G. Recruitment of the nucleolar remodeling complex NoRC establishes ribosomal DNA silencing in chromatin. Mol Cell Biol. 2004;24:1791–1798. doi: 10.1128/MCB.24.4.1791-1798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santoro R, Li J, Grummt I. The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nat Genet. 2002;32:393–396. doi: 10.1038/ng1010. [DOI] [PubMed] [Google Scholar]
- 40.Nemeth A, Strohner R, Grummt I, Langst G. The chromatin remodeling complex NoRC and TTF-I cooperate in the regulation of the mammalian rRNA genes in vivo. Nucleic Acids Res. 2004;32:4091–4099. doi: 10.1093/nar/gkh732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer C, Schmitz KM, Li J, Grummt I, Santoro R. Intergenic transcripts regulate the epigenetic state of rRNA genes. Mol Cell. 2006;22:351–361. doi: 10.1016/j.molcel.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 42. Mayer C, Neubert M, Grummt I. The structure of NoRC-associated RNA is crucial for targeting the chromatin remodelling complex NoRC to the nucleolus. EMBO Rep. 2008 doi: 10.1038/embor.2008.109.. ** The authors show that the RNAs interacting with TIP5 of the NoRC complex have specific structures.
- 43. Santoro R, Schmitz KM, Sandoval J, Grummt I. Intergenic transcripts originating from a subclass of ribosomal DNA repeats silence ribosomal RNA genes in trans. EMBO Rep. 2010;11:52–58. doi: 10.1038/embor.2009.254.. **The authors show that noncoding RNAs initiated in the intergenic spacer act in trans to silence other rRNA gene repeats.
- 44.Zhou Y, Santoro R, Grummt I. The chromatin remodeling complex NoRC targets HDAC1 to the ribosomal gene promoter and represses RNA polymerase I transcription. EMBO J. 2002;21:4632–4640. doi: 10.1093/emboj/cdf460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro R, Grummt I. Epigenetic mechanism of rRNA gene silencing: temporal order of NoRC-mediated histone modification, chromatin remodeling, and DNA methylation. Mol Cell Biol. 2005;25:2539–2546. doi: 10.1128/MCB.25.7.2539-2546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao X, Jacobsen SE. Role of the Arabidopsis DRM Methyltransferases in De Novo DNA Methylation and Gene Silencing. Curr Biol. 2002;12:1138–1144. doi: 10.1016/s0960-9822(02)00925-9. [DOI] [PubMed] [Google Scholar]
- 47.Huynh KD, Lee JT. X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny. Nat Rev Genet. 2005;6:410–418. doi: 10.1038/nrg1604. [DOI] [PubMed] [Google Scholar]
- 48.Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- 49.Flavell RB. The structure and control of expression of ribosomal RNA genes. Oxford Surveys Plant Mol. Cell Biol. 1986;3:252–274. [Google Scholar]
- 50.Grummt I, Roth E, Paule MR. rRNA transcription in vitro is species-specific. Nature. 1982;296:173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- 51.Miesfeld R, Arnheim N. Species-specific rDNA transcription is due to promoterspecific binding factors. Mol. Cell. Biol. 1984;4:221–227. doi: 10.1128/mcb.4.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frieman M, Chen ZJ, Saez-Vasquez J, Shen LA, Pikaard CS. RNA polymerase I transcription in a Brassica interspecific hybrid and its progenitors: tests of transcription factor involvement in nucleolar dominance. Genetics. 1999;152:451–460. doi: 10.1093/genetics/152.1.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen ZJ, Comai L, Pikaard CS. Gene dosage and stochastic effects determine the severity and direction of uniparental rRNA gene silencing (nucleolar dominance) in Arabidopsis allopolyploids. Proc. Natl. Acad. Sci. USA. 1998;95:14891–14896. doi: 10.1073/pnas.95.25.14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reeder RH. Mechanisms of nucleolar dominance in animals and plants. J. Cell Biol. 1985;101:2013–2016. doi: 10.1083/jcb.101.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reeder RH, Roan JG. The mechanism of nucleolar dominance in Xenopus hybrids. Cell. 1984;38:39–44. doi: 10.1016/0092-8674(84)90524-5. [DOI] [PubMed] [Google Scholar]
- 56.Caudy AA, Pikaard CS. Xenopus ribosomal RNA gene intergenic spacer elements conferring transcriptional enhancement and nucleolar dominance-like competition in oocytes. J Biol Chem. 2002;277:31577–31584. doi: 10.1074/jbc.M202737200. [DOI] [PubMed] [Google Scholar]
- 57. Silva M, Pereira HS, Bento M, Santos AP, Shaw P, Delgado M, Neves N, Viegas W. Interplay of ribosomal DNA loci in nucleolar dominance: dominant NORs are up-regulated by chromatin dynamics in the wheat-rye system. PLoS One. 2008;3:e3824. doi: 10.1371/journal.pone.0003824.. *The authors report an upregulation in the dominant class of rRNA genes in addition to the repression of the underdominant class of genes in wheat-rye hybrid lines.
- 58.Lewis MS, Cheverud JM, Pikaard CS. Evidence for nucleolus organizer regions as the units of regulation in nucleolar dominance in Arabidopsis thaliana interecotype hybrids. Genetics. 2004;167:931–939. doi: 10.1534/genetics.103.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis MS, Pikaard DJ, Nasrallah M, Doelling JH, Pikaard CS. Locus-specific ribosomal RNA gene silencing in nucleolar dominance. PLoS One. 2007;2:e815. doi: 10.1371/journal.pone.0000815. [DOI] [PMC free article] [PubMed] [Google Scholar]