Abstract
Purinergic signaling has broad physiological significance to the hearing organ, involving signal transduction via ionotropic P2X receptors and metabotropic G-protein-coupled P2Y and P1 (adenosine), alongside conversion of nucleotides and nucleosides by ecto-nucleotidases and ecto-nucleoside diphosphokinase. In addition, ATP release is modulated by acoustic overstimulation or stress and involves feedback regulation. Many of these principal elements of the purinergic signaling complex have been well characterized in the cochlea, while the characterization of P2Y receptor expression is emerging. The present study used immunohistochemistry to evaluate the expression of five P2Y receptors, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12, during development of the rat cochlea. Commencing in the late embryonic period, the P2Y receptors studied were found in the cells lining the cochlear partition, associated with establishment of the electrochemical environment which provides the driving force for sound transduction. In addition, early postnatal P2Y2 and P2Y4 protein expression in the greater epithelial ridge, part of the developing hearing organ, supports the view that initiation and regulation of spontaneous activity in the hair cells prior to hearing onset is mediated by purinergic signaling. Sub-cellular compartmentalization of P2Y receptor expression in sensory hair cells, and diversity of receptor expression in the spiral ganglion neurons and their satellite cells, indicates roles for P2Y receptor-mediated Ca2+-signaling in sound transduction and auditory neuron excitability. Overall, the dynamics of P2Y receptor expression during development of the cochlea complement the other elements of the purinergic signaling complex and reinforce the significance of extracellular nucleotide and nucleoside signaling to hearing.
Keywords: Purinergic signaling, Cochlear ontogeny, ATP, Spiral ganglion, Hair cells
Introduction
Multiple roles have been proposed for purinergic signaling in the cochlea, including establishment of synaptic transmission during cochlear ontogeny, signaling of noise-damage in supporting cells in the organ of Corti and regulation of electrochemical homeostasis underlying sound transduction [1]. Just prior to the onset of hearing, neonatal rat spiral ganglion neurons exhibit spontaneous activity, which establishes synaptic transmission with the central auditory pathways in the brain. A purinergic signaling mechanism underlies this process which involves release of ATP from epithelial cells within the transient Kölliker's organ (greater epithelial ridge), part of the developing auditory sensory organ. Extracellular ATP activates P2 receptors on the inner hair cells causing depolarization and glutamatergic neurotransmission to the spiral ganglion neurons [2]. Both P2X receptors (ATP-gated ion channels and P2Y (G-protein-coupled) receptors are implicated. ATP release has also been observed in the supporting cells in and adjacent to the organ of Corti [3–5] where the mechanism involves P2Y and P2X receptor signaling that could be induced by hair cell damage. Connexin and pannexin hemichannels are implicated in the ATP release mechanism [6–8]. The stria vascularis in the lateral wall of the cochlea is the source of the K+ flux into scala media that establishes the driving force for sound transduction (the positive endocochlear potential). This tissue is also a source of vesiculated ATP in the marginal cells which is likely to contribute to the elevation of ATP within this cochlear fluid compartment during noise stress [9, 10]. ATP release would activate P2Y receptors on the marginal cells, resulting in reduction in the K+ conductance of these cells (paracrine action) [11]. Along with activation of P2X receptors, this mechanism contributes to a reduction in endocochlear potential [12].
Purinergic signaling in the cochlea involving the P2Y and P2X receptors is regulated by ecto-enzymes, including the ecto-nucleotidases (ecto-nucleoside triphosphate diphosphohydrolases; NTPDase), and ecto-5′-nucleotidase, which dephosphorylate extracellular nucleotides such as ATP and UTP through a cascade of di- and mono-phosphate nucleosides [13]. In the case of hydrolysis of ATP to adenosine, this enables activation of adenosine (P1) receptors. This process also terminates P2X receptor activation and promotes selective activation of P2Y receptors, which have broader sensitivity to purines and pyrimidines (ATP, ADP, AMP, UTP, UDP, and di-adenosine polyphosphates). Eight mammalian P2Y receptors have been identified, and in the case of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11, they directly mediate Ca2+ signaling via the 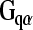 -phospholipase
-phospholipase 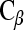 (PLC)-inositol trisphosphate (IP3)-gated Ca2+ store pathway. P2Y12, P2Y13, and P2Y14 receptor signal transduction utilizes
(PLC)-inositol trisphosphate (IP3)-gated Ca2+ store pathway. P2Y12, P2Y13, and P2Y14 receptor signal transduction utilizes 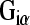 -mediated inhibition of adenylate cyclase and hence reduction in cAMP (reviewed by [14, 15]). Endogenous P2Y receptor signaling may also engage additional pathways, including stimulation of adenylate cyclase (P2Y11), and activation of phospholipase D, phospholipase A2, MEK/MAP kinase, and Rho-dependent kinase (for ERK signaling) [15]. The P2Y–PLC pathway may also mediate Ca2+ entry through transient receptor potential (TRP) channels, gated by membrane-bound diacylglycerol (DAG) [16, 17]. In broad terms, P2Y1 and P2Y11 are ATP preferring [14]; P2Y12 and P2Y13 are ADP preferring [14]; rat P2Y2 and P2Y4 orthologs are equally activated by ATP and UTP [18] and P2Y6 is preferentially activated by UDP, with ADP > ATP [19]; P2Y14 is activated most potently by UDP-glucose [14]. However, the functional significance of the agonist preferences of the P2Y receptors needs to be matched to the endogenous ligand environment. While there is clear evidence for ATP, UTP and UDP-glucose release mechanisms from a variety of stimulated cells (see [20] for a review), determination of the adenine nucleotides and uracil nucleotide species available to the P2Y receptor ligand binding sites is problematic, due to the action of the hydrolyzing ecto-enzymes and ecto-nucleoside diphosphokinase [20], which interconvert the nucleotides. Characterization of endogenous extracellular nucleotide and nucleoside profiles in the cochlea is limited to the detection of ATP release, including evidence that this arises from acoustic overstimulation [10, 21]. Thus, the elements of nucleotide release, hydrolysis, and uptake, provide a diverse ligand profile that acts via the P2X, P2Y, and P1 receptors, as a purinergic signaling complex. A comprehensive analysis of all the elements of such a purinergic signaling complex in the cochlea is therefore necessary.
-mediated inhibition of adenylate cyclase and hence reduction in cAMP (reviewed by [14, 15]). Endogenous P2Y receptor signaling may also engage additional pathways, including stimulation of adenylate cyclase (P2Y11), and activation of phospholipase D, phospholipase A2, MEK/MAP kinase, and Rho-dependent kinase (for ERK signaling) [15]. The P2Y–PLC pathway may also mediate Ca2+ entry through transient receptor potential (TRP) channels, gated by membrane-bound diacylglycerol (DAG) [16, 17]. In broad terms, P2Y1 and P2Y11 are ATP preferring [14]; P2Y12 and P2Y13 are ADP preferring [14]; rat P2Y2 and P2Y4 orthologs are equally activated by ATP and UTP [18] and P2Y6 is preferentially activated by UDP, with ADP > ATP [19]; P2Y14 is activated most potently by UDP-glucose [14]. However, the functional significance of the agonist preferences of the P2Y receptors needs to be matched to the endogenous ligand environment. While there is clear evidence for ATP, UTP and UDP-glucose release mechanisms from a variety of stimulated cells (see [20] for a review), determination of the adenine nucleotides and uracil nucleotide species available to the P2Y receptor ligand binding sites is problematic, due to the action of the hydrolyzing ecto-enzymes and ecto-nucleoside diphosphokinase [20], which interconvert the nucleotides. Characterization of endogenous extracellular nucleotide and nucleoside profiles in the cochlea is limited to the detection of ATP release, including evidence that this arises from acoustic overstimulation [10, 21]. Thus, the elements of nucleotide release, hydrolysis, and uptake, provide a diverse ligand profile that acts via the P2X, P2Y, and P1 receptors, as a purinergic signaling complex. A comprehensive analysis of all the elements of such a purinergic signaling complex in the cochlea is therefore necessary.
While there has been an extensive analysis of P2X receptor expression both in the developing and mature cochlea across several species (for reviews, see [1, 22]), and adenosine receptor and ecto-nucleotidase activity has been established [23–25]. The sites and mechanisms of action of P2Y receptor activity in the cochlea are still emerging. The present study characterizes the expression profile of P2Y receptors exhibiting varied agonist preference during development of the rat cochlea. The functional implications of the findings are considered with respect to the other elements of the purinergic signaling complex.
Materials and methods
Animals
All procedures in this study were approved by the University of Auckland Animal Ethics Committee. Cochlear tissues were obtained from embryonic day 16 and 18 (E16, E18), postnatal (P0–P28) and adult (P49–P56) Wistar rats after the animals were killed with pentabarbitone sodium (120 mg/kg; Nembutal, Virbac Laboratories Ltd. New Zealand) by intraperitoneal injection.
RT-PCR detection of P2Y12 receptor mRNA transcript
This study undertook to localize the expression of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 receptors in the developing rat cochlea utilizing specific antisera for these subtypes. With the exception of P2Y12, expression of all these receptors has previously been confirmed in rat cochlear tissues by RT-PCR [3, 26]. We complemented this in the present study by confirming P2Y12 transcript expression by RT-PCR alongside immunolocalisation of the five P2Y proteins. Total RNA was isolated from whole cochlea, as well as dissected spiral ganglion (P0, P12, and adult), using Trizol™ (Gibco/Invitrogen). First-strand cDNA synthesis was performed using oligo-d(T) primers and Moloney Murine Leukemia Virus (MMLV)-reverse transcriptase. P2Y12-specific primers (GenBank accession no. AF313450; sense 214 5′-CAGGTTCTCTTCCCATTGCT-3′; antisense 871 5′-CAGCAATGATGATGAAAACC-3′) produced the predicted amplicon of 658 bp (after [27]) when PCR was undertaken with Taq DNA polymerase using the following thermal cycler profile: 96°C denaturation, then 35 cycles of 94°C 1 min; 50°C 1 min; 72°C 1 min; extension at 72°C for 7 min.
Antibody specificity
All P2Y receptor antisera had previously demonstrated target epitope specificity by Western Blotting. The polyclonal P2Y1 receptor antiserum (a gift from Dr. Darren Moore, Babraham Institute, Cambridge, UK) was raised in rabbit against a synthetic peptide from the C-terminus of the human P2Y1 protein (363aa–373aa; CPEFKQNGDTSL). There is 10/11aa homology with the corresponding rat P2Y1 sequence. The antiserum has previously been shown by Western Blot to specifically recognize the rat P2Y1 epitope (63-kDa band) and the recombinant hP2Y1 protein [28]. P2Y2, P2Y4, P2Y6, and P2Y12 antisera were obtained from Alomone Labs (Israel), who provided Western Blot data of their specificity. In addition, the P2Y4 antiserum has been used to immunolocalise P2Y4 expression in the cochlear lateral wall tissue of the adult Sprague–Dawley rat and gerbil [29]. In the present study, antibody specificity was confirmed by pre-adsorption of the P2Y receptor antibodies with their respective target peptides, with the exception of P2Y1, where we complemented previous analysis of antibody specificity by undertaking Western Blotting of cochlear tissue. The target peptide pre-adsorption controls utilized peptide antigen at a ratio of 1:1 or 1:2. The target peptide was pre-incubated with respective primary antibody for 4 h at 4°C before being applied to cochlear sections. Controls using omission of the primary antibodies assessed non-specific secondary antibody binding. These controls, along with the temporal patterning of cell specific labeling which was obtained using optimized antisera incubation conditions validates the specificity of the P2Y receptor immunolabeling.
For Western Blotting using the P2Y1 antiserum, adult rat cochleae were isolated from the temporal bone, decapsulated, and homogenized in ice-cold non-reducing buffer (96 mM Tris–base, 20% (v/v) glycerol, 1% (w/v) sodium dodecyl sulfate, pH 6.8). Homogenates were sonicated for 5 min and heated (95–100°C) for 5 min. Supernatants were then collected, after the sample was centrifuged for 2 min. For immunoblot analysis, cochlear protein was separated using a polyacrylamide Tris–HCl gel (10% resolving gel and 4% stacking gel; BioRad, CA, USA) and electrophoretically transferred onto a PVDF membrane (Roche, Mannheim, Germany). The membranes were blocked with 5% (w/v) non-fat milk powder and 5% (v/v) normal goat serum (NGS; Vector Laboratories, Inc., Burlingame, CA) in Tris-buffered saline (TBS; 20 mM Tris–base, 137 mM NaCl, 0.1% Tween 20, pH 7.6) at room temperature for 2 h and then incubated in P2Y1 antibody (1:1,000), diluted in 2% (w/v) non-fat milk powder and 5% (w/w) NGS in TBS for 2 h. The protein bands were visualized by application of horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:8,000) and detected by chemiluminescence (ECL® Western blotting analysis system; Amersham Biosciences). An internal standard protein ladder was used to confirm the predicted 63 kDa size of the P2Y1 antisera-labeled band (Fig. 4k).
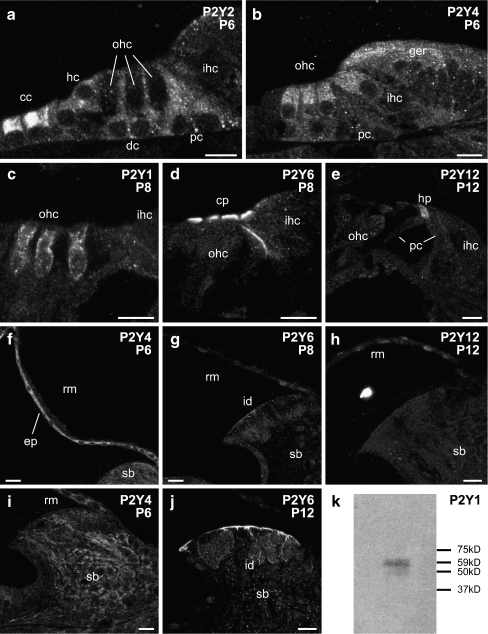
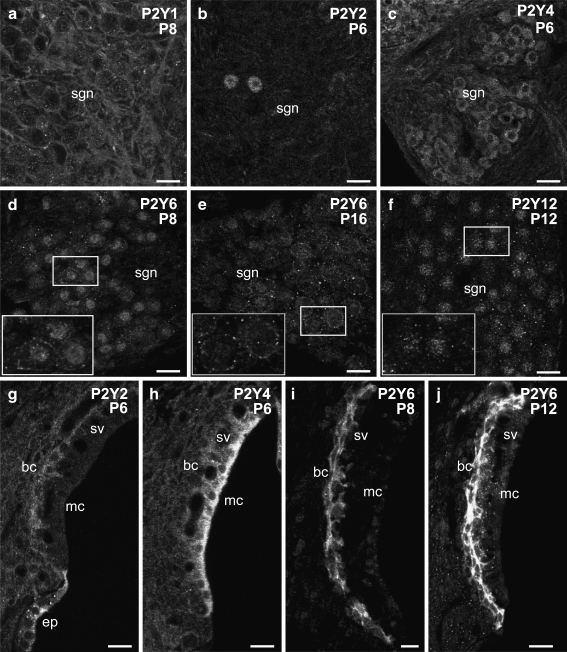
Fig. 4.
Detail of P2Y receptor expression in the rat cochlea between P6 and P12, and western blotting for P2Y1 antiserum in the adult cochlea. a P2Y2 immunolabeling in the organ of Corti at P6 shows that P2Y2 receptor was localized in the supporting cells, including pillar cells, Deiter's cells, Hensen cells, and Claudius cells. b P2Y4 receptor expression in the organ of Corti at P6 was found in the inner and outer hair cells (complementing P2Y2 protein expression) and in the greater epithelial ridge. c P2Y1 labeling was detected on the basolateral wall of outer hair cells at P8. d P2Y6 expression in the organ of Corti was constrained to the cuticular plates of the inner and outer hair cells at P8. e P2Y12 receptor immunolabeling in the organ of Corti was confined to the head plates of the pillar cells. f P2Y4 protein expression in the Reissner's membrane epithelial and mesenchymal cells. g Weak P2Y6 expression was detected in the Reissner's membrane epithelial cells and interdental cells of the spiral limbus. h Dispersed P2Y12 immunolabeling in the epithelial cells and mesenchymal cells of Reissner's membrane, with no signal detected in the spiral limbus. i P2Y4 protein expression was observed in the epithelial cells of the Reissner's membrane and the fibrocytes of the spiral limbus. j Strong P2Y6 labeling was localized in the interdental cells. k Western Blot of P2Y1 immunoblotting of cochlear tissue. Single band detected at size of 63 kDa confirms specificity. Scale bar 20 µm. cc Claudius cell, dc Dieter's cell, ep epithelial cells, ger greater epithelial ridge, hc Hensen cells, hp head plate, id interdental cells, ihc inner hair cells, ohc outer hair cells, pc pillar cells, rm Reissner's membrane, sb spiral limbus
Immunohistochemistry
Cochleae were fixed by transcardial perfusion with normal saline containing heparin (1 mg/ml NaNO2, 0.9% NaCl and 0.02% heparin) and 4% paraformaldehyde (Scharlau Chemie S.A., Spain) in 0.1 M phosphate buffer (pH 7.4). The cochleae were then dissected from the temporal bones and perfused with fixative via the round and oval windows for post-fixing overnight at 4°C. The late postnatal (P20–P28) and adult cochlear tissues, but not the embryonic/early neonatal cochlear tissues, were decapsulated to enable cyrosectioning without decalcification. Decapsulation was achieved by carefully shaving away the bone with a scalpel blade to expose the spiral ligament. This has the advantage of reducing processing time and keeping the tissue treatment comparable across all ages. The tissues were cryoprotected by 10% sucrose for 4 h and then 30% sucrose overnight. After incubation in 30% sucrose and Tissue-Tek optimal cutting temperature compound (O.C.T.; Miles Inc., Diagnostics Division, Elkhart, IN, USA; 1:1) for 1 h, the cochlear tissues were mounted in O.C.T. using dry ice. The tissues were either stored at −80°C, or used immediately for cryosectioning. Cochlear tissues were sectioned using a cryostat microtome (Cryocut 1800, Reichert-Jung, Germany) with a disposable blade (Feather Co. Ltd., Japan) at 50 µm thickness, collected into 0.1 M phosphate-buffered saline (PBS).
All sections were incubated in a blocking/permeabilising solution at 4°C for 2 h (10% NGS and 1% Triton X-100 in 0.1 M PBS) and then incubated at 4°C overnight in the target polyclonal rabbit antibody: P2Y1 antiserum (1:2–4 k dilution); P2Y2 antiserum (3 µg/ml; Alomone Labs, Israel), P2Y4 antiserum (0.6 µg/ml; Alomone Labs), P2Y6 antiserum (0.76 µg/ml; Alomone Labs) and P2Y12 antiserum (4 µg/ml; Alomone Labs) in 0.1 M PBS with 10% NGS and 0.1% Triton X-100. For immunofluorescence, sections were washed four times in 0.1 M PBS before the application of the secondary antibody, Alexa594-conjugated goat anti-rabbit IgG, applied at 4°C for 4 h (1:500; Molecular Probes, Inc., USA. in 5% NGS and 0.1% Triton X-100 in 0.1 M PBS). For immunoperoxidase studies, after PBS washes, sections were incubated in biotinylated secondary antibody (1:200 in 5% NGS and 0.1% Triton X-100 with 0.1 M PBS; biotinylated goat anti-rabbit immunoglobulin; Vector Laboratories) for 40 min, followed by incubation with avidin–biotin–HRP complex for 40 min. The immunoreactivity was detected using a 3, 3-diaminobenzidine tetrahydrochloride (DAB) peroxidase substrate kit (Vector Laboratories, Inc.) with a 2–3-min development time. After washes, the cochlear sections were mounted in anti-fade mounting medium CitiFluor (Agar Scientific, UK) on glass slides (ProbeOn Plus Microscope Slides; Fisher Scientific, PA, USA) and stored at 4°C.
Immunofluorescence images were acquired with a confocal microscope (TSC4D, Leica, Germany). Bright-field images were obtained using a Zeiss Axioskop microscope (Axioskop2 mot plus; Zeiss, Germany) with Nomarski differential interference contrast optics, and a digital camera (AxioCam HRc, Zeiss, Germany). All images were processed using Adobe Photoshop software to convert confocal images to grayscale, and in the case of color micrographs from immunoperoxidase staining, the images were color-matched to the original histological sections. All images presented here were single optical sections unless otherwise stated.
The results are qualitative, rather than semi-quantitative, and reflect our assessment of relative immunofluorescence signal between cell types in the same section and with comparison of signal levels of the same cell types at different developmental ages, in batch-processed, using similar laser excitation and detector settings on the microscope. Thus in Table 1, which provides a summary of the results for P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 antisera, expression in different cochlear tissues is scored as absent, weak, moderate or strong, based on the relative immunofluorescence intensity.
Table 1.
Developmental profile of P2Y receptor expression in the rat cochlea
| E16 | E18 | P0 | P6–12 | P20 | Adult | ||
|---|---|---|---|---|---|---|---|
| Organ of Corti | |||||||
| Sensory cells | Inner hair cells | – | – | Y2 (m); Y4 (s); Y6 (m); Y12 (s) | Y4 (s) | Y4 (s) | Y4 (m) |
| Outer hair cells | – | – | Y2 (m); Y4 (s); Y6 (m); Y12 (s) | Y1 (s); Y4 (s) | Y1 (s); Y4 (s); Y6 (m) | Y1 (s); Y4 (m) | |
| Synaptic region | – | – | Y2 (s) | – | Y4 (m) | Y4 (s) | |
| Greater epithelial ridge | – | Y2 (w); Y4 (m); Y6 (w) | Y2 (w); Y4 (s); Y6 (w) | Y4 (w) | – | – | |
| Cuticular plate | – | – | Y6 (s) | Y6 (s) | Y6 (m) | Y2 (s); Y6 (s) | |
| Tunnel crossing fibers | – | – | – | – | Y4 (s) | Y4 (s) | |
| Supporting cells | Deiters’ cells | – | – | – | Y2 (s) | – | – |
| Hensen's cells | – | – | – | Y2 (s) | – | – | |
| Pillar cells | – | – | – | Y2 (s) | Y2 (m); Y4 (m); Y6 (m) | Y2 (s); Y4 (w); Y6 (s) | |
| - Headplate | – | – | – | Y12 (m) | Y2 (m); Y6 (s) | Y2 (s); Y6 (s) | |
| - Footplate | – | – | – | – | Y2 (m); Y6 (m) | Y2 (s); Y6 (s) | |
| Claudius’ cells | – | – | Y2 (m) | Y2 (s) | Y2 (m) | Y2 (w) | |
| - Mesenchymal cells | – | – | – | – | – | Y1 (s) | |
| Phalangeal cells | – | – | – | Y4 (s) | – | – | |
| Stria vascularis | – | – | Y6 (m) | – | – | – | |
| Marginal cells | – | – | Y4 (s) | Y4 (s) | Y4 (m) | Y4 (m) | |
| Intermediate cells | – | – | – | – | – | Y4 (m) | |
| Basal cells | – | – | – | Y1 (s); Y2 (m–s); Y6 (m–s) | Y1 (w); Y2 (s); Y6 (s) | Y2 (s); Y6 (s) | |
| Spiral ligament | – | – | – | Y1 (m) | Y4 (m) | Y4 (m) | |
| Spiral prominence | – | – | – | – | Y4 (s) | Y4 (s) | |
| –Epithelial cells | – | – | – | Y2 (m) | Y2 (s) | Y2 (s) | |
| Outer Sulcus | – | – | – | Y2 (m) | Y2 (s) | Y2 (s) | |
| Spiral limbus | – | – | Y4 (m) | Y4 (m) | Y4 (m) | Y4 (m) | |
| –Interdental cells | – | – | Y6 (w) | Y1 (s); Y2 (m); Y6 (w) | Y1 (m); Y6 (m) | Y1 (w); Y6 (m) | |
| Reissner's membrane | –Epithelial cells | – | – | Y4 (s); Y6 (w) | Y1 (s); Y4 (s); Y6 (w); Y12 (m) | Y1 (s); Y4 (s); Y6 (w); Y12 (w) | Y1 (m); Y4 (m); Y6 (w); Y12 (w) |
| –Mesenchymal cells | – | – | – | Y12 (m) | Y12 (w) | Y12 (w) | |
| Spiral ganglion | Neurons | – | Y4 (w); Y12 (m) | Y4 (s); Y6 (m); Y12 (m) | Y1 (m); Y2 (m); Y4 (s); Y6 (m); Y12 (m) | Y1 (w); Y2 (m); Y4 (s); Y6 (m); Y12 (m) | Y1 (w); Y2 (w); Y4 (s); Y6 (w); Y12 (m) |
| Satellite cells | – | – | – | – | Y4 (s) | Y4 (s) | |
| Intraganglionic bundles | – | Y2 (s) | Y2 (s) | – | Y4 (s) | Y4 (m) |
w weak, m moderate, s strong
Results
Rat cochlear tissue was examined for P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 receptor immunolabeling at different developmental stages from E16 to adult. It was notable that immunolabeling of all five P2Y receptors emerged in the late embryonic period. Subsequently, rapid divergence of expression by these receptors corresponded to the period of cellular differentiation from P0 to the onset of hearing (approximately P12, [30]). Beyond the second postnatal week to the adult, the P2Y receptor expression profile exhibited only minor changes within the cochlear tissues.
Late embryonic P2Y receptor expression
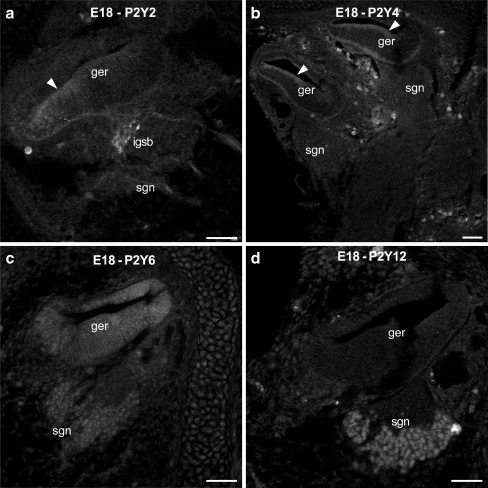
No immunolabeling was detected for any of the five examined P2Y receptors at E16. At E18, some P2Y2 labeling was evident in the intraganglionic spiral bundle (efferent fibers; Fig. 1a), but not in the spiral ganglion afferent neurons (SGN). Weak labeling was also present in the sensory epithelium adjacent to the greater epithelial ridge. P2Y4 receptor immunolabeling was present broadly across the sensory epithelium and the greater epithelial ridge (Fig. 1b). P2Y6 labeling was more diffuse and distributed throughout the otocyst and the SGN (Fig. 1c). P2Y12 expression was constrained to the SGN cell bodies (Fig. 1d). P2Y1 expression was not detected in embryonic cochlear tissue.
Fig. 1.
Immunolabeling of P2Y2, P2Y4, P2Y6 and P2Y12 receptors in the rat cochlea at E18. a P2Y2 protein expression was detected in the intraganglionic spiral bundle (efferent fibers), but not in the spiral ganglion neurons. In addition, weak labeling was observed in the sensory epithelium (arrowhead) adjacent to the greater epithelial ridge. b P2Y4 immunolabeling was only present on the sensory epithelium and greater epithelial ridge (arrowheads). c P2Y6 immunolabeling was weak, but evenly distributed within the otocyst and the spiral ganglion. d P2Y12 protein expression was constrained to the spiral ganglion neurons. Scale bars 50 µm. ger greater epithelial ridge, igsb intraganglionic spiral bundle, sgn spiral ganglion neurons
P2Y receptor expression at birth (P0)
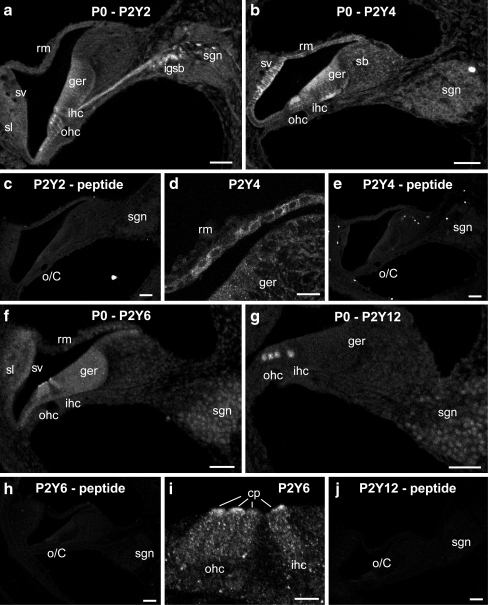
At birth, the P2Y receptor expression in the spiral ganglion was observed for all the P2Y receptors, except P2Y1 (Fig. 2a,b,f,g). P2Y2 SGN labeling was weak, however there was pronounced labeling of the neurite projections to the organ of Corti via the habenula perforata and labeling in the (efferent) intraganglionic spiral bundle region (Fig. 2a). Within the organ of Corti, P2Y2 labeling included the inner (IHC) and outer (OHC) hair cells and the Deiters’ cells, extending laterally to include the Claudius cells. P2Y2 labeling of the greater epithelial ridge was diffuse (Fig. 2a), compared with stronger P2Y4 signal (Fig. 2b). The hair cells were also labeled for P2Y4, with higher expression in the outer hair cells (Fig. 2b). P2Y6 specifically labeled the cuticular plate region of the inner and outer hair cells (Fig. 2f,i). As for P2Y6, P2Y12 immunolabeling in the greater epithelial ridge was constrained to the inner and outer hair cells, but in this case, to their basal (neural) pole (Fig. 2g). The lateral wall of the cochlea includes the spiral ligament and the stria vascularis. The spiral ligament is a connective tissue primarily consisting of several classes of fibrocytes, while the stria vascularis consists of basal, intermediate and marginal epithelial cells [31, 32]. The stria vascularis showed pronounced P2Y4 immunolabeling throughout the tissue, while P2Y6 labeling was diffuse (Fig. 2b,f). The spiral ligament supporting the stria vascularis, exhibited only diffuse low level P2Y2, P2Y4 and P2Y6 labeling (Fig. 2a,b,f). Reissner's membrane epithelial cells showed pronounced P2Y4 labeling (Fig. 2b,d), where none was evident at early ages, nor with the other P2Y receptor antibodies at this stage. The labeling with the antisera was blocked by pre-adsorption with the target peptides (Fig. 2c,e,h,j).
Fig. 2.
Immunolabeling of P2Y2, P2Y4, P2Y6, P2Y12 receptors at P0. a P2Y2 immunolabeling was detected in the both inner and outer hair cells in the great epithelial ridge, intraganglionic spiral bundle, spiral ganglion neurons and their nerve fibers innervating hair cells. b The distribution of P2Y4 receptor included the marginal and intermediate cells of the stria vascularis, epithelial cells of the Reissner's membrane, both inner and outer hair cells in the greater epithelial ridge, fibrocytes in the spiral limbus and spiral ganglion neurons. d A close-up of P2Y4 immunolabeling on the epithelial cells of the Reissner's membrane. f Weak P2Y6 immunofluorescence was found in the greater epithelial ridge, both inner and outer hair cells and perinuclear region of spiral ganglion neurons. Strong expression of P2Y6 receptor was constrained to the cuticular plates of inner and outer hair cells. g P2Y12 protein expression was limited to the basal pole of inner and outer hair cells and the perinuclear region of spiral ganglion neurons. i P2Y6 immunolabeling in the hair cells showed prominent expression in the cuticular plates of the hair cells. c, e, h, j Lack of immunolabeling in the negative controls, using pre-adsorption of the P2YR antibodies with respective target peptides demonstrated the specificity of these P2Y receptors protein expression. Scale barsa–c, e–h, and j 50 µm; d, i 20 µm. cp cuticular plate, ger greater epithelial ridge, ihc inner hair cells, igsb intraganglionic spiral bundle, o/C organ of Corti, ohc outer hair cells, rm Reissner's membrane, sb spiral limbus, sgn spiral ganglion neurons, sl spiral ligament, sv stria vascularis
P2Y receptor expression around the onset of hearing (P6–P12)
Cochlear tissues were largely differentiated by P6–P12. During this period, the immunolabeling of the examined P2Y receptors continued to differentiate between the different cochlear structures. For example, in the organ of Corti, expression of P2Y2 changed from being pronounced in the hair cells at P0, to being absent in these cells, but having significant signal in the adjacent supporting cells: the Deiters’ cells, pillar cells, Hensen's cells, and Claudius cells (Figs. 3b and 4a). P2Y12 expression was down-regulated in the hair cells, while developing in the head plates of the pillar cells (Fig. 4e). However, the hair cells maintained strong expression of P2Y4 (Figs. 3c and 4b), P2Y6 (cuticular plate region, Figs. 3d and 4d), and at P8, P2Y1 expression developed along the lateral walls of the outer hair cells (Figs. 3a and 4c). P2Y6 receptor labeling also developed along the confluence of the inner and outer pillar cells (Figs. 3d and 4d).
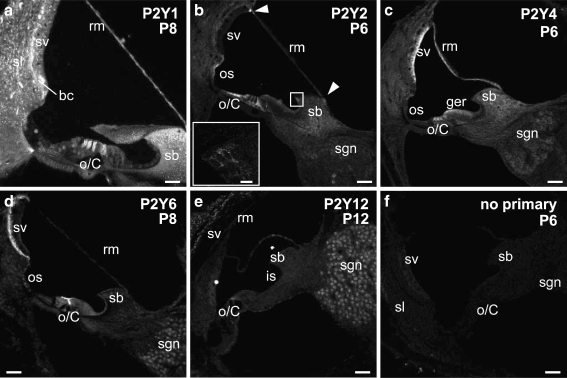
Fig. 3.
The protein expression pattern of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 receptors in the rat cochlea prior to the onset of hearing. a P2Y1 immunolabeling at P8 was detected extensively in the cochlear partition, including Reissner's membrane, spiral limbus and spiral ligament, basal cells of the stria vascularis. P2Y1 was also found in the hair cells in the organ of Corti. b P2Y2 labeling at P6 was observed in the pillar cells and Deiters’ cells in the organ of Corti, the interdental cells and fibrocytes of the spiral limbus, the epithelial cells of the spiral prominence, and a small subgroup of spiral ganglion neurons. Furthermore, the arrowheads point at the expression at the insertion points of Reissner's membrane into stria vascularis and spiral limbus. Inset shows P2Y2 expression in the interdental cells of the spiral limbus. c P2Y4 receptor expression at P6 rat cochlea was distributed in the hair cells in the organ of Corti, Reissner's membrane, stria vascularis, and the spiral ganglion neurons. d P2Y6 receptor protein expression in the P8 cochlea was detected in the cuticular plates of the hair cells in the organ of Corti and in the basal cells of the stria vascularis at a high level, but the expression in the Reissner's membrane epithelial cells and the interdental cell in the spiral limbus was weak. e P2Y12 immunolabeling was established at P12, and its expression was limited to the head plates of pillar cells in the organ of Corti, dispersed expression in Reissner's membrane epithelial and mesenchymal cells, and the perinuclear region of spiral ganglion neurons. f Negative control, omitting the primary antibody, at P6 cochlea. Scale bar 50 µm; inset ofb: 10 µm. bc basal cells, is inner sulcus, o/C organ of Corti, os outer sulcus, rm Reissner's membrane, sb spiral limbus, sgn spiral ganglion neurons, sl spiral ligament, sv stria vascularis
P2Y receptor expression in Reissner's membrane expanded postnatally. While P2Y4 expression was maintained in the epithelial cells facing the endolymphatic compartment (scala media; Figs. 3c and 4f), this was supplemented by strong P2Y1 expression that occurred in both the epithelial cells and also the mesenchymal cells facing scala vestibuli (perilymph; Fig. 3a). P2Y6 and P2Y12 expression was similar to P2Y1, but weaker (Figs. 3d,e and 4g,h).
The spiral limbus showed strong P2Y1 labeling, while P2Y2, P2Y4, P2Y6 expression was weaker and tended to be selective for particular cell types (Figs. 3a,c,d and 4f,g,i,j). Within this structure, the interdental cells, facing scala media, showed P2Y1 (Fig. 3a), P2Y2 (Fig. 3b—see inset) and P2Y6 expression, which was up-regulated from P8 (Fig. 4g) to P12 (Fig. 4j). The fibrocytes within the limbus were most strongly immunolabelled for P2Y1, P2Y2 and P2Y4 (Fig. 4i).
In the P6–P12 period, the greater epithelial ridge, which is the source of ATP release that establishes neurotransmission between the inner hair cells and spiral ganglion neurons [2] differentiates into the inner sulcus region. P2Y4 expression is pronounced here (Figs. 3c and 4b), with more diffuse P2Y6 expression (Fig. 3d). This contrasts with the outer sulcus cell region, where P2Y2 expression is pronounced (Fig. 3b), and again, more diffuse P2Y6 expression is present (Fig. 3d).
In the lateral wall of the cochlea, prominent P2Y1 immunolabeling was seen in the fibrocytes in the spiral ligament around P8 (Fig. 3a), whilst P2Y2, P2Y4, and P2Y6 expression was marginally above the background level from birth (Fig. 3b,c,d). The stria vascularis developed stratified P2Y receptor expression in the early neonatal period. The P2Y4 expression became localized to the marginal cells by P6 (Figs. 3c and 5h). P2Y1 and P2Y6 were strongly expressed in the basal cells (Figs. 3a,d and 5i), along with weak P2Y2 expression (Figs. 3b and 5g). P2Y6 expression in the basal cells continued to be up-regulated between P8 and P12 (Fig. 5i,j). The type II fibrocytes [33] at the insertion point of Reissner's membrane showed strong P2Y2 expression at P6 (Fig. 3b). Omission of primary antisera resulted in loss of these specific labeling profiles in P6–P20 cochlear tissues (Fig. 3f), indicating clearance of the fluorescence-labeled secondary antibody.
Fig. 5.
Detail of expression pattern of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 in the spiral ganglion neurons and the lateral wall before the onset of hearing. a P2Y1 immunolabeling at P8 was detected in both the spiral ganglion neurons and the satellite cells encapsulating the neurons. b Only a few spiral ganglion neurons (most likely Type II) were labeled using the P2Y2 antibody at P6. c P2Y4 receptor expression was found in the spiral ganglion neurons at P6. d P2Y6 expression in the spiral ganglion neurons at P8. Inset shows predominantly perinuclear and to a lesser extent membrane-bound P2Y6 immunolabeling of the neurons. e P2Y6 protein expression in the P16 spiral ganglion neurons. Inset illustrates that the P2Y6 labeling was primarily in the plasma membrane of the neurons at this later age. f P2Y12 immunolabeling remained in the perinuclear region of spiral ganglion neurons, as demonstrated in the inset. g P2Y2 protein expression in the P6 lateral wall included the basal cells of the stria vascularis and the epithelial cells of the spiral prominence. h P2Y4 expression in the P6 lateral wall was limited to marginal cells of the stria vascularis. i P2Y6 immunolabeling was limited to basal cells of the stria vascularis, with processes extending laterally at P8. j Elevated expression of P2Y6 in the basal cells of the stria vascularis from P8 to P12. Scale barsa–j, 15 µm. bc basal cells, ep epithelial cells, mc marginal cells, sgn spiral ganglion neurons, sv stria vascularis
Between P6–P12, the spiral ganglion exhibited expression of all five P2Y receptors. P2Y1 immunolabeling surrounded the SGN somata (Fig. 5a). This most likely reflects labeling of the glia (satellite) cells. P2Y2 immunolabeling was present in a small number of SGN, possibly indicative of type II afferents that innervate the outer hairs via the outer spiral bundle (Fig. 5b). P2Y4, P2Y6 and P2Y12 expression was apparent in the SGN somata, with different sub-cellular compartmentalization (Fig. 5c,d,e,f). P2Y6 labeling largely resolved from perinuclear to membrane-bound by P16 (Fig. 5e). In contrast, P2Y12 immunolabeling of the SGN remained predominantly perinuclear (Fig. 5f). RT-PCR analysis of P2Y12 expression confirmed the presence of this mRNA transcript in whole cochlea total RNA samples and also RNA isolated from dissected spiral ganglion tissue, at P12 and adult. The controls, including omission of reverse transcriptase, were negative (data not shown).
P2Y receptor expression in the adult cochlea
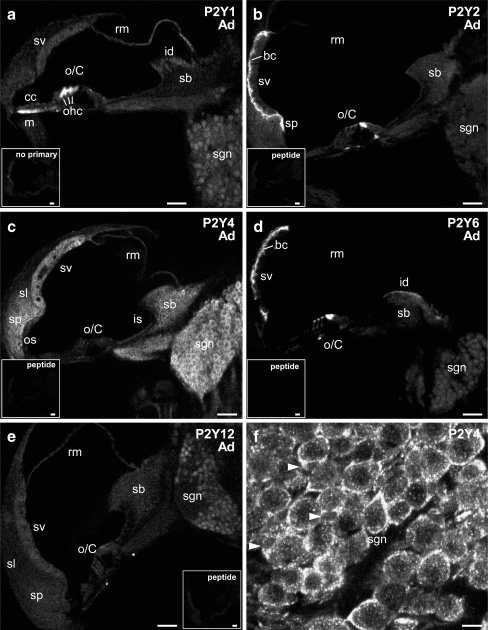
In the adult organ of Corti, the hair cells expressed a broad range of P2Y receptors. P2Y1 protein immunolabeling was evident at a high level on the basolateral walls of the outer hair cells, as for P8 (Figs. 6a and 7a); however, inner hair cell expression was lost (Fig. 7a). P2Y2 expression in the inner and outer hair cells, evident at P0, but absent at P6, returned to the cuticular plate region in the adult (Figs. 6b and 7b). P2Y6 showed similar polarization of expression to the cuticular plate region that was seen with P2Y2 expression (Figs. 6d and 7c, compare with 3C). P2Y4 was evenly distributed throughout both types of hair cells (Figs. 6c and 7d,e). Of note was the pronounced P2Y4 immunolabeling of the efferent crossing fibers and their terminals at the base of the outer hair cells (Fig. 7d,e). The outer spiral bundles (type II SGN) were not labeled, whereas signal was evident in the inner spiral plexus (type I SGN synaptic terminals beneath the inner hair cells; Fig. 7d,e). The heads of the pillar cells, facing scala media expressed P2Y2 (Figs. 6b and 7b) and P2Y6 (Figs. 6d and 7c). To a lesser degree, this expression also appeared in the foot plates of these cells. Compared with the neonatal tissue, the other supporting cells showed down-regulation of P2Y receptor expression. P2Y4 expression was maintained in the inner sulcus region (Fig. 6c), albeit at a lower level (compare with Fig. 3c), and also developed in the outer sulcus cell region (Fig. 6c). In the outer suclus region, P2Y2 expression was reduced to the spiral prominence (Fig. 6b). P2Y1 labeling was diffuse outside the hair cells, with the exception of a region of strong mesenchymal cell expression immediately below the Claudius cells (Fig. 6a).
Fig. 6.
The expression profile of P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 receptors in the adult cochlea. a P2Y1 immunolabeling was detected in the outer hair cells in the organ of Corti, mesenchymal cells juxtaposed to the Claudius cells, the epithelial cells in the Reissner's membrane, the interdental cells of the spiral limbus and the cell bodies of spiral ganglion neurons. b The protein expression of P2Y2 receptor in the organ of Corti included the hair cells and pillar cells in the organ of Corti. In the lateral wall, strong P2Y2 immunolabeling was observed in the basal cells of the stria vascularis, the epithelial cells of the spiral prominence. Weak signal was also detected in a few spiral ganglion neurons as seen at earlier ages. c Strong P2Y4 immunolabeling was widely distributed in the adult cochlea. The labeling detected in the organ of Corti was comparatively weaker but is detailed in the following figure. Diffuse labeling was found in the stria vascularis, spiral ligament, spiral prominence, spiral limbus and osseous spiral lamina. Strong P2Y4 expression was also observed in the spiral ganglion. d P2Y6 receptor expression, similar to P2Y2 receptor, was localized in the hair cells and pillar cells in the organ of Corti, and the basal cells of the stria vascularis. P2Y6 signal in the interdental cells in the spiral limbus was stronger. Weak labeling was detected in the spiral ganglion neurons. e P2Y12 protein was confined to the mesenchymal cells of Reissner's membrane at a low expression level and the perinuclear region of spiral ganglion neurons at a moderate expression level. f Intense P2Y4 immunolabeling was found in the cell bodies of spiral ganglion neurons and satellite cells that encapsulate the neuron somata. Satellite cells are indicated by arrowheads. Insets include no primary antibody negative control (a) and peptide pre-adsorption negative control (b–e), which demonstrate the specificity of the P2Y receptor immunolabeling. Scale bars 50 µm. bc basal cells, cc Claudius cells, id interdental cells, is inner sulcus, m mesenchymal cells, o/C organ of Corti, ohc outer hair cells, os outer sulcus, rm Reissner's membrane, sb spiral limbus, sgn spiral ganglion neurons, sl spiral ligament, sp spiral prominence, sv stria vascularis
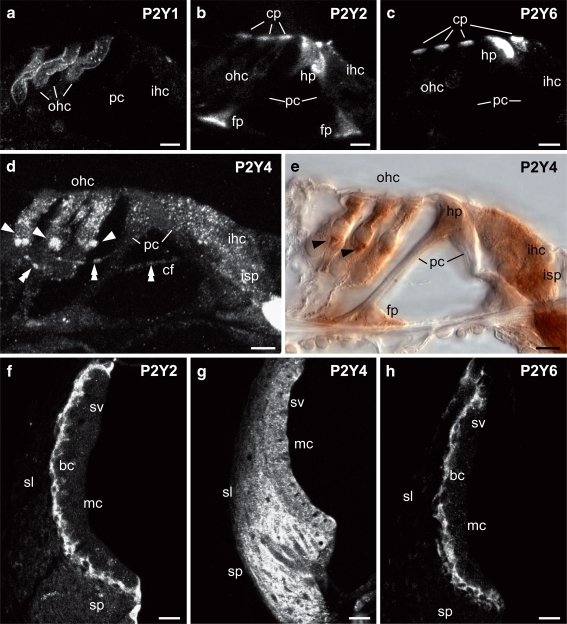
Fig. 7.
Detail of P2Y receptor expression in the adult rat cochlea. a Strong P2Y1 immunolabeling on the lateral wall of outer hair cells. b Intense P2Y2 immunolabeling in the organ of Corti was identified in the cuticular plate of inner and outer hair cells, and both head and foot plates of the pillar cells. c P2Y6 protein expression in the organ of Corti, similar to P2Y2 receptor, was found in the cuticular plate region of both the inner and outer hair cells and head and foot plates of the pillar cells. d, e P2Y4 protein expression shown using confocal immunofluorescence and immunoperoxidase, respectively. This illustrated that P2Y4 protein was expressed by both the inner and outer hair cells and by the tunnel crossing fibers (indicated by double arrowheads) terminating on the outer hair cells as large synaptic boutons (indicated by arrowheads). In addition, P2Y4 labeling was also detected in the inner spiral plexus, where afferent fibers terminate on the inner hair cells. Weak P2Y4 immunolabeling was also observed in the pillar cells. d is a maximum intensity projection of several confocal images. f–h P2Y2, P2Y4, and P2Y6 expression in the lateral wall. Both P2Y2 and P2Y6 receptors were detected in the basal cells of the stria vascularis (f, h). The processes of the basal cells are shown extending into the intermediate cell region. g P2Y4 labeling was diffuse throughout the stria vascularis, spiral ligament, and spiral prominence. Scale barsa–e, 10 µm; f–h, 20 µm. bc basal cells, cf tunnel crossing fibers, cp cuticular plate, fp footplate, hp head plate, ihc inner hair cells, isp inner spiral plexus, mc marginal cells, ohc outer hair cells, pc pillar cells, sl spiral ligament, sp spiral prominence, sv stria vascularis
Reissner's membrane P2Y receptor expression in adult tissue had a qualitative profile of P2Y1 > P2Y4 > P2Y12 (Fig. 6a,c,e). P2Y6 expression (Fig. 6d) was down-regulated from the neonatal tissue. The spiral limbus fibrocytes exhibited strong labeling for P2Y4 (Fig. 6c). The other P2Y receptor subtypes exhibited signals close to background in this region. The strongest interdental cell labeling was for P2Y6 (Fig. 6d), with P2Y1 and P2Y4 expression also evident in these cells (Fig. 6a,c).
The lateral wall showed strong expression of P2Y4 in fibrocytes (Figs. 6c and 7g), with minimal immunolabeling by P2Y1 and P2Y2. Within the stria vascularis, P2Y2 and P2Y6 expression was strong in the basal cell region, whereas P2Y4 expression was relatively homogeneous across basal, intermediate and marginal cells (Figs. 6b,c,d and 7f,g,h).
Within the adult cochlear spiral ganglion, the somata showed significant upregulation of P2Y4 expression compared with the labeling seen in neonatal SGN (Fig. 6c,f). The satellite cells that encapsulate the SGN also appeared to exhibited strong P2Y4 expression (subject to confirmation with a satellite cell marker). In comparison, P2Y1 expression in these cells was down-regulated (Fig. 6a). P2Y2 expression in putative type II SGN was maintained at a low level (Fig. 6b). P2Y6 immunolabeling in adult SGN (Fig. 6d) was comparable to the weak signal recorded at P16. P2Y12 immunolabeling in the cochlea was most pronounced in the SGN (Fig. 6e), and was comparable to the signal obtained with the P2Y1 antibody. As for the neonatal tissue, immunolabeling was blocked in adult cochlear tissue, by pre-adsorption of the antisera with their respective target peptides, and by omission of primary antisera (insets to Fig. 6a–e).
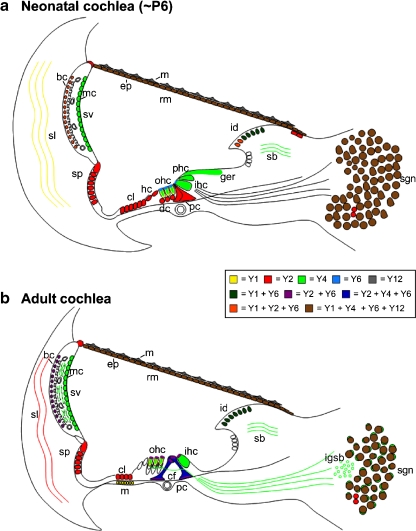
Schematic summaries of the P2YR expression profiles in radial cross-sections of the rat cochlea just prior to the onset of hearing (P6) and in the adult are provided in Fig. 8. Tissue-specific tabulation of our subjective assessment of P2YR expression for different ages of the cochlea is provided in Table 1.
Fig. 8.
Schematic diagrams summarize the P2Y receptor expression pattern in the neonatal (a) and adult (b) cochlea. bc basal cells, cf tunnel crossing fibers, cl Claudius cells, cp cuticular plate, ep epithelial cells, fp footplate, hp head plate, id interdental cells, igsb intraganglionic bundle, ihc inner hair cells, isp inner spiral plexus, m mesenchymal cells, mc marginal cells, ohc outer hair cells, rm Reissner's membrane, pc pillar cells, sb spiral limbus, sgn spiral ganglion neurons, sl spiral ligament, sp spiral prominence, sv stria vascularis
Discussion
The key findings of this study concern the dynamic interplay between expression of the five closely related P2Y receptors within the highly differentiated cochlear tissue. It was surprising that none of the receptors exhibited significant expression at E16, as P2Y receptor expression is pronounced in the developing CNS [34, 35]. In the cochlea, neuronal P2Y receptor expression did not commence until E18. During maturation of the hearing organ, P2Y receptor expression became increasingly compartmentalized—even to a sub-cellular level in the case of the sensory hair cells and some supporting cells. Our findings point to a fundamental issue with regard to P2Y receptor signaling, namely, the question of the physiological significance of having such a diversity of P2Y receptor subtypes expressed within the same cells, or adjacent cell types? While the differential sensitivity of these receptors to various purines and pyrimidines may be relevant to the activation profile—clearly highly compartmentalized intracellular signaling cascades are also likely to be relevant. Given the extensive evidence of involvement of purinergic signaling in the regulation of sound transduction and neurotransmission in the cochlea (for reviews, see [1, 36]), the present study demonstrates a broad representation of P2Y receptors in this tissue, which may provide insight into the process of P2Y receptor signal transduction in other neural and secretory tissues. Much is known about the physiology of the hearing organ that may enable us to at least partially tease out the significance of this P2Y receptor diversity in relation to the other elements of the purinergic signaling complex.
Our data on P2Y receptor expression in the developing rat cochlea may shed light on the recent observations of ATP-induced ATP release. This has been identified in the neonatal rat organ of Corti [3, 4]. Either exogenously applied nanomolar concentrations of ATP or UTP, or focal ATP release via laser ablation of sensory hair cells initiated Ca2+ waves through the lateral supporting cells (Hensen cells; via the P2Y–PLC–IP3 pathway). This phenomenon could be blocked by extracellular apyrase—which hydrolyses ATP, and the PLC inhibitor U73122. The findings, including block of the Ca2+ wave propagation using carbenoxolone, a gap junction blocker, suggested that the connexin hemichannel conduit for the ATP release in the cochlea [7, 37] is coupled to P2Y receptor signaling [3]. Our data showing prominent P2Y2 receptor expression at this site is consistent with the initial immunolocalization of P2Y2 to the Hensen's cell region by Gale et al. [3]. That study demonstrated that members of the mitogen-activated protein kinases (MAPK) pathway were correlated with expression of the P2Y2 receptors and an ATP-dependent Ca2+ wave, as part of an injury response mechanism. The response was primarily driven by release of stored Ca2+ and ATP release by the connexin hemichannels. In addition to activation of c-Jun N-terminal kinase (p-JNK), ERK 1/2 (extracellularly regulated kinases 1 and 2) was co-ordinated through the supporting cells (predominantly Deiters’ cells) via the spread of a P2 receptor-dependent Ca2+ signal [5]. Recent work described in this issue (Lahne and Gale, this issue) further details the involvement of both P2Y and P2X receptors in signaling injury in the cochlea.
Study of the neonatal rat organ of Corti has also revealed spontaneous pulsatile ATP release from the columnar epithelial cells of the transient Kölliker's organ (greater epithelial ridge), located medial to the inner hair cells [2]. The release mechanism is likely to engage the same extracellular ATP-mediated ATP release process and has been shown to provide local paracrine activation of P2 receptors on the inner hair cells. The inner hair cells release glutamate neurotransmitter in synchrony with the pulses of ATP, which establishes spiral ganglion neuron-mediated transmission to the auditory brainstem [2]. This activity is thought to consolidate the synaptic modeling of the cochlear nuclei. Our data suggest that P2Y4 receptor expression by the greater epithelial ridge cells are involved in this ATP-induced ATP release. P2Y4 receptor expression by the inner hair cells may also complement the implicated P2X receptor action to drive synaptic transmission.
In the adult cochlear tissue of guinea pigs, rats, and mice, P2X2 receptor expression is prominent in the hair cells and supporting cells of the organ of Corti, and the cells lining scala media, with the exception of the marginal cells of the stria vascularis [38–41]. These receptors assemble as ATP-gated ion channels that contribute to the regulation of the electrochemical driving force for sound transduction. It seems likely that noise stress induces ATP release into the endolymphatic compartment [10] which activates this mechanism, as microinjection of ATP into scala media produces a reduction in the cochlear partition resistance (reflecting the shunt of K+ out of scala media via the ATP-gated ion channels) and a concomitant fall in endocochlear potential (driving sound transduction through the hair cells) [12]. This paracrine adaptive mechanism also involves P2Y receptor signaling. As noted, the stria vascularis is the only tissue facing the endolymphatic compartment that does not express P2X receptor/ATP-gated ion channels. Studies by Marcus et al. [11, 29, 42] have revealed that the marginal cells of the stria vascularis provide a conductance pathway for K+ flux into scala media via KCNQ1/KCNE1 ion channels. The movement of K+ out of the stria vascularis and into the endolymph is important to regulation of endolymph potassium concentration and the positive endocochlear potential. The KCNE1 subunit has a protein kinase C regulatory domain which can be activated via the P2Y4 receptor–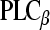 –diacylglycerol (DAG)– protein kinase C (PKC) pathway [11]. As we confirmed in the present study, P2Y4 co-localizes on the apical membrane of the strial marginal cells with KCNE1, whereas P2Y2 is localized in a separate, basal cell layer of this specialized ion transport epithelium [29]. Our present study also identified strong P2Y6 expression in the basal cell region, and showed that for P2Y4, all three cell layers of the stria vascularis expressed the protein. As part of the purinergic signal-plex, ecto-NTPDase2, which preferentially hydrolyses nucleoside triphosphates, is highly expressed in the basal cells, and with noise exposure, this expression is up-regulated in all three cell layers [24, 43, 44]. Activation of the P2Y4 receptor at the marginal cells, presumably by paracrine release of ATP and UTP [9, 10], would produce an inhibition of the KCNQ1/KCNE1 K+ channel and therefore reduce the cation flux which supports EP. This would complement the P2X2 receptor-mediated shunt of K+ out of scala media. The establishment of this triumvirate of basal P2Y2 and P2Y6 expression and over-layered P2Y4 expression in the stria vascularis at the start of the second postnatal week may relate to the maturation of purinergic signaling linked to the concomitant elevation of endolymphatic K+ concentration and endocochlear potential detected by Bosher and Warren [45] at this age.
–diacylglycerol (DAG)– protein kinase C (PKC) pathway [11]. As we confirmed in the present study, P2Y4 co-localizes on the apical membrane of the strial marginal cells with KCNE1, whereas P2Y2 is localized in a separate, basal cell layer of this specialized ion transport epithelium [29]. Our present study also identified strong P2Y6 expression in the basal cell region, and showed that for P2Y4, all three cell layers of the stria vascularis expressed the protein. As part of the purinergic signal-plex, ecto-NTPDase2, which preferentially hydrolyses nucleoside triphosphates, is highly expressed in the basal cells, and with noise exposure, this expression is up-regulated in all three cell layers [24, 43, 44]. Activation of the P2Y4 receptor at the marginal cells, presumably by paracrine release of ATP and UTP [9, 10], would produce an inhibition of the KCNQ1/KCNE1 K+ channel and therefore reduce the cation flux which supports EP. This would complement the P2X2 receptor-mediated shunt of K+ out of scala media. The establishment of this triumvirate of basal P2Y2 and P2Y6 expression and over-layered P2Y4 expression in the stria vascularis at the start of the second postnatal week may relate to the maturation of purinergic signaling linked to the concomitant elevation of endolymphatic K+ concentration and endocochlear potential detected by Bosher and Warren [45] at this age.
P2Y receptor expression in the inner and outer sulcus and interdental cells has also been linked to this regulation of ion homeostasis [46]. Pharmacological profiling of the ATP and UTP mediated short-circuit current in the outer sulcus is consistent with a P2Y2 or P2Y4 receptor-mediated increase in cation uptake in addition to the P2X2 receptor path [47, 48]. P2Y14 receptor expression also occurs in these regions postnatally [49]. The P2Y receptor-mediated cation return was prominent in the neonatal rat cochlea and down-regulated to adult levels by the end of the second postnatal week [48]. This finding is consistent with our identification of the dynamics of P2Y2 expression at this site, and may be reconciled with the P2Y4 pharmacological designation of Lee et al. [48] by considering that in the rat P2Y2 and P2Y4 receptors have comparable ATP and UTP agonist sensitivity [18]. In the inner sulcus and interdental cell region, where P2Y4 and P2Y6 expression was pronounced, this site is known to exhibit substantial ecto-NTPDase3 expression [50]. Ecto-NTPDase3 preferentially hydrolyses ATP at approximately three times the rate of ADP hydrolysis [51]. This enables prolonged activation of ADP/UDP-preferring receptors which is well-matched to the agonist profile for P2Y6 [19]. Thus release of extracellular nucleotides into the endolymph, and modification by ecto-NTPDases, likely provide an intrinsic humoral regulatory system engaging both P2Y and P2X receptors in response to high-level acoustic stimulation that likely expands the dynamic range for sound transduction by providing adaptation of hearing sensitivity.
Our data showed that from P0, the hair cells developed highly compartmentalized expression of several different P2Y receptors. In the adult, this resolved to the localization of P2Y2 and P2Y6 in the cuticular plate region, with prominent basolateral membrane expression of P2Y1 (in outer hair cells). P2Y4 expression was distributed in both the outer and inner hair cells (see Fig. 7). This sub-cellular compartmentalization may parallel the compartmentalization of P2X receptors in the hair cells. As noted, P2X2 receptors are confined to the apical surface, particularly the stereocilia of the hair cells [41, 52], while it seems likely that P2X7 receptor protein is localized on the basolateral surface of the outer hair cells [53] and may influence prestin-mediated electromotility [37]. Ion imaging has indicated that extracellular ATP invokes apically mediated Ca2+ entry, via the P2X2 receptors [54, 55], however, this is complemented by apically localized P2Y receptor signaling that causes a release of Ca2+ stored within Hensen's body, in the cuticular plate region of the outer hair cells, via the PLC–IP3 pathway [55]. Our data indicate that this would likely be mediated by both P2Y2 and P2Y6 receptors. Synthesis of membrane-bound DAG following activation of these P2Y receptors, may also trigger Ca2+ entry via co-localized canonical transient receptor potential channels (TRPC3) [56, 57]. This localized Ca2+ influx may influence the mechanoelectrical transducer channel conductance [55], or more generally, the multi-modal cation influx may affect neurotransmission, and for outer hair cells, voltage-dependent electromotility.
The spiral ganglion neurons expressed P2Y receptors from E18. P2Y4 expression developed progressively, to have the strongest expression in the adult (within the somata and at the afferent terminals of the spiral plexus beneath the inner hair cells), with lower levels of immunolabeling of P2Y1, P2Y6, and P2Y12 evident in the SGN. Both P2Y4 and P2Y1 were also expressed in the satellite cells. P2Y2 labeling in the cross-sections was typically limited to just a few neurons, possibly indicating a selectivity for the 5% of adult SGN that comprise the type II afferent innervating the outer hair cells [58]. P2Y4 expression was also apparent pre-synaptically, at the efferent tunnel crossing fibers and their cholinergic synapses at the base of the outer hair cells (Fig. 7d,e). Earlier electrophysiological investigations of purinergic signaling in spiral ganglion neurons had confirmed that both P2X and P2Y receptors mediate inward currents. The SGN P2X receptors exhibit desensitizing currents in response to micromolar ATP [59–61], while the P2Y receptors are activated by nanomolar ATP and UTP concentrations, and via intracellular second messenger signaling, engage a slowly developing non-selective cation conductance [62]. The identity of this cation conductance is thought to be a TRP channel which provides cross-talk with other metabotropic receptors involving the GPCR- PLC - DAG pathway, including the muscarinic acetylcholine receptor and the substance P receptor [17, 63]. Given the high level of TRPC3 recently identified in guinea pig and mouse SGN somata [57, 64], this channel is the most likely candidate, akin to the situation in the outer hair cells.
In summary, this study is the first to describe the dynamic changes in the expression of the P2Y receptors, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12, in the highly differentiated cochlear tissue during development. The demonstration that all five P2Y receptors are compartmentally expressed in the cochlear partition, the organ of Corti and the spiral ganglion neurons further substantiates a role for the purinergic signaling complex and associated downstream signaling cascades in the establishment and maintenance of hearing.
Acknowledgments
This study is funded by the Health Research Council of New Zealand and the Marsden Fund (Royal Society of New Zealand). Our thanks to the University of Auckland Biomedical Imaging Resource Unit for supporting the confocal microscopy.
References
- 1.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32:128–141. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 3.Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr Biol. 2004;14:526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium. 2007;41:77–86. doi: 10.1016/j.ceca.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Lahne M, Gale JE. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci. 2008;28:4918–4928. doi: 10.1523/JNEUROSCI.4914-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang W, Ahmad S, Shestopalov VI, Lin X. Pannexins are new molecular candidates for assembling gap junctions in the cochlea. NeuroReport. 2008;19:1253–1257. doi: 10.1097/WNR.0b013e32830891f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White PN, Thorne PR, Housley GD, Mockett B, Billett TE, Burnstock G. Quinacrine staining of marginal cells in the stria vascularis of the guinea-pig cochlea: a possible source of extracellular ATP? Hear Res. 1995;90:97–105. doi: 10.1016/0378-5955(95)00151-1. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;12:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 11.Marcus DC, Sunose H, Liu J, Bennett T, Shen Z, Scofield MA, Ryan AF. Protein kinase C mediates P2U purinergic receptor inhibition of K+ channel in apical membrane of strial marginal cells. Hear Res. 1998;115:82–92. doi: 10.1016/S0378-5955(97)00180-9. [DOI] [PubMed] [Google Scholar]
- 12.Thorne PR, Munoz DJ, Housley GD. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the guinea pig. J Assoc Res Otolaryngol. 2004;5:58–65. doi: 10.1007/s10162-003-4003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Devel Res. 2001;52:44–56. doi: 10.1002/ddr.1097. [DOI] [Google Scholar]
- 14.Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Therap. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Kolen K, Slegers H. Integration of P2Y receptor-activated signal transduction pathways in G protein-dependent signalling networks. Purinergic Signalling. 2006;2:451–469. doi: 10.1007/s11302-006-9008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci USA. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulon D, Jagger DJ, Lin X, Davis RL. Neuromodulation in the spiral ganglion: shaping signals from the organ of corti to the CNS. J Membr Biol. 2006;209:167–175. doi: 10.1007/s00232-005-0841-9. [DOI] [PubMed] [Google Scholar]
- 18.Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholas RA, Lazarowski ER, Watt WC, Li Q, Boyer J, Harden TK. Pharmacological and second messenger signalling selectivities of cloned P2Y receptors. J Auton Pharmacol. 1996;16:319–323. doi: 10.1111/j.1474-8673.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 20.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 21.Wangemann P. Ca2+-dependent release of ATP from the organ of Corti measured with a luciferin–luciferase bioluminescence assay. Audit Neurosci. 1996;2:187–192. [Google Scholar]
- 22.Ito K, Dulon D (2010) Purinergic signalling in cochleovestibular hair cells and afferent neurons. Purinergic Signalling doi:10.1007/s11302-010-9183-x [DOI] [PMC free article] [PubMed]
- 23.Vlajkovic SM, Thorne PR, Housley GD, Munoz DJ, Kendrick IS. Ecto-nucleotidases terminate purinergic signalling in the cochlear endolymphatic compartment. NeuroReport. 1998;9:1559–1565. [PubMed] [Google Scholar]
- 24.Vlajkovic SM, Thorne PR, Sevigny J, Robson SC, Housley GD. Distribution of ectonucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Hear Res. 2002;170:127–138. doi: 10.1016/S0378-5955(02)00460-4. [DOI] [PubMed] [Google Scholar]
- 25.Vlajkovic SM, Abi S, Wang CJ, Housley GD, Thorne PR. Differential distribution of adenosine receptors in rat cochlea. Cell Tissue Res. 2007;328:461–471. doi: 10.1007/s00441-006-0374-2. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira M, Butlen D, Ferrary E, Sterkers O, Escoubet B. Identification of uridine 5′-triphosphate receptor mRNA in rat cochlear tissues. Acta Otolaryngol. 2000;120:156–159. doi: 10.1080/000164800750000810. [DOI] [PubMed] [Google Scholar]
- 27.Jin J, Tomlinson W, Kirk IP, Kim YB, Humphries RG, Kunapuli SP. The C6-2B glioma cell P2Y(AC) receptor is pharmacologically and molecularly identical to the platelet P2Y12 receptor. Br J Pharmacol. 2001;133:521–528. doi: 10.1038/sj.bjp.0704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000;421:374–384. doi: 10.1002/(SICI)1096-9861(20000605)421:3<374::AID-CNE6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Sage CL, Marcus DC. Immunolocalization of P2Y4 and P2Y2 purinergic receptors in strial marginal cells and vestibular dark cells. J Membr Biol. 2002;185:103–115. doi: 10.1007/s00232-001-0116-z. [DOI] [PubMed] [Google Scholar]
- 30.Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69:236–242. doi: 10.1016/0378-5955(93)90113-F. [DOI] [PubMed] [Google Scholar]
- 31.Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear Res. 1991;56:53–64. doi: 10.1016/0378-5955(91)90153-Z. [DOI] [PubMed] [Google Scholar]
- 32.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165(1–2):1–9. doi: 10.1016/S0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 33.Splepecky N (1996) Structure of the mammalian cochlea. In: Dallos P et al (eds) Handbook of auditory research: the cochlea. Springer, pp 44–129
- 34.Meyer MP, Clarke JD, Patel K, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn. 1999;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Cheung KK, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228:254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- 36.Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells—beyond the transducer. J Membr Biol. 2006;209:89–118. doi: 10.1007/s00232-005-0835-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Housley GD, Ryan AF. Cholinergic and purinergic neurohumoral signalling in the inner ear: a molecular physiological analysis. Audiol Neurootol. 1997;2:92–110. doi: 10.1159/000259233. [DOI] [PubMed] [Google Scholar]
- 39.Housley GD, Luo L, Ryan AF. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J Comp Neurol. 1998;393:403–414. doi: 10.1002/(SICI)1096-9861(19980420)393:4<403::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.King M, Housley GD, Raybould NP, Greenwood D, Salih SG. Expression of ATP-gated ion channels by Reissner's membrane epithelial cells. NeuroReport. 1998;9:2467–2474. doi: 10.1097/00001756-199808030-00008. [DOI] [PubMed] [Google Scholar]
- 41.Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcus DC, Sunose H, Liu J, Bennett T, Shen Z, Scofield MA, Ryan AF. Protein kinase C mediates P2U purinergic receptor inhibition of K+ channel in apical membrane of strial marginal cells. Hear Res. 1998;115:82–92. doi: 10.1016/S0378-5955(97)00180-9. [DOI] [PubMed] [Google Scholar]
- 43.Vlajkovic SM, Thorne PR, Sevigny J, Robson SC, Housley GD. NTPDase1 and NTPDase2 immunolocalization in mouse cochlea: implications for regulation of p2 receptor signaling. J Histochem Cytochem. 2002;50:1435–1442. doi: 10.1177/002215540205001102. [DOI] [PubMed] [Google Scholar]
- 44.Vlajkovic SM, Housley GD, Munoz DJ, Robson SC, Sevigny J, Wang CJ, Thorne PR. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 2004;126:763–773. doi: 10.1016/j.neuroscience.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Bosher SK, Warren RL. A study of the electrochemistry and osmotic relationships of the cochlear fluids in the neonatal rat at the time of the development of the endocochlear potential. J Physiol. 1971;212:739–761. doi: 10.1113/jphysiol.1971.sp009354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spicer SS, Thomopoulos GN, Schulte BA. Structural evidence for ion transport and tectorial membrane maintenance in the gerbil limbus. Hear Res. 2000;143:147–161. doi: 10.1016/S0378-5955(00)00037-X. [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci. 2001;21:9168–9174. doi: 10.1523/JNEUROSCI.21-23-09168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Heo JH, Kim CH, Chang SO, Kim CS, Oh SH. Changes in P2Y4 receptor expression in rat cochlear outer sulcus cells during development. Hear Res. 2007;228:201–211. doi: 10.1016/j.heares.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 49.O'Keeffe MG, Thorne PR, Housley GD, Robson SC, Vlajkovic SM. Developmentally regulated expression of ectonucleotidases NTPDase5 and NTPDase6 and UDP-responsive P2Y receptors in the rat cochlea. Histochem Cell Biol 133:425–436 [DOI] [PubMed]
- 50.Vlajkovic SM, Vinayagamoorthy A, Thorne PR, Robson SC, Wang CJ, Housley GD. Noise-induced up-regulation of NTPDase3 expression in the rat cochlea: Implications for auditory transmission and cochlear protection. Brain Res. 2006;1104:55–63. doi: 10.1016/j.brainres.2006.05.094. [DOI] [PubMed] [Google Scholar]
- 51.Smith TM, Kirley TL. Cloning, sequencing, and expression of a human brain ecto-apyrase related to both the ecto-ATPases and CD39 ecto-apyrases1. Biochim Biophys Acta. 1998;1386:65–78. doi: 10.1016/s0167-4838(98)00063-6. [DOI] [PubMed] [Google Scholar]
- 52.Housley GD, Greenwood D, Ashmore JF. Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc R Soc Lond B Biol Sci. 1992;249:265–273. doi: 10.1098/rspb.1992.0113. [DOI] [PubMed] [Google Scholar]
- 53.Nikolic P, Housley GD, Thorne PR. Expression of the P2X7 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the developing and adult rat cochlea. Audiol Neurootol. 2003;8:28–37. doi: 10.1159/000067891. [DOI] [PubMed] [Google Scholar]
- 54.Housley GD, Raybould NP, Thorne PR. Fluorescence imaging of Na+ influx via P2X receptors in cochlear hair cells. Hear Res. 1998;119:1–13. doi: 10.1016/S0378-5955(97)00206-2. [DOI] [PubMed] [Google Scholar]
- 55.Mammano F, Frolenkov GI, Lagostena L, Belyantseva IA, Kurc M, Dodane V, Colavita A, Kachar B. ATP-Induced Ca2+ release in cochlear outer hair cells: localization of an inositol triphosphate-gated Ca2+ store to the base of the sensory hair bundle. J Neurosci. 1999;19:6918–6929. doi: 10.1523/JNEUROSCI.19-16-06918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raybould NP, Jagger DJ, Kanjhan R, Greenwood D, Laslo P, Hoya N, Soeller C, Cannell MB, Housley GD. TRPC-like conductance mediates restoration of intracellular Ca2+ in cochlear outer hair cells in the guinea pig and rat. J Physiol. 2007;579:101–113. doi: 10.1113/jphysiol.2006.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tadros SF, Kim Y, Phan PA, Birnbaumer L, Housley GD. TRPC3 ion channel subunit immunolocalization in the cochlea. Histochem Cell Biol. 2010;133:137–147. doi: 10.1007/s00418-009-0653-6. [DOI] [PubMed] [Google Scholar]
- 58.Hafidi A, Despres G, Romand R. Ontogenesis of type II spiral ganglion neurons during development: peripherin immunohistochemistry. Int J Dev Neurosci. 1993;11:507–512. doi: 10.1016/0736-5748(93)90024-8. [DOI] [PubMed] [Google Scholar]
- 59.Salih SG, Housley GD, Burton LD, Greenwood D. P2X2 receptor subunit expression in a subpopulation of cochlear type I spiral ganglion neurones. NeuroReport. 1998;9:279–282. doi: 10.1097/00001756-199801260-00019. [DOI] [PubMed] [Google Scholar]
- 60.Salih SG, Jagger DJ, Housley GD. ATP-gated currents in rat primary auditory neurones in situ arise from a heteromultimetric P2X receptor subunit assembly. Neuropharmacology. 2002;42:386–395. doi: 10.1016/S0028-3908(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 61.Jagger DJ, Housley GD. Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice. J Physiol. 2003;552:525–533. doi: 10.1111/j.1469-7793.2003.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito K, Dulon D. Nonselective cation conductance activated by muscarinic and purinergic receptors in rat spiral ganglion neurons. Am J Physiol Cell Physiol. 2002;282:C1121–1135. doi: 10.1152/ajpcell.00364.2001. [DOI] [PubMed] [Google Scholar]
- 63.Ito K, Rome C, Bouleau Y, Dulon D. Substance P mobilizes intracellular calcium and activates a nonselective cation conductance in rat spiral ganglion neurons. Eur J Neurosci. 2002;16:2095–2102. doi: 10.1046/j.1460-9568.2002.02292.x. [DOI] [PubMed] [Google Scholar]
- 64.Phan PA, Tadros SF, Kim Y, Birnbaumer L, Housley GD. Developmental regulation of TRPC3 ion channel expression in the mouse cochlea. Histochem Cell Biol. 2010;133:437–448. doi: 10.1007/s00418-010-0686-x. [DOI] [PubMed] [Google Scholar]