Abstract
Gap junction-mediated K+ recycling in the cochlear supporting cell has been proposed to play a critical role in hearing. However, how potassium ions enter into the supporting cells to recycle K+ remains undetermined. In this paper, we report that ATP can mediate K+ sinking to recycle K+ in the cochlear supporting cells. We found that micromolar or submicromolar levels of ATP could evoke a K+-dependent inward current in the cochlear supporting cells. At negative membrane potentials and the resting membrane potential of −80 mV, the amplitude of the ATP-evoked inward current demonstrated a linear relationship to the extracellular concentration of K+, increasing as the extracellular concentration of K+ increased. The inward current also increased as the concentration of ATP was increased. In the absence of ATP, there was no evoked inward current for extracellular K+ challenge in the cochlear supporting cells. The ATP-evoked inward current could be inhibited by ionotropic purinergic (P2X) receptor antagonists. Application of pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS, 50 µM) or pre-incubation with an irreversible P2X7 antagonist oxidized ATP (oATP, 0.1 mM) completely abolished the ATP-evoked inward current at the negative membrane potential. ATP also evoked an inward current at cell depolarization, which could be inhibited by intracellular Cs+ and eliminated by positive holding potentials. Our data indicate that ATP can activate P2X receptors to recycle K+ in the cochlear supporting cells at the resting membrane potential under normal physiological and pathological conditions. This ATP-mediated K+ recycling may play an important role in the maintenance of cochlear ionic homeostasis.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-010-9184-9) contains supplementary material, which is available to authorized users.
Keywords: ATP, Potassium, P2x receptor, Purinergic signaling, Gap junction, Connexin, Cochlea, Deafness
Introduction
Supporting cells in the cochlea provide physical support and nutrition to hair cells and also play an important role in the maintenance of cochlear ionic homeostasis [1, 2]. It has been hypothesized that supporting cells in the cochlea like glia cells in the brain absorb or sink K+ ions, which hair cells release during mechano-electrical transduction, and transport them back to the endolymph via intracellular gap junctional communication [3–9]. However, the detailed mechanism by which potassium ions enter into the cochlear supporting cells to recycle K+ remains unclear.
ATP is an important extracellular signaling molecule. In the cochlea, it has been reported that ATP can evoke inward currents and raise the intracellular Ca++ concentration in the outer and inner hair cells, thereby modifying sound transduction and neurotransmission [10–13]. ATP can activate purinergic (P2) receptors to produce inward cationic currents [14, 15]. P2 receptors have two subgroups: ATP-gated ionotropic (P2X) receptors and G-protein-coupled metabotropic (P2Y) receptors. Both P2X and P2Y receptors are expressed in the cochlea, including supporting cells [16–23]. Recently, we have demonstrated that gap junctional hemichannels in the cochlear supporting cells can release ATP [24], which can activate P2x receptors in the outer hair cells (OHCs) to induce Ca++ influx and modulate OHC electromotility [24, 25]. P2X receptors have a cationic permeability, permeable to K+ ions [15]. In this study, the effect of ATP on K+ recycling in the cochlear supporting cells was investigated. We found that the micromolar and submicromolar levels of ATP can induce a significant K+-dependent inward current in the cochlear supporting cells at the resting membrane potential. Our new findings indicate that ATP can mediate K+ sinking and recycling in the cochlear supporting cells and plays an important role in cochlear ionic homeostasis.
Materials and methods
Animal preparation and cochlear supporting cell isolation
Cochlear supporting cells were freshly isolated from adult guinea pigs (250–400 g, n = 43) as previously described [8, 26]. Briefly, the temporal bones were removed after decapitation. The otic capsule was isolated and dissected in normal extracellular solution (in mM: 130 NaCl, 5 KCl, 1.47 MgCl2, 2 CaCl2, 25 dextrose, and 10 HEPES; 300 mOsm, pH 7.2) to reveal the organ of Corti. The sensory epithelium was micro-dissected by a sharpened needle. The isolated sensory epithelium was dissociated by trypsin (1 mg/ml) for 5–10 min. The dissociated cells were then transferred to a dish for recording. All experimental procedures were conducted at room temperature (23°C) in accordance with the policies of the University of Kentucky Animal Care & Use Committee.
Patch-clamp recording and data processing
Single dissociated cochlear supporting cell was selected and recorded under the whole cell configuration (Fig. 1a) using an Axopatch 200B patch clamp amplifier (Molecular Devices, CA, USA). Patch pipettes were filled with an intracellular solution that contained (in mM) 140 KCl, 5 EGTA, 2 MgCl2, and 10 HEPES, pH 7.2, with initial resistance of 2.5–3.5 MΩ in bath solution. Data were collected by jClamp software (SciSoft, New Haven, CT, USA) [7–9, 25]. The signal was filtered by a four-pole low-pass Bessel filter with a cutoff frequency of 2 kHz and digitized utilizing a Digidata 1322A (Molecular Devices, CA, USA).
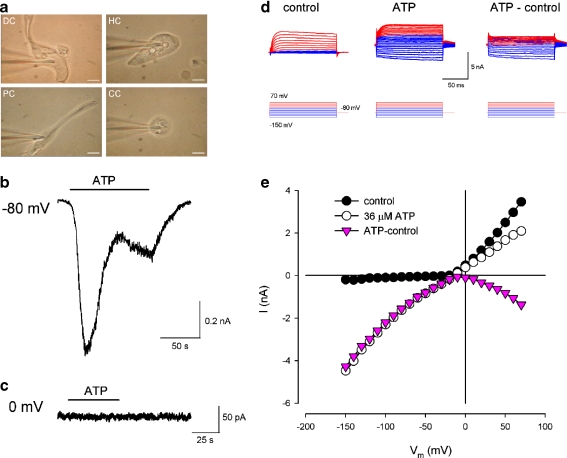
Fig. 1.
Cochlear supporting cells and ATP-evoked inward current. a Micrographs of isolated single cochlear supporting cell and patch clamp recording. DC Deiters’ cell, HC Hensen cell, PC pillar cell, CC Claudius cell. Scale bars, 10 μm. b, c Current traces evoked by ATP in a Deiters’ cell. The cell membrane potential was clamped at −80 mV (b) and 0 mV (c). The horizontal bars represent the application of 36 µM ATP. d, e Evoked currents in a Deiters’ cell for voltage step stimulation. Red and blue colors represent current responses to the positive and negative voltage step stimuli, respectively. The current–voltage (I–V) relations were plotted by average values of the steady-state currents in last 20 ms of the voltage step stimulation. Pink color represents the I–V curve of the subtracted inward current evoked by ATP
Data were analyzed with jClamp and plotted by SigmaPlot software (SPSS Inc. Chicago) for presentation. Membrane potential (Vm) was corrected for pipette series resistance (Rs). Error bars represent SE.
Potassium challenge and chemical perfusion
All chemicals were purchased from Sigma-Aldrich (St. Louis, USA). A Y-tube perfusion system was used for the application of ATP and chemicals [25]. The potassium challenge was achieved by perfusion with high K+ extracellular solutions, which were prepared by replacement of NaCl with KCl in the normal extracellular solution. The solution osmolarity was kept constant at 300 mOsm.
Results
ATP-evoked inward current in the cochlear supporting cells
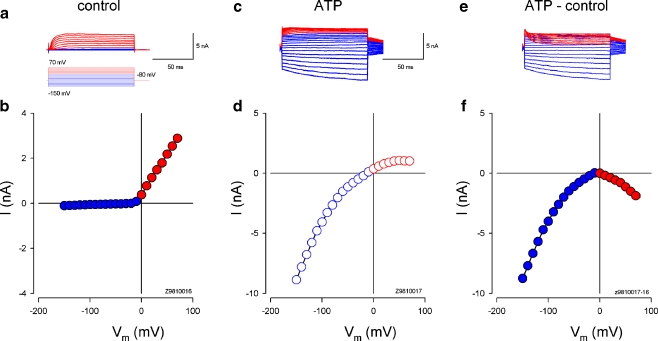
The organ of Corti of guinea pigs contains four types of supporting cells, i.e., Deiters’ cell, pillar cells, Hensen cells, and Claudius cells, with their own morphological characteristics (Fig. 1a; also see [26]). ATP could evoke the inward currents in all tested cochlear supporting cells (n > 100, Figs. 1 and 2). The evoked inward currents in the cochlear supporting cells show two phases: a large, quick phase followed by a delayed, developing phase (Fig. 1b). This is a characteristic of P2X receptor activity [15]. The evoked inward current was large at the resting membrane potential of −80 mV and became invisible at the membrane potential of 0 mV (Fig. 1b, c). The inward current was also visible at cell depolarization, showing a bell shape for the evoked I–V curves (Figs. 1e and 2f). The evoked inward current increased when the cell was hyperpolarized and depolarized and was large at the negative membrane potential (Figs. 1d, e and 2c–f).
Fig. 2.
ATP-evoked inward current in the cochlear supporting cells. a, b Current responses of a Hensen cell to voltage step stimulation. Red and blue colors represent the current responses to positive and negative voltage step stimuli, respectively. The I–V curve was plotted by average values of the steady-state currents in the last 20 ms of voltage step stimulation. c, d Current trace and I–V curve under 36 μM ATP perfusion. e, f ATP-evoked currents and I–V curve. The current response was obtained by subtraction of the control current response from that under ATP application. The inward current increased when the cell was depolarized and hyperpolarized. Rs, 6.9 MΩ
Potassium dependence of ATP-evoked inward current in the cochlear supporting cells
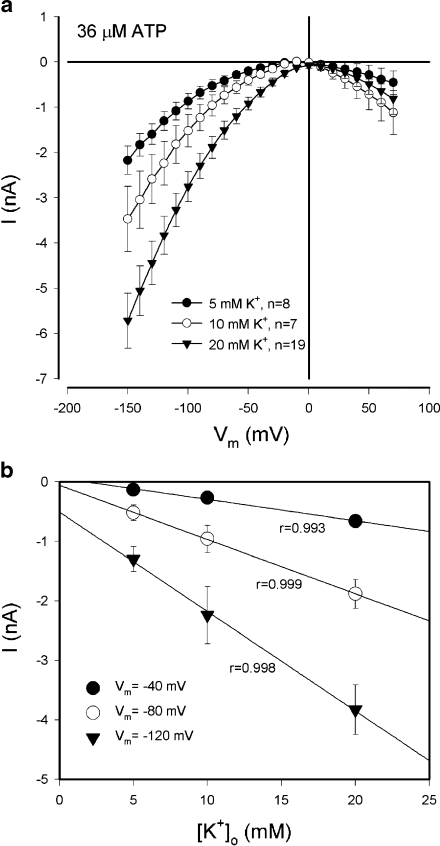
The evoked inward current depended on extracellular K+ (Fig. 3). As the extracellular concentration of K+ was increased, the ATP-evoked inward current increased (Fig. 3a). At the resting membrane potential of −80 mV, the amplitudes of the ATP-evoked inward currents at 5, 10, and 20 mM extracellular K+ concentrations were −0.52 ± 0.13 (n = 8),−0.96 ± 0.23 (n = 7), and −1.89 ± 0.24 (n = 19) nA, respectively. The regression analysis shows good linear relationships (r > 0.99) between the amplitudes of inward currents and the extracellular concentrations of K+ at negative membrane potentials (Fig. 3b). The slope was 0.036, 0.091, and 0.167 nA/mM at the holding potentials of −40, −80, and −120 mV, respectively, and increased as the cell became more hyperpolarized.
Fig. 3.
K+ dependence in the ATP-evoked inward currents in the cochlear supporting cells. aI–V relations evoked by ATP (36 µM) at different extracellular concentrations of K+. The evoked inward currents at negative membrane potentials increased as the extracellular K+ concentration was increased. b The increase in the amplitude of the inward current at negative membrane potentials has linear relationships to the extracellular concentration of K+ ([K+]o). Solid lines represent data fitted by a linear function (y = ax + b; a = −0.036, −0.091, and −0.167 nA/mM for Vm = −40, −80, and −120 mV, respectively). r is the coefficient of linear regression. Error bars represent SE
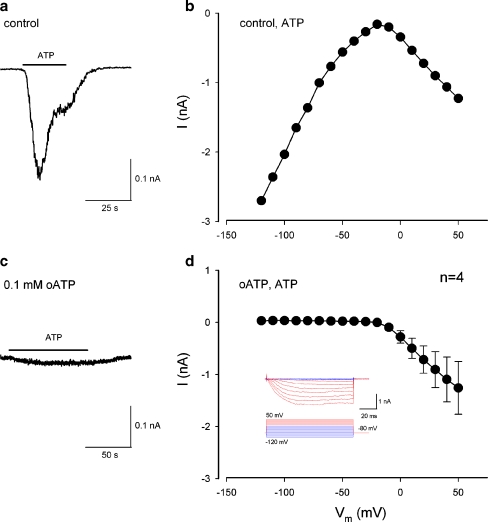
ATP dependence of the inward current in the cochlear supporting cells
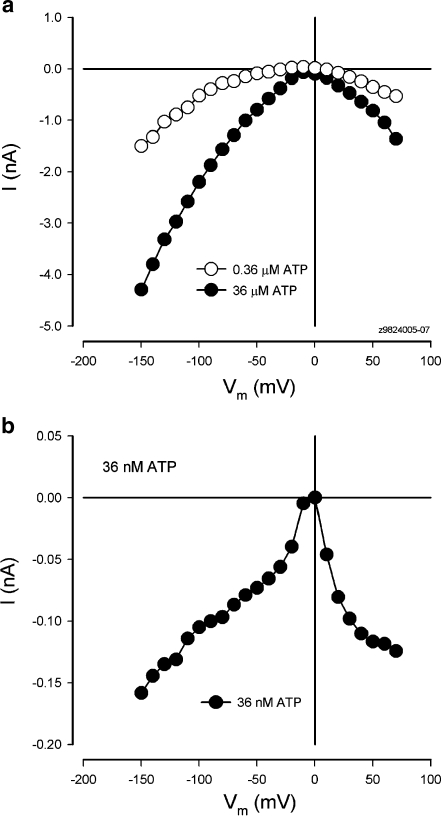
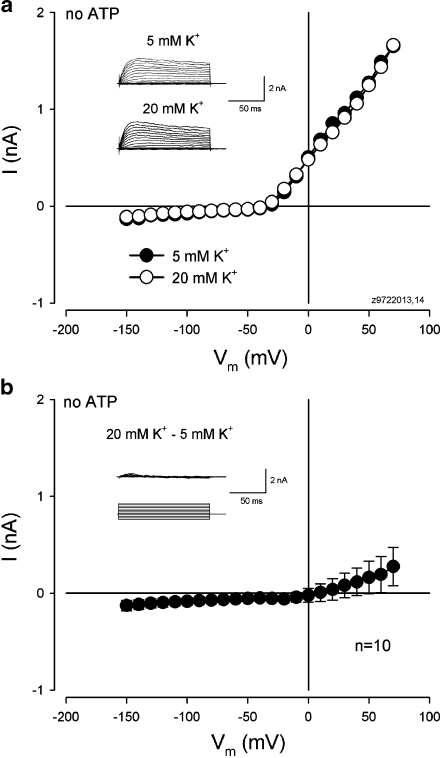
Figure 4 shows the evoked inward current in the cochlear supporting cell by application of micromolar and submicromolar levels of ATP. The inward current could be evoked by nanomolar ATP at the physiological level (Fig. 4b) and increased as the concentration of ATP was increased (Fig. 4a). However, in the absence of ATP, extracellular K+ challenge could not evoke an apparent inward current in the cochlear supporting cells (Fig. 5). There was no visible inward current evoked by increasing the extracellular concentration of K+ from 5 to 20 mM in the absence of ATP in all ten cells tested (Fig. 5b). Actually, a small outward current is visible at cell depolarization since high extracellular K+ challenge can cause cell depolarizing. Therefore, ATP is required for high K+-induced inward currents in the cochlear supporting cells.
Fig. 4.
Physiological level of ATP evoked inward currents in the cochlear supporting cells. a Inward currents in a Hensen cell evoked by micromolar and submicromolar levels of ATP. b Nanomolar ATP evoked inward current in a Hensen cell
Fig. 5.
Absence of ATP cannot evoke an inward current for high extracellular K+ challenge in the cochlear supporting cells. aI–V relations of a Hensen cell at normal (5 mM) and high (20 mM) extracellular concentrations of K+ in the absence of ATP. Inset Current traces to voltage step stimuli at 5 and 20 mM extracellular concentrations of K+. Rs, 5.97 MΩ. b Response for high (20 mM) extracellular K+ challenge in the absence of ATP. The curve is obtained from the subtraction of the current response at 5 mM [K+]o from that at 20 mM [K+]o and averaged. Inset Current traces obtained after subtraction from the inset in a. There is no inward current visible
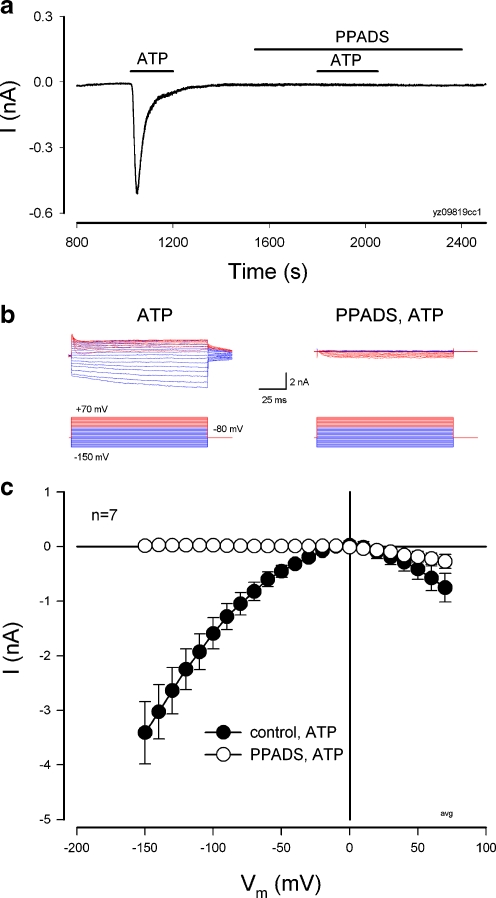
Blockage of inward current by P2X receptor antagonists
The ATP-induced inward current could be blocked by P2X receptor antagonists (Figs. 6 and 7 and Electronic supplementary material (ESM) Fig. S1). Figure 6 shows that pre-application of pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS, 50 µM) inhibited the ATP-evoked current in the cochlear supporting cells. PPADS completely abolished the ATP-evoked inward current at the negative membrane potential (Fig. 6b, c and ESM Fig. S1). The ATP-evoked inward current at the positive membrane potential was reduced but still visible (Fig. 6b, c and Fig. S1), indicating that other mechanisms may also contribute to the production of the inward current at cell depolarization.
Fig. 6.
Inhibition of the ATP-evoked inward current in the cochlear supporting cells by a P2X receptor blocker PPADS. a Current trace in a Hensen cell evoked by ATP before and after perfusion of PPADS. Horizontal bars represent ATP (36 µM) and PPADS (50 µM) perfusion. Pre-application of PPADS inhibited ATP to evoke inward current. The cell was held at −80 mV. b Current traces of a Deiters’ cell for ATP application at voltage step stimulation with and without PPADS (50 µM) treatment. The current traces were subtracted by control current responses. See ESM Fig. S1 for other current traces and I–V curves. cI–V relations for applications of 36 µM ATP before (control) and after treatment of PPADS (50 µM). The data were averaged from the responses of the same supporting cells to ATP (36 µM) before and after application of PPADS (50 µM). PPADS completely inhibited the ATP-evoked inward current at the negative membrane potential
Fig. 7.
Blockage of the ATP-evoked inward current at negative membrane potentials by a P2X7 antagonist, oxidized ATP (oATP). a, b ATP-evoked inward current in a pillar cell. Horizontal bar in a represents the application of 36 µM ATP. Membrane potential was held at−80 mV. Rs, 5.7 MΩ. c, d Inhibition of the ATP inward currents at negative membrane potentials by pretreatment of oATP. The cells were pre-incubated with 0.1 mM oATP for 45 min. Horizontal bar in c represents the application of 36 µM ATP. The cell membrane potential was held at −80 mV. Inset in d shows ATP-evoked current traces after pre-incubation with oATP. Red and blue colors represent the current responses to positive and negative voltage step stimuli, respectively
Oxidized ATP (oATP), which can irreversibly block the P2X7 receptor, also completely blocked the ATP-evoked inward current at the negative membrane potential in the cochlear supporting cells (Fig. 7, four experiments). After the pre-incubation of 0.1 mM of oATP for 45 min, the ATP-evoked inward current at the negative membrane potential was completely blocked (Fig. 7c, d). Similarly, the ATP-evoked inward current retained at cell depolarization (Fig. 7d).
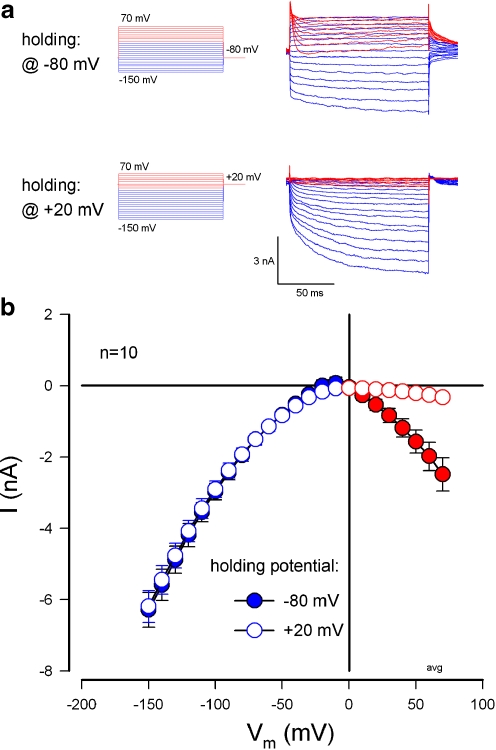
Inhibition of the ATP-evoked inward current at cell depolarization by intracellular Cs+ and positive holding potentials
Positive holding potential and intracellular Cs+ could eliminate the ATP-evoked inward current at cell depolarization, but little affected the inward current at negative membrane potentials (Figs. 8 and 9 and ESM Fig. S2). Figure 8 shows that holding potential of +20 mV eliminated the ATP-evoked inward current at cell depolarization. However, the evoked inward current at the negative membrane potential was not affected (Fig. 8 and ESM Fig. S2).
Fig. 8.
Elimination of ATP-evoked inward currents at cell depolarization by positive holding potential inactivation. a Current traces of a Claudius cell for voltage step stimulation from −150 to +70 mV at holding potentials of −80 and +20 mV. Red and blue colors represent the ATP-evoked current responses to positive and negative voltage step stimuli, respectively. The current traces were submitted by control responses (see ESM Fig. S2). The cell was perfused with 36 µM ATP. bI–V relations evoked by ATP at the holding potentials of −80 and +20 mV in the cochlear supporting cells. The curves were averaged from the responses of the same cells holding at −80 and +20 mV to the 20 mM [K+]o challenge in the presence of 36 µM ATP. Positive holding potential abolished the ATP-evoked inward currents at cell depolarization, but little affected the inward currents at negative membrane potentials
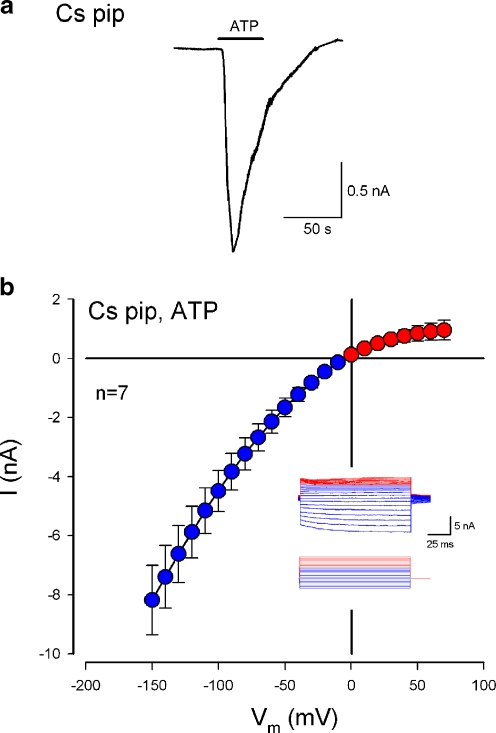
Fig. 9.
Inhibition of the ATP-evoked inward current at positive membrane potentials by intracellular Cs+. The cell was recorded on the whole cell configuration using a Cs pipette, which was filled with the Cs-based intracellular solution that 140 mM K+ was replaced by 140 mM Cs+. a Inward current evoked by ATP (36 µM) with Cs-pipette at a Claudius cell. The cell was held at −40 mV. b ATP-evoked I–V relation in the cochlear supporting cells with the Cs pipette. Inset Subtracted ATP-evoked current trace. Red and blue colors represent the responses to positive and negative voltage stimuli, respectively. See ESM Fig. S3 for other current traces and I–V curves. The ATP-evoked current at cell depolarization was reversed and became outward
Figure 9 shows the ATP-evoked current in the cochlear supporting cells recorded by Cs+ patch pipette. Cs+ can block potassium channels. ATP-evoked inward current at the negative membrane potential was still visible and large in the Cs+ pipette recording (Fig. 9 and ESM Fig. S3). However, the evoked inward current at cell depolarization disappeared (Fig. 9b), revealing the rectification of the P2X receptor conductance.
Discussion
In this study, we found that ATP evoked a K+-dependent inward current in the cochlear supporting cells (Figs. 1, 2, 3, and 4). The evoked inward current increased as the extracellular concentration of K+ was increased (Fig. 3). In the absence of ATP, there was no evoked inward current for extracellular K+ challenge in the cochlear supporting cells (Fig. 5). These data indicate that ATP can induce K+ sinking to recycle K+ in the cochlear supporting cells.
ATP physiologically exists in the cochlear endolymph and perilymph. Under normal physiological conditions, the cochlear endolymph and perilymph contain nanomolar amounts of extracellular ATP [27]. It has been found that cochlear ATP is mainly released from cochlear supporting cells via gap junction hemichannels [24]. In the local area near the cell surface, the ATP concentration would be high and can reach micromolar levels [24, 28]. In this study, we found that the application of submicromolar and nanomolar ATP could evoke inward currents in the cochlear supporting cells (Fig. 4). Moreover, our records show that the evoked inward current increased as the cell became hyperpolarized (Figs. 1, 2, 3, and 4), demonstrating linear relationships to the extracellular concentration of K+ at negative membrane potentials (Fig. 4). The slope increased as cells were hyperpolarized (Fig. 4). Hence, this ATP-mediated K+ sinking may be able to function under normal physiological conditions and play an important role in the cochlea for K+ recycling.
This ATP-evoked inward current was inhibited by P2X receptor antagonists (Figs. 6 and 7 and ESM Fig. S1), indicating that ATP sinks K+ through the activation of the P2X receptors. Multiple expression of P2X isoforms have been identified in the cochlea, including supporting cells [18, 20, 21, 23, 29, 30]. P2X2, P2X4, and P2X7 are the predominant isoforms. These P2X isoforms can form homomeric and heteromeric channels to influx cations when ATP binds to the binding site [15, 31]. The recorded inward current also shows slow desensitization (Figs. 1b, 6a, 7a, and 9a), which is a known characteristic of P2X receptor activity [15]. PPADS and oATP completely inhibited the ATP-evoked inward current in the cochlear supporting cells at negative membrane potentials, but had little effect on the evoked inward current at cell depolarization (Figs. 6 and 7c, d and ESM Fig. S1). This is also consistent with previous reports that inward currents pass more readily than outward currents through the P2X receptors, a characteristic referred to as inward rectification [32, 33].
The ATP-evoked inward current was also visible at cell depolarization (Figs. 1, 2, 3, 4, 6, 7, 8, and 9) and insensitive to PPADS and oATP applications (Figs. 6 and 7 and ESM Fig. S1), implying that other mechanisms also exist beyond the P2X receptor activity. We found that the inward current at cell depolarization was eliminated by deactivation of positive holding potentials (Fig. 8) and could be inhibited by intracellular Cs+ (Fig. 9), suggesting that other K+-dependent channels, such as Ca++-activated K (KCa) channels [34], may be involved. Currently, the detailed mechanism underlying this evoked inward current at cell depolarization remains unclear. Further studies are required.
The evoked inward currents increased as the ATP concentration was increased (Fig. 4). We have reported that cochlear gap junctional hemichannels can release ATP and inositol 1,4,5-trisphosphate (IP3) [24, 35]. Such release increased under mechanical stimulation. Moreover, we have recently reported that a new gap junction gene family Pannexin is extensively expressed in the inner ear [36]. Pannexins mainly assemble functional hemichannels, which can also release ATP [37]. It has also been reported that noise can increase the ATP level in the cochlea [38]. Load sound stimulation increases the potassium level around the hair cells [39]. Such increase in ATP release can in turn enhance K+ sinking in the cochlear supporting cells (Figs. 1, 2, 3, and 4), conferring protection from K+ toxicity. Recently, we have found that current or voltage changes in Deiters’ cells can modify outer hair cell electromotility [40], which is an active cochlear amplifier and can increase auditory sensitivity and frequency selectivity in mammals. Thus, this ATP-mediated K+ sinking mechanism may play an important role in protecting the cochlea from noise damage and also have an implication in hearing regulation.
Cochlear supporting cells are well coupled by gap junctions [5, 41–43]. Dysfunction of gap junctions can induce a high incidence of hearing loss [1]. For a long time, researchers have hypothesized that the inner ear gap junctions mediate K+ transport back to the endolymph [2–5, 7–9]. In this study, we found that ATP can induce K+ sinking in the cochlear supporting cells. This is the first step for K+ transport and provides direct evidence for K+ recycling in the cochlea through supporting cells.
ATP can also activate P2X receptors to mediate cation absorption in the hair cells and outer sulcus cells in the cochlear lateral wall [44, 45] and other signaling events in the cochlea [46]. In previous studies, we have reported that gap junctions and hemichannels in the cochlea can release ATP and IP3 to mediate or control nutrient and energy supplies in the cochlea [24, 26, 35, 47, 48]. In this study, we found that ATP can activate P2X receptors to mediate K+ sinking and recycling in the cochlear supporting cells. Therefore, ATP not only mediates the cochlear nutrition supplies but also plays an important role in the maintenance of the cochlear ionic homeostasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 190 kb)
(DOC 87 kb)
(DOC 68 kb)
Acknowledgments
This work was supported by NIDCD DC 05989.
References
- 1.Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006;209:177–186. doi: 10.1007/s00232-005-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mistrik P, Ashmore J. The role of potassium recirculation in cochlear amplification. Curr Opin Otolaryngol Head Neck Surg. 2009;17:394–399. doi: 10.1097/MOO.0b013e328330366f. [DOI] [PubMed] [Google Scholar]
- 3.Santos-Sacchi J, Dallos P. Intercellular communication in the supporting cells of the organ of Corti. Hear Res. 1983;9:317–326. doi: 10.1016/0378-5955(83)90034-5. [DOI] [PubMed] [Google Scholar]
- 4.Santos-Sacchi J. Isolated supporting cells from the organ of Corti: some whole cell electrical characteristics and estimates of gap junctional conductance. Hear Res. 1991;52:89–98. doi: 10.1016/0378-5955(91)90190-K. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol. 1995;191:101–118. doi: 10.1007/BF00186783. [DOI] [PubMed] [Google Scholar]
- 6.Spicer SS, Schulte BA. Evidence for a medial K+ recycling pathway from inner hair cells. Hear Res. 1998;118:1–12. doi: 10.1016/S0378-5955(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhao HB, Santos-Sacchi J. Effect of membrane tension on gap junctional conductance of supporting cells in Corti’s organ. J Gen Physiol. 1998;112:447–455. doi: 10.1085/jgp.112.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao HB, Santos-Sacchi J. Voltage gating of gap junctions in cochlear supporting cells: evidence for nonhomotypic channels. J Membr Biol. 2000;175:17–24. doi: 10.1007/s002320001051. [DOI] [PubMed] [Google Scholar]
- 9.Zhao HB. Directional rectification of gap junctional voltage gating between dieters cells in the inner ear of guinea pig. Neurosci Lett. 2000;296:105–108. doi: 10.1016/S0304-3940(00)01626-8. [DOI] [PubMed] [Google Scholar]
- 10.Ashmore JF, Ohmori H. Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol. 1990;428:109–131. doi: 10.1113/jphysiol.1990.sp018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa T, Akaike N, Kimitsuki T, Komune S, Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990;63:1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- 12.Dulon D, Mollard P, Aran JM. Extracellular ATP elevates cytosolic Ca2+ in cochlear inner hair cells. NeuroReport. 1991;2:69–72. doi: 10.1097/00001756-199102000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sugasawa M, Erostegui C, Blanchet C, Dulon D. ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol. 1996;491:707–718. doi: 10.1113/jphysiol.1996.sp021251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 16.Housley GD, Greenwood D, Ashmore JF. Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc R Soc Lond B. 1992;249:265–273. doi: 10.1098/rspb.1992.0113. [DOI] [PubMed] [Google Scholar]
- 17.Housley GD, Luo L, Ryan AF. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J Comp Neurol. 1998;393:403–414. doi: 10.1002/(SICI)1096-9861(19980420)393:4<403::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Bobbin RP. P2X receptors in cochlear Deiters’ cells. Br J Pharmacol. 1998;124:337–344. doi: 10.1038/sj.bjp.0701848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarlebark LE, Housley GD, Thorne PR. Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X2 receptor subunits in adult and developing rat cochlea. J Comp Neurol. 2000;421:289–301. doi: 10.1002/(SICI)1096-9861(20000605)421:3<289::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Jarlebark L, Housley GD, Raybould NP, Vlajkovic S, Thorne PR. ATP-gated ion channels assembled from P2X2 receptor subunits in the mouse cochlea. NeuroReport. 2002;13:1979–1984. doi: 10.1097/00001756-200210280-00030. [DOI] [PubMed] [Google Scholar]
- 22.Parker MS, Nkeiruka NO, Bobbin RP. Localization of the P2Y4 receptor in the guinea pig organ of Corti. J Am Acad Audiol. 2003;14:286–295. [PubMed] [Google Scholar]
- 23.Szucs A, Szappanos H, Toth A, Farkas Z, Panyi G, Csernoch L, Sziklai I. Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig. Hear Res. 2004;196:2–7. doi: 10.1016/j.heares.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu N, Zhao HB. ATP activates P2x receptors and requires extracellular Ca++ participation to modify outer hair cell nonlinear capacitance. Pflugers Arch. 2008;457:453–461. doi: 10.1007/s00424-008-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao HB. Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signaling and metabolic communications. Eur J Neurosci. 2005;21:1859–1868. doi: 10.1111/j.1460-9568.2005.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz DJ, Thorne PR, Housley GD, Billett TE. Adenosine 5′-triphosphate (ATP) concentrations in the endolymph and perilymph of the guinea-pig cochlea. Hear Res. 1995;90:119–125. doi: 10.1016/0378-5955(95)00153-5. [DOI] [PubMed] [Google Scholar]
- 28.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolic P, Housley GD, Thorne PR. Expression of the P2X7 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the developing and adult rat cochlea. Audiol Neurootol. 2003;8:28–37. doi: 10.1159/000067891. [DOI] [PubMed] [Google Scholar]
- 30.Ji N, Zhao HB (2005) Expressions of ATP-gated purinergic (P2) receptors in the cochlear outer hair cells. The 28th Association for Research in Otolaryngology Annual Meeting, New Orleans, LA, 19–24 February. Available at http://www.aro.org
- 31.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z, Hume RI. Two mechanisms for inward rectification of current flow through the purinoceptor P2X2 class of ATP-gated channels. J Physiol. 1998;507:353–364. doi: 10.1111/j.1469-7793.1998.353bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara Y, Keceli B, Nakajo K, Kubo Y. Voltage- and [ATP]-dependent gating of the P2X(2) ATP receptor channel. J Gen Physiol. 2009;133:93–109. doi: 10.1085/jgp.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raybould NP, Jagger DJ, Housley GD. Positional analysis of guinea pig inner hair cell membrane conductances: implications for regulation of the membrane filter. J Assoc Res Otolaryngol. 2001;2:362–376. doi: 10.1007/s101620010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gossman DG, Zhao HB. Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: a novel mechanism of IP3 intercellular signaling. Cell Commun Adhes. 2008;15:305–315. doi: 10.1080/15419060802357217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang XH, Streeter M, Liu YP, Zhao HB. Identification and characterization of pannexin expression in the mammalian cochlea. J Comp Neurol. 2009;512:336–546. doi: 10.1002/cne.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shestopalov VI, Panchin Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci. 2008;65:376–394. doi: 10.1007/s00018-007-7200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone BM, Patuzzi R, Syka J, Syková E. Stimulus-related potassium changes in the organ of Corti of guinea-pig. J Physiol. 1989;408:77–92. doi: 10.1113/jphysiol.1989.sp017448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu N, Zhao HB. Modulation of outer hair cell electromotility by cochlear supporting cells and gap junctions. PLoS ONE. 2009;4:e7923. doi: 10.1371/journal.pone.0007923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 42.Zhao HB, Yu N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J Comp Neurol. 2006;499:506–518. doi: 10.1002/cne.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu YP, Zhao HB. Cellular characterization of Connexin26 and Connnexin30 expression in the cochlear lateral wall. Cell Tissue Res. 2008;333:395–403. doi: 10.1007/s00441-008-0641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Housley GD, Raybould NP, Thorne PR. Fluorescence imaging of Na+ influx via P2X receptors in cochlear hair cells. Hear Res. 1998;119:1–13. doi: 10.1016/S0378-5955(97)00206-2. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci. 2001;21:9168–9174. doi: 10.1523/JNEUROSCI.21-23-09168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32:128–141. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhao HB (2003) Biophysical properties and functional analysis of inner ear gap junctions for deafness mechanisms of nonsyndromic hearing loss. Proceedings of the 9th International Meeting on Gap Junctions, Cambridge, UK, August 23–28
- 48.Zhao HB (2005) What is the function of connexin 26 in the cochlea? Potassium recycling or intercellular signaling and nutrient/energy supplies? In: Lim DJ (ed) Meniere’s disease and inner ear homeostasis disorders. Proceedings of the 5th International Symposium. April, 2005, Los Angeles, CA, pp 254–255
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 190 kb)
(DOC 87 kb)
(DOC 68 kb)