Abstract
Extracellular adenosine triphosphate (ATP) has profound effects on the cochlea, including an effect on the regulation of the endocochlear potential (EP). Noise-induced release of ATP into the endolymph activates a shunt conductance mediated by P2X2 receptors in tissues lining the endolymphatic compartment, which reduces the EP and, consequentially, hearing sensitivity. This may be a mechanism of adaptation or protection from high sound levels. As inaction of such a process could contribute to hearing loss, this study examined whether the action of ATP on EP changes with age and noise exposure in the mouse. The EP and the endolymphatic compartment resistance (CoPR) were measured in mice (CBA/CaJ) aged between 3 and 15 months. The EP and CoPR declined slightly with age with an associated small, but significant, reduction in auditory brainstem response thresholds. ATP (100–1,000 μM) microinjected into the endolymphatic compartment caused a dose-dependent decline in EP correlated to a similar decrease in CoPR. This was blocked by pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate, consistent with a P2X2 receptor-mediated shunt conductance. There was no substantial difference in the ATP response with age. Noise exposure (octave-band noise 80–100 decibels sound pressure level (dBSPL), 48 h) in young animals induced an upregulation of the P2X2 receptor expression in the organ of Corti and spiral limbus, most noticeably with the 90-dB exposure. This did not occur in the aged animals except following exposure at 90 dBSPL. The EP response to ATP was muted in the noise-exposed aged animals except following the 90-dB exposure. These findings provide some evidence that the adaptive response of the cochlea to noise may be reduced in older animals, and it is speculated that this could increase their susceptibility to noise-induced injury.
Keywords: Ageing, Noise, Endocochlear potential, Auditory brainstem response, CBA/CaJ, Immunohistochemistry, P2X2 receptors
Introduction
Extracellular purines (adenosine triphosphate, ATP) exert a variety of profound effects on the mature and developing cochlea, acting via both P2X and P2Y receptors in sensory, neural and secretory tissues [1–4]. Purinergic signalling appears to be involved in the regulation of hearing sensitivity under stress conditions, acting via the P2X2 subtype of the receptor in tissues lining the endolymphatic compartment [2, 5]. There is increasing evidence that ATP is a neuromodulator at the primary synapse between the inner hair cell and auditory afferent neurones [6] and is a neurotransmitter at the type II synapse with the outer hair cells [7] which regulates the generation of spontaneous neural activity in the developing cochlea [8]. ATP acts as a vasoactive signal, regulating cochlear blood flow [9], and appears to be involved in regulating cochlear mechanics via an action on the Deiters’ supporting cells of the auditory sensory organ [10–12]. There is also recent evidence that ATP may be involved in modulating neurite growth in primary afferent neurones [13] and synaptogenesis of the primary afferent synapse, acting via the P2X3 receptor, the expression of which closely follows the development and maturation of the synapse in the developing rat and mouse [14, 15]. Intercellular communication between supporting cells of the organ of Corti is regulated by extracellular ATP [16].
Of all the actions of ATP, the regulation of hearing sensitivity by extracellular ATP in the endolymphatic compartment is the most profound and well defined. Exogenous ATP delivered to the scala media in guinea pigs [5] results in a dose-dependent decrease in the endocochlear potential (EP) which is highly correlated with a decrease in resistance of the endolymphatic compartment (cochlear partition resistance, CoPR). This is due to the action of ATP on the ATP-gated ion channels (P2X2 receptors) present on the apical surface of hair cells and some supporting cells of the organ of Corti and epithelial cells of Reissner’s membrane [17]. This is supported by the attenuation of the response with the application of the broadly selective P2X2 antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) [5]. Activation of these receptors by ATP leads to increased potassium flux through these ion channels, causing cellular depolarisation, and the decreased resistance rapidly reduces the EP. ATP also acts through P2Y receptors on the marginal cells of the stria vascularis to regulate potassium secretion into the endolymph [18].
It has been proposed that ATP released into the endolymph from cells of the organ of Corti or the stria vascularis, following noise or hypoxia, acts via these P2X receptors on cells lining the endolymphatic compartment to limit hearing sensitivity [2, 5]. This is accomplished by a combined depolarising action on the sensory cells and suppression of the EP to reduce the driving force for sound transduction by hair cells. It is postulated that this purinergic pathway is an adaptive, protective mechanism to enable the cochlea to cope with a broad range of sound levels [2, 4, 5]. Such a mechanism may offer protection, over time, to the sensory cells by reducing their activity under noisy conditions and thus protecting them from damage, for example, from activity-induced free radical exposure. Further evidence in support of this comes from our studies showing increased expression of the P2X2 receptor subunit in the organ of Corti with extended loud sound exposure (3 days, 90 decibels sound pressure level, dBSPL) [19] and preliminary evidence of a decreased noise-induced temporary threshold shift (TTS) in mice lacking the P2X2 receptor [20]. The decreased TTS following noise exposure in this case reflects the fact that in the normal mouse, there is an ATP-mediated reduction in sensitivity which manifests as a TTS.
The rationale for this study was to determine if the P2X2 expression and ATP-mediated changes in EP and CoPR are altered with age and whether noise exposure has the same influence on P2X2 expression in young and old mice. Previous studies have shown that the action of ATP in other tissues generally declines with age and this is due to a differential change in the expression levels of the P2 receptors [21–27]. A similar age-related decline in the responsiveness of the cochlea to ATP, and hence a reduction in its putative protective or adaptive influence, may have implications for the development of hearing loss from ageing.
Methods
Animals
Mice (CBA/CaJ strain) of either sex, aged between 3 and 18 months, were used in this study. The majority of animals were studied at 3–6 and 12–15 months of age. Auditory function was assessed using the auditory brainstem response (ABR), the EP and CoPR, and the expression levels of the P2X2 receptors were determined using immunohistochemistry. Some animals at each age group were also subjected to noise, and the functional, molecular and histological measurements were repeated. All experimental procedures were approved by the University of Auckland Animal Ethics Committee.
ABR recording
Mice were lightly anesthetised with a combination of Ketamine (38 mg/kg) and Domitor (0.5 mg/kg) administered intraperitoneally. Animals were placed on a thermostatically controlled electric blanket during the ABR recordings, and all measurements were undertaken inside a sound-isolating chamber using TDT System II evoked potentials (Tucker Davis Technologies, USA). The ABR was recorded from subdermal needle electrodes placed at the vertex (reference), base of pinna (active), and base of the contralateral pinna (ground) and attached to a DB4 amplifier. Responses to clicks (alternating polarity square wave stimulus, 10-μs duration) and tone bursts (4, 10, 16, 22, 28 and 32 kHz, 10-ms duration, 1.5-ms rise–fall time, phase alternating) delivered by a closed-field system using an electrostatic loudspeaker (Beyer) were amplified (100,000×) and filtered (100 Hz–3 kHz) and then averaged (1,024 times) before being displayed. At each frequency, the amplitude of the signal was attenuated in 5-dB steps until all the wave I–V complex of the averaged ABR waveforms were no longer distinguishable by eye from background in recorded traces. This was taken as the ABR threshold.
Noise exposure
Mice were exposed to different levels of noise (80, 90 and 100 dBSPL, 4.5- to 9-kHz octave band noise) for 48 h (12-h light/dark cycle) in a sound booth. They had free access to food and water during the exposure. Control animals were taken from the animal facility where ambient noise levels were approximately 50–60 dBSPL.
EP and CoPR measurements
Animals were anesthetised with urethane (1.6 g/kg, i.p.) and body temperature was maintained using a thermostatically controlled electric blanket. A tracheotomy was performed and the animal was artificially ventilated with room air. The cochlea was surgically exposed through a ventral approach, and two adjacent holes (40–80 μm in diameter) were made in the bone overlying the scala media of the basal turn to insert glass micropipettes (5- to 7-μm tip diameter) for the EP and CoPR measurements and for current injection (Fig. 1). Two single-barrelled glass micropipettes (5- to 10-μm tip diameter) were inserted into the scala media in adjacent holes, one for voltage measurement (EP) and to inject ATP and the other for delivering current. Both the micropipettes were coupled to a Teflon-coated Ag–AgCl wire. Two Teflon-coated Ag–AgCl wires were also inserted in the cervical muscles to serve as ground references for the voltage measurement and current injection. The EP electrode was attached to an amplifier (Grass Instruments, USA), amplified 10× and digitised via a DigiData 1200 series interface (Axon Instruments, USA) and recorded on computer using pClamp software (v8.2, Axon Instruments). To ensure proper positioning of the current micropipette within the scala media, EP was monitored as the pipette was advanced, and once a stable value was obtained, the electrode was connected to the current source. Current was generated by a custom-made constant current stimulator using pClamp software (v8.2, Axon Instruments). Square current pulses (1 μA, 0.5-s duration at 1-s intervals) were continuously applied to the electrode positioned in the scala media and referenced to the ground electrode in neck muscle.
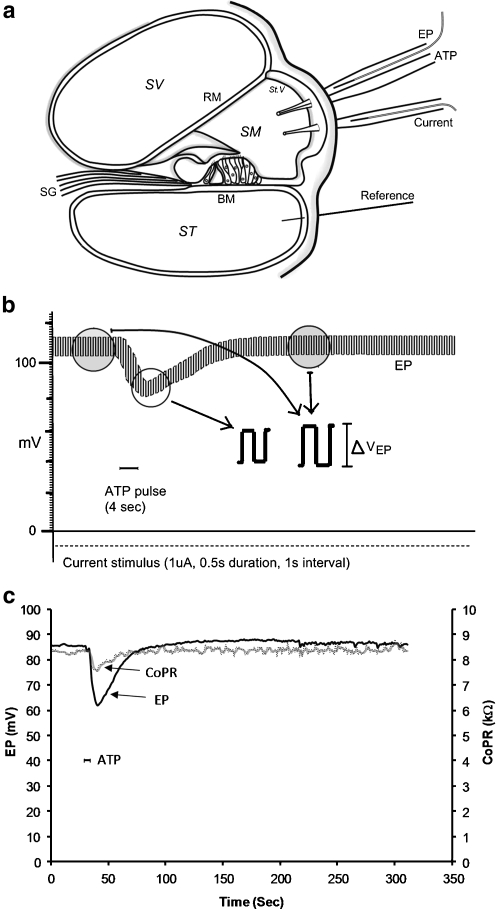
Fig. 1.
a Diagram showing the method for measuring the endocochlear potential (EP) and cochlear partition resistance (CoPR) from electrodes inserted through holes in the otic capsule into the scala media. b Trace of the EP showing a transient decline in EP with ATP microinjection (100 μM, 20 nL). Current pulses induced a change in voltage from which the CoPR was calculated. This voltage change was smaller during the introduction of ATP (reflecting decreased CoPR). c Changes in the CoPR and EP with time calculated for a single ATP microinjection (20 nL, 100 μM). SV scala vestibuli, ST scala tympani, SM scala media, RM Reissner’s membrane, St.V stria vascularis, BM basilar membrane, EP endocochlear potential SG spiral ganglion
ATP (100 or 1,000 μM) within different volumes of artificial endolymph (20–150 nl; composition in mM: K+ 154, Na+ 1, Cl− 142, Ca++ 1, Mg++ 0.5, HCO−3 12, glucose 4) was injected into the scala media using computer-controlled pressure pulses to produce declines in EP of different magnitudes as described previously [5]. Whilst current pulses were continuously delivered, ATP was injected into the scala media for 4 and 30 s to produce a steady-state reduction in EP (Fig. 1). The total amount of ATP applied varied across experiments between 20 × 10−13 and 150 × 10−13 mol. Similar volumes of artificial endolymph alone were injected into the scala media to act as controls for volume and pressure effects.
Current-induced voltage shifts in EP were measured before, during and after the ATP and control pulses (pClamp software) and used to calculate the CoPR (R = ΔVEP/I). A minimum of three CoPR values were averaged to provide each CoPR data point. The percentage changes in EP and CoPR were calculated relative to the initial EP measurement before the test or control pulses. Because insertion of the multiple electrodes into the scala media caused some tissue damage, which often resulted in lower EP values, only experiments with EP values ≥55 mV were accepted for analysis.
Immunohistochemistry
Mice were euthanised with sodium pentobarbital (90 mg/kg, i.p.) and were perfused endocardially with 4% paraformaldehyde (PFA) in a 0.1 M phosphate buffer (PB, pH 7.4). Both auditory bullae were extracted and opened and then the round and oval windows were punctured for perfusion of fixative. The cochleae were fixed overnight in 4% PFA and then decalcified in 5% ethylenediaminetetraacetic acid disodium salt solution for 7 days. After cryoprotection overnight in 30% sucrose, they were rinsed in 0.1 M buffer PB, snap-frozen in isopentane at −80°C and cryosectioned at 30 μm. Sections were washed three times in 0.1 M phosphate-buffered saline (PBS). They were then incubated in blocking and permeabilising solution for 1 h (PBS with 5% bovine serum albumin, 10% normal horse serum, 1% Triton X-100) and then incubated overnight in anti-P2X2 rabbit anti-rat antibody (Alomone; 0.3 μg/ml; 1:750 dilution). Following this, they were washed with 0.1 M PBS and incubated for 2 h in Alexa 488 donkey anti-rabbit secondary antibody (2 μg/ml, 1:200 dilution; Invitrogen) in antibody solution (10% normal horse serum, 0.1% Triton X-100 in 0.1 M PBS), washed with 0.1 M PBS and mounted on glass slides with mounting medium (Citifluor). Control sections omitted the primary antibody. Further controls were undertaken using the P2X2 receptor knockout mice which showed no specific signal. Sections were imaged using an SP2 laser scanning confocal microscope (Leica Leisertechnik, Heidelberg, Germany).
Sections were excited with a 488-nm wavelength argon–krypton laser and image acquisition (emission band-pass 530 nm) controlled by Scanware software (Leica). A series of six to ten optical sections were collected for each specimen at 2 μm, and image analysis was performed on an optical section from the centre of the stack. Immunostaining presented in figures is representative of two to four individual experiments. For semiquantitative analysis of receptor expression (see below), all sections were treated equally and incubated with primary and secondary antibodies for the same time. All sections were then examined using the same microscope settings
Analysis
Images were analysed using identical acquisition parameters, and immunolabelling was semi-quantified using ImageJ (v.1.38x, NIH, USA). Specific regions in the cochlea (the spiral limbus, organ of Corti, spiral ganglion) were selected as regions of interest (ROI), and the mean pixel intensity was obtained from the immunostaining intensity histograms. Mean pixel intensity of the background in the same image was deducted from the ROI value as background control. For each cochlea, up to six sections were analysed. The mean pixel intensities were averaged per animal, and the final intensity of the cell type of interest per experimental group was expressed as a mean ± SEM (n = 2–4 animals per group).
Statistical analysis was performed using a Student’s t test or ANOVA, and an α level of 0.05 was required for significance. Unless otherwise specified, results were expressed as the mean ± SE of the mean.
Results
Effect of age on auditory function (ABR) in CBA mice
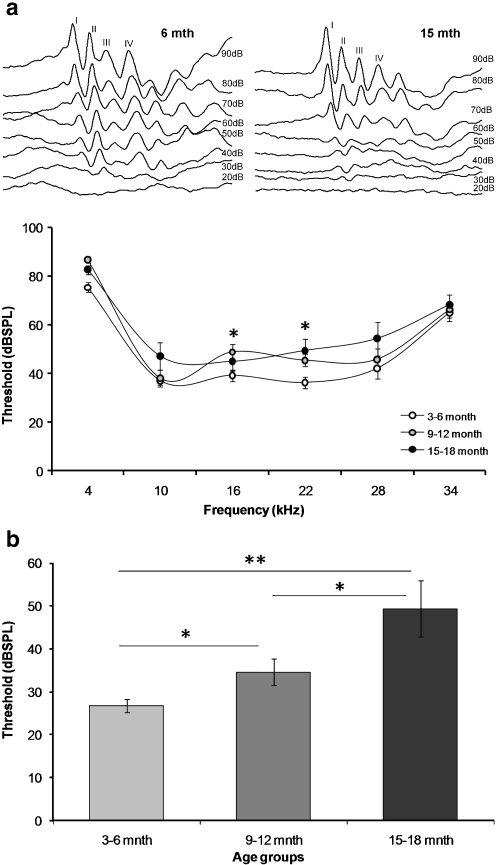
Reproducible ABR waveforms were recorded from mice of all ages, and there were no apparent differences in the waveform morphology between the groups (Fig. 2a). The baseline ABR thresholds (dBSPL) for the click stimulus were significantly greater (p < 0.05) in the middle and older age groups (Fig. 2b). The mean ABR thresholds to tones were also significantly greater in the older age group at frequencies 16 and 22 kHz (p < 0.05, Fig 2b).
Fig. 2.
Examples of the ABR waveforms in young and older mice (a) and the ABR thresholds (dBSPL) of CBA/CaJ mice at different ages in response to pure tones (frequency range 4–34 kHz) and auditory clicks (b). Data presented as mean ± SEM; *p < 0.05; **p < 0.01; Student’s t test, comparing against young mice (3–6 months)
Effect of noise on auditory function (ABR)
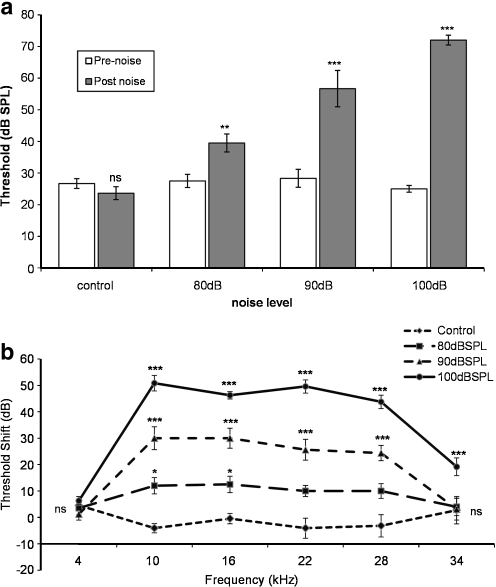
Auditory thresholds following noise exposure were measured in young animals only within 1 h after the cessation of noise exposure. Exposure to 4.5- to 9-kHz octave band noise for 48 h at 80 dBSPL had a small (5–10 dB) but significant (p < 0.01) effect on the ABR thresholds to clicks and pure tones (Fig. 3a, b). The effect was more profound with the higher level noise (90 and 100 dBSPL) exposures, which increased the average click thresholds by 25 and 45 dB (p < 0.001, Fig. 3a), respectively, and across the frequency range with significant threshold shifts at frequencies from 10 to 28 kHz (p < 0.05 and p < 0.001, Fig. 3b). These experiments showed that the range of noise exposures used subsequently in the biophysical analysis of cochlear partition regulation produced titrated threshold shifts.
Fig. 3.
ABR thresholds in response to click stimuli (a) and pure tones (b) in young mice after noise exposure (4.5–9 kHz; 80, 90 and 100 dB SPL, 48 h). Data presented as mean ± SEM; *p < 0.05; ***p < 0.001; Student’s t test
Changes in EP and CoPR with age and noise exposure
Young mice had a mean EP of 123.2 ± 1.5 mV (n = 15), which was significantly (8.6%) higher than older mice (12–15months; 112.7 ± 4.4 mV; n = 11, p < 0.05). The mean baseline CoPR recorded in young mice was also significantly higher (10.8%, p < 0.01) than older mice (7.52 ± 0.13 kΩ; n = 17 and 6.71 ± 0.26 kΩ; n = 11, respectively). Exposure to broadband noise at each of the levels (80, 90 and 100 dBSPL for 48 h) did not have any observable effect on either the EP or CoPR values within any age group, measured hours later.
Effect of ATP on EP and CoPR
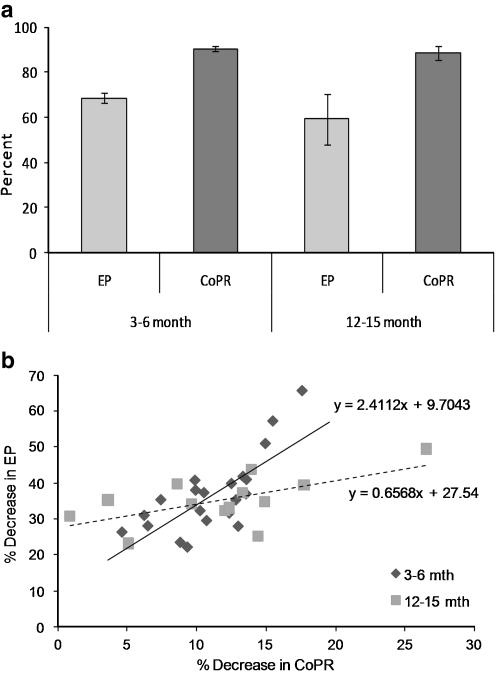
Microinjection of ATP into the scala media caused a fall in both the EP and CoPR of groups of young (3–6 months) and older (12–15 months) mice (Fig. 1c), which was not significantly different across the age groups (Fig. 4a). Both the EP and CoPR decreased sharply with the microinjection of ATP and then slowly recovered to baseline. As previously described for the guinea pig [5], these changes in EP and CoPR were blocked by the application of PPADS, a P2X2R antagonist, confirming that the ATP effect was P2X2R-mediated (pilot studies undertaken on C57/BL6 mice, Table 1). There was no significant change in the EP or CoPR with injection of equivalent volumes of artificial endolymph without ATP. The magnitude of the change in EP and CoPR was dose-dependent, and the change in EP was linearly correlated with the fall in CoPR (Fig. 4b). There was no significant difference in the change in the EP and CoPR in animals across age for the same amount of ATP. However, the slope of the relationship between EP and CoPR was steeper in younger than older animals (Fig. 4b), indicating that a small change in CoPR related to a larger change in EP for the younger animals.
Fig. 4.
Influence of exogenous ATP injected into the endolymph. a Change in EP (% maximum EP, mean ± SEM) and CoPR following injection of ATP (100 μM, 20 nL) into the scala media for young (3–6 months) and older (12–15 months) mice. b Relationship between the percentage change in the endocochlear potential (EP) and cochlear partition resistance (CoPR) in young (3–6 months, triangles) and older (15–18 months, squares) mice
Table 1.
Pre-infusion of scala media with the P2X antagonist PPADS (1,000 μM, 50 nL) prior to ATP (100 μM, 10 nL) caused a significant reduction in the magnitude of change in EP and CoPR
| No PPADS | PPADS | |||
|---|---|---|---|---|
| Before ATP | During ATP | Before ATP | During ATP | |
| EP | 118.8 ± 4.2 | 99.1 ± 4.2* | 110.7 ± 3.9 | 106.5 ± 4.5 |
| CoPR | 6.8 ± 0.2 | 6.1 ± 0.1* | 7.2 ± 0.3 | 6.8 ± 0.3 |
Data presented as mean ± SEM
*p < 0.01 (when compared between before and during ATP)
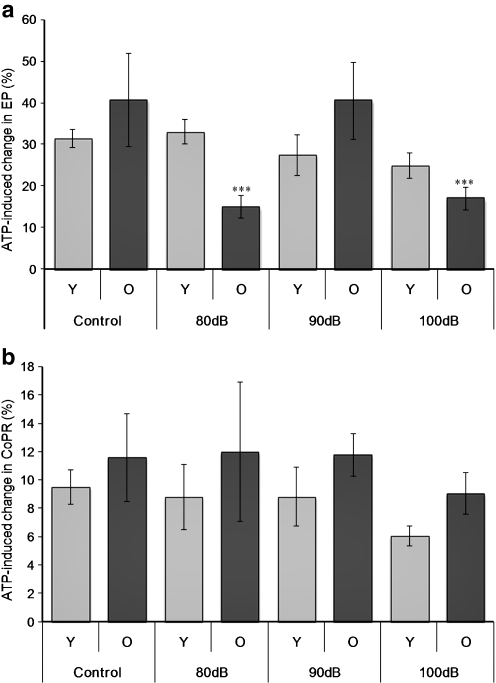
Noise exposure had different influences on the ATP-induced changes in EP, but no significant effect on the CoPR values across age. For the young animals, there was no significant change in the response to ATP following any level of noise exposure, although a declining response was observed with increasing level of noise exposure (Fig. 5a). In contrast, older animals showed a significant (p < 0.001) reduction in the magnitude of the EP response following both 80- and 100-dBSPL noise exposures, but not after 90 dBSPL. However, there was no correlation with changes in the CoPR (Fig. 5b).
Fig. 5.
Relationship between the percentage change in the endocochlear potential (EP) (a) and cochlear partition resistance (CoPR) (b) in young (Y, 3–6 months, light bars) and older (O, 12–15 months, dark bars) mice in response to different levels of noise exposure (4.5–9 kHz, 80, 90, 100 dBSPL). Mean ± SEM, ***p < 0.001
P2X2 receptor expression
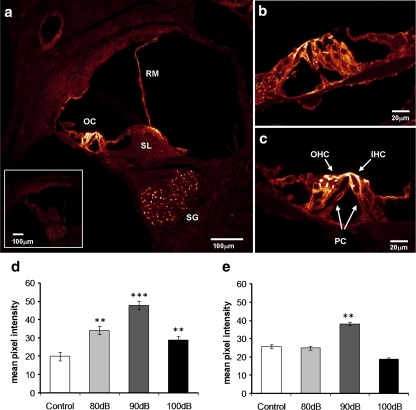
Immunohistochemistry of sections of the cochlea showed expression of P2X2 receptor subunits in the organ of Corti, spiral ganglion, spiral limbus region and Reissner’s membrane (Fig. 6a). Within the organ of Corti, expression was confined to the outer hair cells, predominantly the apical pole of the cells and the Deiters’ and pillar supporting cells (Fig. 6b). There was no immunostaining when the primary antibody was omitted (or in P2X2 knockout mice). Semi-quantitative analysis of P2X2R intensity of immunolabelling was undertaken in the organ of Corti, spiral ganglion and spiral limbus. We did not quantify the expression in Reissner’s membrane because of the variability in the orientation of this tissue in cross-sections. In the young mice, there was an increase in P2X2R expression in the spiral limbus and organ of Corti, but no change in the spiral ganglion after noise exposure. The mean staining intensity increased significantly (p < 0.01, p < 0.001) in the organ of Corti by 50–125%, which was greatest after the 90-dBSPL noise exposure (Fig. 6d). Older mice showed no increase in the P2X2R expression, except at 90-dBSPL exposure which was significant (p < 0.01) in the organ of Corti (Fig. 6e).
Fig. 6.
Immunolocalisation of P2X2R in the cochlea. a Low magnification image of a cross-section of the cochlea from a 100-dB noise-exposed mouse (15 months) showing greatest expression in the hair cells and supporting cells of the organ of Corti, the spiral limbus, Reissner’s membrane and the spiral ganglion cell bodies. The inset shows the absence of immunofluorescence in controls (primary antibody omitted). b, c Higher magnification images of the organ of Corti of young (4 months old) (b) and older (15 months old) (c) mice after exposure to narrow-band noise at 100 dBSPL. Noise exposure caused a significant increase in the P2X2R expression in the organ of Corti in young animals (3–6 months) (d), which was greatest after 90-dBSPL noise. The intensity of P2X2R immunostaining was increased in older animals (12–15 months) exposed to noise (E), but only after the 90-dBSPL exposure. Mean ± SEM, **p < 0.01, ***p < 0.001; one-way ANOVA followed by Student’s t test. SG spiral ganglion, SL spiral limbus, OC organ of Corti, IHC inner hair cells, OHC outer hair cells, PC pillar cells, RM Reissner’s membrane
Discussion
This study investigated the influence of age and noise on ATP-mediated effects on the endocochlear potential and cochlear compartment resistance as a surrogate measure of P2X2R-mediated influences on cochlear function in CBA/CaJ mice. Firstly, these data confirm that the mouse shows ATP-induced changes in EP which are P2X2R-mediated and correlated with changes in cochlear compartment resistance (CoPR) similar to the guinea pig [5] and rat [28]. The responsiveness of the control (non-noise-exposed) cochlea to exogenous ATP did not change with age. However, with increasing levels of noise exposure, there was an upregulation of P2X2R in the organ of Corti and spiral limbus in the young animals which was not apparent in the cochleae of aged animals, except for a small increase after 90-dBSPL exposure. In keeping with this, exogenous ATP had a much smaller influence on the EP of the aged noise-exposed cochlea than younger animals, whilst there was no difference in ATP-mediated reduction in CoPR with age. These data suggest that purinergic signalling pathways in the cochleae of younger animals are more responsive to noise compared to older animals, but this may arise from non-P2X2 receptor-mediated mechanisms. Overall the P2X2 receptor pathway, as measured by ΔCoPR, was robust across the ages.
The small increase in auditory thresholds as indicated by the ABR in the CBA/CaJ mice with age is in keeping with other studies, and this strain has been shown to maintain hearing well into advanced age [29]. There was a significant, albeit small, drop in the magnitude of EP in older mice, which has also been reported by Ohlemiller and Gagnon [30]. As EP provides a component of the driving force for hair cell transduction, EP magnitude and auditory threshold, as measured by sensitivity of individual spiral ganglion neurons, are intimately correlated [31]. It is possible that the decrease in EP contributes to the observed loss of auditory sensitivity with age, but an age-related physiological effect on sensory tissues and neural pathways cannot be excluded. The EP is reliant on the function of lateral wall tissues as well as the magnitude of the ion conductance in tissues lining the scala media. The EP is thought to be the result of the large positive potential generated in the stria vascularis [32]. A decrease in tissue-resistive pathways would be expected to cause a reduction in the EP [33]. Whilst it is not possible to dissect the specific reason for the change in EP with age, a decrease in CoPR would imply that a change in resistive pathways in the tissues lining the endolymphatic space is likely to contribute to the decrease.
Interestingly, exposure to high noise levels of 80, 90 or 100 dBSPL did not have any effect on the baseline EP and CoPR in both young and old mice when measured several hours after the exposure. This is also in keeping with some previous reports that indicate that there is a minimal, chronic change in EP after noise exposure. However, this is in contrast to the transient decline in EP that occurs during the exposure [34–36], which probably relates to the opening of the transduction channels and possibly an increase in P2X2R activation and decrease in compartment resistance (unpublished observations). However, these changes in EP are small and revert to baseline values within seconds or minutes after termination of the exposure. The more permanent effect of noise on EP has been studied here, and the data are somewhat equivocal. Many studies that have used mild to moderate noise exposures have not been able to record any decrease in EP immediately or soon after noise exposure [37–42]. In contrast, Ide and Morimitsu [43, 44] reported a significant drop in EP, which recovered to baseline after 5 days following exposure to intense sound (145-dB pure tones, 4 h) in guinea pig. Another study [45] demonstrated a decline in EP after 20–80 min of exposure to 125-dB white noise. Recently, Ohlemiller [46] reported that the effect of noise on EP in the mouse is strain-specific, which could indicate that the effect of noise on the EP generating mechanism may well be more variable and strain-specific than had been first thought. Notably, all animal models presently known to undergo an age-associated reduction in EP feature an environmental or stochastic component such that at most, half of the subjects exhibit an abnormally low EP at advanced ages. Examples of this include the CBA/CaJ mouse strain used in this study, suggesting considerable variability in individual responses within a strain. A dose-dependent decline in the EP with exogenous ATP in the scala media of the mouse confirms previous findings in guinea pig [5] and rat [47], implying that purinergic regulation of the EP is a generalised phenomenon in rodents and probably other mammals. This is now well established to be the result of activation of an ATP-mediated K+ shunt conductance through tissues of the cochlear partition (tissues lining the endolymphatic space) and which is confirmed by the decrease in compartment resistance (CoPR) associated with the opening of P2X2 channels. ATP-gated ion channels composed of P2X2 subunits have been identified in the epithelial cells of Reissner’s membrane [17], which separates the endolymph of the scala media from the perilymph of the scala vestibuli, the stereocilia and surface of the hair cells, and the surface of supporting cells of the organ of Corti [48]. Recovery of the response to exogenous ATP is due to the rapid metabolism of ATP by ectonucleotidases (NTPDases) [49–51]. The change in CoPR relative to the change in EP was smaller in mice compared with that observed in guinea pigs [5].
ATP activation of this shunt conductance is proposed to decrease sound transduction during high sound exposure by reducing EP as well as a direct depolarisation of hair cells [48]. As a consequence, there is a decrease in the hair cell sensitivity and expansion of the hair cell dynamic range, which may lead to sensory adaptation during loud sound exposure as well as protection from noise-induced injury [4, 5, 48]. Expression of P2X2R increases with chronic noise exposure in the rat [19], supporting the view that the ATP-mediated shunt conductance contributes to the adaptation of the cochlea to loud sound or its protection to sound injury. Although caution must be applied to the accuracy of semiquantitative immunohistochemistry analysis, the present study using similar techniques to our previous studies demonstrates that this increase in expression also occurs in the young mouse, which has not been observed previously. However, the increase in expression was substantially muted in older mice across all noise levels. This substantially reduced expression of P2X2R was also mirrored by a substantially reduced change in EP in response to exogenous ATP at 80 and 100 dBSPL. However, given that there was no difference in CoPR response to ATP injection into the scala media with age, the decline in EP response to ATP in noise-exposed older mice may arise from changes in other elements of the purinergic signalling complex in the cochlea.
Although there was considerable variability in the responses to ATP across age and with noise exposure among animals in this study, our data suggest that purinergic signalling is enhanced by noise in young animals more than in older animals. Furthermore, this is more significant at lower sound levels than at high levels. The 80- and 90-dB levels were selected to straddle the exposures that produce minimal to temporary changes in hearing sensitivity, whereas the 100-dBSPL exposure should induce some permanent tissue injury. The increase in expression of purinergic signalling with the lower levels of noise may reflect a physiological and protective adaptation which may be suppressed with tissue injury that would occur with the higher level exposures. A similar exposure dependence occurs with both NTPDase [50] and P2X7 (unpublished observation) upregulation. The fact that P2X2R upregulation with noise exposure is very much reduced in older animals suggests an age-dependent pattern of purinergic response to noise stress. The implication of this difference in the purinergic responses between young and old animals is not clear. If the ATP-mediated process contributes to the susceptibility and adaptability of the cochlea to noise, then it could be speculated that older animals would be more susceptible and less adaptable in noise. This lack of adaptation could contribute to the age-related cochlear injury and hearing loss by making the cochlea “less resistant” to noise exposure.
In summary, this study has demonstrated that ATP signalling in the mouse cochlea is less consistent and robust in the ageing mouse, and this is associated with a reduced upregulation of P2X2 receptors with noise. Such findings imply that the ageing cochlea may be less adaptive to noise, which could enhance its susceptibility to injury from noise exposure.
The findings prompt a broader consideration of the contribution of related components of the purinergic signalling complex to hearing physiology as the cochlea ages.
Acknowledgements
This research was supported by grants from the Deafness Research Foundation and Auckland Medical Research Foundation.
Abbreviations
- ABR
Auditory brainstem response
- ATP
Adenosine 5′-triphosphate
- CoPR
Cochlear partition resistance
- dBSPL
Decibels sound pressure level
- EP
Endocochlear potential
- PPADS
Pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate
- PTS
Permanent threshold shift
- TTS
Temporary threshold shift
Footnotes
Ravindra S. Telang and Vinthiya Paramananthasivam contributed equally to the work.
References
- 1.Housley GD, Thorne PR. Purinergic signalling: an experimental perspective. J Auton Nerv Syst. 2000;81:139–145. doi: 10.1016/S0165-1838(00)00116-8. [DOI] [PubMed] [Google Scholar]
- 2.Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Jarlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Munoz DJ, Thorne PR. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neuro-otol. 2002;7:55–61. doi: 10.1159/000046865. [DOI] [PubMed] [Google Scholar]
- 3.Thorne PR, Munoz DJ, Nikolic P, Mander L, Jagger DJ, Greenwood D, Vlajkovic S, Housley GD. Potential role of purinergic signalling in cochlear pathology. Audiol Neuro-otol. 2002;7:180–184. doi: 10.1159/000058307. [DOI] [PubMed] [Google Scholar]
- 4.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32:128–141. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Thorne PR, Munoz DJ, Housley GD. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the guinea pig. J Assoc Res Otolaryngol. 2004;5:58–65. doi: 10.1007/s10162-003-4003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sueta T, Paki B, Everett AW, Robertson D. Purinergic receptors in auditory neurotransmission. Hear Res. 2003;183:97–108. doi: 10.1016/S0378-5955(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 7.Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 9.Munoz DJB, McFie C, Thorne PR. Modulation of cochlear blood flow by extracellular purines. Hear Res. 1999;127:55–61. doi: 10.1016/S0378-5955(98)00161-0. [DOI] [PubMed] [Google Scholar]
- 10.Bobbin RP. ATP-induced movement of the stalks of isolated cochlear Deiters’ cells. NeuroReport. 2001;12:2923–2926. doi: 10.1097/00001756-200109170-00034. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Bobbin RP. P2X receptors in cochlear Deiters’ cells. Br J Pharmacol. 1998;124:337–344. doi: 10.1038/sj.bjp.0701848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skellett RA, Chen C, Fallon M, Nenov AP, Bobbin RP. Pharmacological evidence that endogenous ATP modulates cochlear mechanics. Hear Res. 1997;111:42–54. doi: 10.1016/S0378-5955(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood D, Jagger DJ, Huang LC, Hoya N, Thorne PR, Wildman SS, King BF, Pak K, Ryan AF, Housley GD. P2X receptor signaling inhibits BDNF-mediated spiral ganglion neuron development in the neonatal rat cochlea. Development. 2007;134:1407–1417. doi: 10.1242/dev.002279. [DOI] [PubMed] [Google Scholar]
- 14.Huang LC, Greenwood D, Thorne PR, Housley GD. Developmental regulation of neuron-specific P2X3 receptor expression in the rat cochlea. J Comp Neurol. 2005;484:133–143. doi: 10.1002/cne.20442. [DOI] [PubMed] [Google Scholar]
- 15.Huang LC, Ryan AF, Cockayne DA, Housley GD. Developmentally regulated expression of the P2X3 receptor in the mouse cochlea. Histochem Cell Biol. 2006;125:681–692. doi: 10.1007/s00418-005-0119-4. [DOI] [PubMed] [Google Scholar]
- 16.Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium. 2007;41:77–86. doi: 10.1016/j.ceca.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.King M, Housley GD, Raybould NP, Greenwood D, Salih SG. Expression of ATP-gated ion channels by Reissner’s membrane epithelial cells. NeuroReport. 1998;9:2467–2474. doi: 10.1097/00001756-199808030-00008. [DOI] [PubMed] [Google Scholar]
- 18.Sage CL, Marcus DC. Immunolocalization of P2Y4 and P2Y2 purinergic receptors in strial marginal cells and vestibular dark cells. J Membr Biol. 2002;185:103–115. doi: 10.1007/s00232-001-0116-z. [DOI] [PubMed] [Google Scholar]
- 19.Wang JC, Raybould NP, Luo L, Ryan AF, Cannell MB, Thorne PR, Housley GD. Noise induces up-regulation of P2X2 receptor subunit of ATP-gated ion channels in the rat cochlea. NeuroReport. 2003;14:817–823. doi: 10.1097/00001756-200305060-00008. [DOI] [PubMed] [Google Scholar]
- 20.Housley GD, Thorne PR, Vlajkovic SM, Morton-Jones RT, Khakh BS, Cockayne DA, Ryan AF (2008) ATP-mediated humoral inhibition of sound transduction supplants neural efferent inhibition at high sound levels as the mechanism for expanding the dynamic range of hearing. In: Santi PA (ed) 31st Annual Mid Winter Research Meeting of the Association for Research in Otolaryngology, vol. 31, Phoenix, Arizona, USA, 600, pp 204–205
- 21.Afework M, Burnstock G. Localization of P2X receptors in the guinea pig adrenal gland. Cells Tissues Organs. 2000;167:297–302. doi: 10.1159/000016793. [DOI] [PubMed] [Google Scholar]
- 22.Afework M, Burnstock G. Age-related changes in the localization of P2X (nucleotide) receptors in the rat adrenal gland. Int J Dev Neurosci. 2000;18:515–520. doi: 10.1016/S0736-5748(00)00023-X. [DOI] [PubMed] [Google Scholar]
- 23.Koga T, Takata Y, Kobayashi K, Fujii K, Nagao T, Fujishima M. Age-related changes in P2-purinergic receptors on vascular smooth muscle and endothelium. Hypertension. 1992;19:286–289. doi: 10.1161/01.hyp.19.3.286. [DOI] [PubMed] [Google Scholar]
- 24.Konishi C, Naito Y, Ohara N. Age-related changes in adenosine 5′-triphosphate-induced constriction of isolated, perfused mesenteric arteries of rats. Life Sci. 1999;64:1265–1273. doi: 10.1016/S0024-3205(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 25.Krishnaraj R. Negative modulation of human NK cell activity by purinoceptors. 1. Effect of exogenous adenosine triphosphate. Cell Immunol. 1992;141:306–322. doi: 10.1016/0008-8749(92)90150-N. [DOI] [PubMed] [Google Scholar]
- 26.Krishnaraj R. Negative modulation of human NK cell activity by purinoceptors. 2. Age-associated, gender-specific partial loss of sensitivity to ATP. Cell Immunol. 1992;144:11–21. doi: 10.1016/0008-8749(92)90221-A. [DOI] [PubMed] [Google Scholar]
- 27.Ragazzi E, Chinellato A, Pandolfo L, Froldi G, Caparrotta L, Aliev G, Prosdocimi M, Fassina G. Endothelial nucleotide-mediated aorta relaxation in aged Watanabe heritable hyperlipidemic rabbits. J Cardiovasc Pharmacol. 1995;26:119–126. doi: 10.1097/00005344-199507000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Thorne PR, Chung M, Muñoz DJB, Wit HP, Housley GD (2006) Regulation of the endocochlear potential by ATP. 43rd Inner Ear Biology Workshop, Montpellier, France, p 53
- 29.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/S0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohlemiller KK, Gagnon PM. Genetic dependence of cochlear cells and structures injured by noise. Hear Res. 2007;224:34–50. doi: 10.1016/j.heares.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sewell WF. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear Res. 1984;14:305–314. doi: 10.1016/0378-5955(84)90057-1. [DOI] [PubMed] [Google Scholar]
- 32.Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol. 2002;282:C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- 33.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/S0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 34.Salt AN, Konishi T. Effects of noise on cochlear potentials and endolymph potassium concentration recorded with potassium-selective electrodes. Hear Res. 1979;1:343–363. doi: 10.1016/0378-5955(79)90005-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Li Q, Dong W, Chen J. Effects of various noise exposures on endocochlear potentials correlated with cochlear gross responses. Hear Res. 1992;59:31–38. doi: 10.1016/0378-5955(92)90099-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang L. Effects of furosemide on endocochlear potentials, auditory action potentials and summating potentials and the changes of inner ear pathology. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1992;27(70–2):124. [PubMed] [Google Scholar]
- 37.Benitez LD, Eldredge DH, Templer JW. Temporary threshold shifts in chinchilla: electrophysiological correlates. J Acoust Soc Am. 1972;52:1115–1123. doi: 10.1121/1.1913222. [DOI] [PubMed] [Google Scholar]
- 38.Boettcher FA, Gratton MA, Schmiedt RA. Effects of noise and age on the auditory system. Occup Med. 1995;10:577–591. [PubMed] [Google Scholar]
- 39.Boettcher FA, Mills JH, Dubno JR, Schmiedt RA. Masking of auditory brainstem responses in young and aged gerbils. Hear Res. 1995;89:1–13. doi: 10.1016/0378-5955(95)00116-X. [DOI] [PubMed] [Google Scholar]
- 40.Boettcher FA, Schmiedt RA. Distortion-product otoacoustic emissions in Mongolian gerbils with resistance to noise-induced hearing loss. J Acoust Soc Am. 1995;98:3215–3222. doi: 10.1121/1.413811. [DOI] [PubMed] [Google Scholar]
- 41.Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma YL, Gerhardt KJ, Curtis LM, Rybak LP, Whitworth C, Rarey KE. Combined effects of adrenalectomy and noise exposure on compound action potentials, endocochlear potentials and endolymphatic potassium concentrations. Hear Res. 1995;91:79–86. doi: 10.1016/0378-5955(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 43.Ide M, Morimitsu T. Long term effects of intense sound on endocochlear DC potential. Auris Nasus Larynx. 1990;17:1–10. doi: 10.1016/s0385-8146(12)80014-9. [DOI] [PubMed] [Google Scholar]
- 44.Ide M, Morimitsu T. Long-term effects of intense sound on hair cells of Corti’s organ and endocochlear DC potential. Auris Nasus Larynx. 1990;17:61–67. doi: 10.1016/s0385-8146(12)80187-8. [DOI] [PubMed] [Google Scholar]
- 45.Wang JA, Dong WJ, Chen JS. Changes in endocochlear potential during anoxia after intense noise exposure. Hear Res. 1990;44:143–149. doi: 10.1016/0378-5955(90)90076-2. [DOI] [PubMed] [Google Scholar]
- 46.Ohlemiller KK. Recent findings and emerging questions in cochlear noise injury. Hear Res. 2008;245:5–17. doi: 10.1016/j.heares.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorne PR, Chung M, Munoz DJB, Wit H, Telang RS, Paramananthasivam V, Vlajkovic SM, Housley GD (2007) Regulation of the endocochlear potential by ATP in rodents: species differences. 7th IBRO World Congress of Neuroscience, Melbourne, Australia
- 48.Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VCM, Cannell MB, Soeller C, Christie DL, S-i U, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlajkovic SM, Thorne PR, Housley GD, Munoz DJ, Kendrick IS. Ecto-nucleotidases terminate purinergic signalling in the cochlear endolymphatic compartment. NeuroReport. 1998;9:1559–1565. [PubMed] [Google Scholar]
- 50.O’Keeffe MG, Thorne PR, Housley GD, Robson SC, Vlajkovic SM (2010) Distribution of NTPDase5 and NTPDase6 and the regulation of P2Y receptor signalling in the rat cochlea. Purinergic Signaling. doi:10.1007/s11302-010-9190-y [DOI] [PMC free article] [PubMed]
- 51.Vlajkovic SM, Housley GD, Munoz DJ, Robson SC, Sevigny J, Wang CJ, Thorne PR. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 2004;126:763–773. doi: 10.1016/j.neuroscience.2004.04.023. [DOI] [PubMed] [Google Scholar]