Abstract
The normal intervertebral disc (IVD) is a poorly innervated organ supplied only by sensory (mainly nociceptive) and postganglionic sympathetic (vasomotor efferents) nerve fibers. Interestingly, upon degeneration, the IVD becomes densely innervated even in regions that in normal conditions lack innervation. This increased innervation has been associated with pain of IVD origin. The mechanisms responsible for nerve growth and hyperinnervation of pathological IVDs have not been fully elucidated. Among the molecules that are presumably involved in this process are some members of the family of neurotrophins (NTs), which are known to have both neurotrophic and neurotropic properties and regulate the density and distribution of nerve fibers in peripheral tissues. NTs and their receptors are expressed in healthy IVDs but much higher levels have been observed in pathological IVDs, thus suggesting a correlation between levels of expression of NTs and density of innervation in IVDs. In addition, NTs also play a role in inflammatory responses and pain transmission by increasing the expression of pain-related peptides and modulating synapses of nociceptive neurons at the spinal cord. This article reviews current knowledge about the innervation of IVDs, NTs and NT receptors, expression of NTs and their receptors in IVDs as well as in the sensory neurons innervating the IVDs, the proinflammatory role of NTs, NTs as nociception regulators, and the potential network of discogenic pain involving NTs.
Keywords: intervertebral disc, nerve fibers, neurotrophins, pain
Introduction
The intervertebral disc (IVD) of vertebrates, including humans, is the main joint between two consecutive vertebrae in the vertebral column. Each IVD consists of three different structures: (i) a gelatinous core called the nucleus pulposus (NP); (ii) an outer ring of fibrous tissue named the annulus fibrosus (AF) that surrounds the NP; and (iii) two endplates of hyaline cartilage that cover the upper and lower surface of both the AF and NP, and serve as an interface between the disc and the vertebrae (for a review see Roberts et al. 2006; Raj, 2008). The cells that form the AF, particularly in the outer region, are fibroblast-like and arranged parallel to the collagen fibers, whereas those in the inner AF are chondrocyte-like. The NP contains collagen fibers that are randomly distributed and elastin fibers that are radially organized embedded in a highly hydrated aggrecan-containing gel. There are a few chondrocyte-like cells in the NP.
The normal IVD is considered as an organ that is poorly innervated supplied only by sensory and sympathetic perivascular nerve fibers. Most of the studies performed in different animal species, including human, have demonstrated that nerve fibers in IVDs are found mostly in the periphery of the AF (see for references Edgar, 2007; Raj, 2008). The sensory fibers that innervate the IVD are mainly nociceptive and, to a lesser extent, proprioceptive. The postganglionic sympathetic fibers are considered vasomotor efferents (see Aoki et al. 2004a,b;). Nevertheless, in human degenerated IVDs, as well as in animal models of IVD degeneration, the number of nerve fibers in the IVD increases (see Johnson et al. 2007); what is associated with pain of IVD origin, however, and the mechanisms responsible for nerve growth and hyperinnervation of the lumbar IVD have not been fully elucidated.
Among the molecules that are presumably involved in IVD hyperinnervation are growth factors that are members of the neurotrophin (NT) family and that are known to be neurotrophic and neurotropic factors (Levi-Montalcini, 1987; Barbacid, 1994, 1995; Huang & Reichardt, 2001). It has been proposed that the degenerating IVD can synthesize and release ‘neurogenic’ factors that attract nerve fibers to it (see for references Colombini et al. 2008; Hadjipavlou et al. 2008) and, as the availability of NTs regulates the density and distribution of sensory and sympathetic nerve fibers in peripheral tissues, NTs are good candidates as mediators in this pathogenic mechanism. However, although most of the currently available evidence supports a positive correlation between increased innervation, discogenic pain and NTs, it remains unclear whether nerve ingrowth is a consequence of the action of neurotrophic/neurotropic factors in the IVD or a side effect of the normal wound-healing events in injured IVDs (Olmarker, 2005).
In recent years different components of the NT system, both NTs and their signaling receptors (NTRs), have been found in healthy IVDs in innervated regions and in regions lacking sensory or sympathetic innervation (Gruber et al. 2008; Purmessur et al. 2008). Furthermore, increased levels of NTs and NTRs were observed in IVDs with symptoms of degeneration and in IVDs from patients suffering discogenic pain (Freemont et al. 2002b; Purmessur et al. 2008). These data strongly suggest that, as occurs in other tissues, there is a positive correlation between levels of expression of NTs and density of innervation in the IVD (Yamauchi et al. 2007).
In addition to their role during the development of the nervous system, NTs also play a role in inflammatory responses and in pain transmission. In fact, NTs, and in particular nerve growth factor (NGF), can act as cytokines (Levi-Montalcini et al. 1996) influencing development, differentiation, chemotaxis and mediator release of inflammatory cells, as well as fibroblast activation, through complex networks that involve other proinflammatory cytokines (Bonini et al. 2003). Furthermore, NTs are known to play key roles in immunocompetent cells by participating in inflammatory responses (Tessarollo, 1998; Sariola, 2001; Vega et al. 2003, 2004) and in sensing pain, as NT signaling increases expression of pain-related peptides, which are modulators of the synapses of the spinal cord pain pathways (Bennet, 2001; Pezet & McMahon, 2006; Merighi et al. 2008).
From the data above four main questions arise. First, is there a correlation between NT levels in the IVD and the density and distribution of sensory nerves? Second, are the resident cells in the IVD a source of NTs and why are these molecules expressed in non-innervated tissues like the IVD? Third, do NTs have a non-nervous role regulating inflammatory processes or as pain modulators in the degenerating IVD? Fourth, what is the role of NTs in the pathogenesis of discogenic pain?
What is discogenic pain? The vertebral column is a complex group of nerves, joints, muscles, tendons and ligaments, all which are capable of causing pain. However, a tissue can generate pain only if it is innervated. Thus, we consider discogenic pain as the pain originating from chemically or mechanically damaged IVDs due to irritation of the nerves innervating the IVDs. It can be distinguished from other types of pain, which usually originate from muscles, bones, vertebral joints other than IVDs or other structures in the spine. A damaged IVD releases nociceptive molecules and growth factors that promote nerve ingrowth into the disc (see Hurri & Karppinen, 2004; Takahashi et al. 2008; Freemont, 2009). This view is supported by findings that injections of local anesthetics in damaged IVDs eliminate acute pain (Hyodo et al. 2005).
Sensory innervation of intervertebral discs
Origin of the intervertebral disc nerves
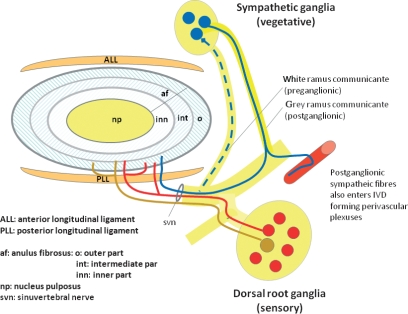
Although the IVD is usually referred to as aneural, nerve fibers have been observed within it, often accompanying blood vessels but also independently. The IVD is innervated by branches of the sinuvertebral nerve, by nerves derived from the ventral rami of spinal nerves or by nerves derived from gray rami communicantes (see for references Takahasi et al. 2009). Furthermore, IVDs also receive innervation from two dense nerve interconnected plexuses located in the anterior and posterior common vertebral ligaments (Bogduk, 1983; Groen et al. 1990; Suseki et al. 1998) (Fig. 1).
Fig. 1.
Schematic representation of the innervation of the intervertebral disc (IVD). Nociceptive sensory fibers originate in the dorsal root ganglia (DRGs) (red) and postganglionic sensory nerve fibers (blue) enter the outer part of the annulus fibrosus. Nerves in the IVD arise from the sinuvertebral nerve, from spinal nerves or from grey rami communicantes. In addition, mechanical nerve fibers originate in the DRGs and coming from the anterior and posterior longitudinal ligament innervate the external layers of the annulus fibrosus of the IVD.
Although the innervation of the IVD has been extensively studied, the impossibility of performing experiments in humans limits research and most of the available data have been obtained from experimentation performed in rats. The origin and paths of sensory nerves innervating the IVD have been established using the retrograde fluorogold tracing method.
It is accepted that the lumbar IVDs, which are the main source of discogenic pain in humans, are innervated segmentarily (see Aoki et al. 2004a,b;). However, the ventral segments of the rat lower lumbar IVDs are innervated by upper (L1–L2) dorsal root ganglion (DRG) neurons (Takahashi et al. 1993, 1996; Morinaga et al. 1996; Sameda et al. 2003) and the nerve fibers innervating the posterolateral portion of the IVD (the region of the IVD in which lesions occur most frequently in humans) come from the upper and lower DRG (T13–L6) but are mainly localized in L1–L2 DRGs (Ohtori et al. 1999; Aoki et al. 2004a). Nerve fibers reach the IVD through the sinuvertebral nerves or from branches of the paravertebral sympathetic trunks (Nakamura et al. 1996b; Suseki et al. 1998; Takahashi et al. 1998, 2003; Ohtori et al. 1999, 2001a,b).
Extrapolation of these data from rat to human must be performed with caution; however, the observations made in rats could explain why the blockade of some nerves is effective in abolishing discogenic pain (Connally & Sanders, 1991; Nakamura et al. 1996a).
Characterization of nerve fibers innervating healthy intervertebral discs
The pioneer descriptions of the IVD innervation using silver impregnation methods reported that nerves are limited to the more external lamellae of the AF and that intradiscal nerve fibers are rare (Roofe, 1940; Ehrenhaft, 1943; Wiberg, 1947; Pederson et al. 1956). These observations were made on normal IVDs from a large series of animal species, including humans (Fig. 2, top). In the last decades, using specific methods to detect neuronal markers or specific peptides expressed by nociceptive and sympathetic nerve fibers, the pattern of innervation of IVDs was confirmed when free nerve fibers that express protein gene product 9.5, neurofilament (NFP) 68-kDa protein, growth axonic protein 43, substance P (SP) or calcitonin gene-related peptide (CGRP) were detected in the AF (see for references Ohtori et al. 2002; Aoki et al. 2005; Takahasi et al. 2009).
Fig. 2.
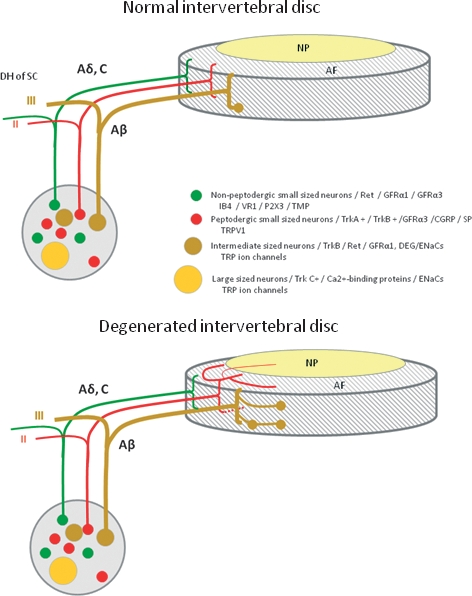
Schematic representation of the innervation of normal (top) and degenerated (bottom) intervertebral discs (IVDs), as well as the origin of sensory nerve fibers that innervate them. In the normal IVD, innervation is restricted to the outer layers of the annulus fibrosus (AF) and consists of small nerve fibers (red and green) and some large fibers forming mechanoreceptors (brown). In the degenerated IVD, nerve fibers are increased in number and they enter the inner layers of the AF and even the nucleus pulposus (NP). Furthermore, in these conditions, the density of mechanoreceptors in the superficial layers of IVDs is increased. Dorsal root ganglia (DRGs) contain different types of sensory neurons that project to the IVD and to the dorsal horn of the spinal cord (DH of SC). Thin myelinated Aδ fibers and unmyelinated C fibers arise from small neurons (red and green), which, in the spinal cord, synapse in lamina I and II and mediate nociception. The myelinated Aβ fibers (brown) arise from intermediate neurons; at the periphery they form slowly and rapidly adapting low-threshold mechanoreceptors, and synapse in lamina III and IV in the dorsal horn of the spinal cord; they mediate sensations of touch, pressure and vibration. Most of the sensory nerve fibers innervating the IVD are Aδ or C fibers. They originate from small peptidergic neurons expressing TrkA/TrkB (the receptor for nerve growth factor/brain-derived neurotrophic factor; red) or non-peptidergic neurons expressing the common signaling receptor for glial cell-derived neurotrophic factor family of neurotrophic factors (Ret) (red). Neurons in DRGs can be differentiated based on their pattern of expression of receptors for neurotrophic factors, pattern of expression of different ion channels primarily of the degenerin/epithelial sodium channels (DEG/ENaCs) (ENaCα, β and γ; acid-sensing ion channel (ASIC)1, ASIC2 and ASIC3) and transient receptor potential (TRP) (TRPA1, TRPC1, TRPC6 and TRPV1-4) families, and peptide content. CGRP, calcitonin gene-related peptide; GFRα1 and GFRα3, glial cell-line-derived neurotrophic receptor subtypes α1 and α3; P2X3, ATP-gated ion channel subtype P2X3; SP, substance P; TMP, thiamine monophosphatase; VR1, vanilloid receptor subtype 1.
In the human normal IVD, protein gene product 9.5-positive nerve fibers, either associated with blood vessels or distant from them, innervate the outer layers of the AF (Coppes et al. 1990; Konttinen et al. 1990; Ashton et al. 1994; Roberts et al. 1995; Brown et al. 1997; Palmgren et al. 1999; Willenegger et al. 2005). These fibers are also positive for acetylcholinesterase, NFP, SP, CGRP, vasoactive intestinal polypeptide (VIP), neuropeptide Y, C-flanking peptide and synaptophysin (Ashton et al. 1994; Coppes et al. 1997; Palmgren et al. 1999). The nerves entering the rat IVD have an identical expression pattern (Weistein et al. 1988; McCarthy et al. 1991, 1992; McCarthy, 1993).
In addition to nociceptive nerve fibers, human IVDs contain mechanoreceptors in the outer two to three lamellae of the AF. Their morphology resembles Pacinian corpuscles, Ruffini endings and, most frequently, Golgi tendon organs (Roberts et al. 1995).
Sensory fibers in the IVD arise from neurons localized in DRGs. The vertebrate DRGs contain heterogeneous neuronal populations, which can be classified according to the neuronal size, ultrastructural features (large pale, small dark and intermediate), neuropeptide and neurotransmitter content, cytoskeleton composition, cytosolic proteins, ion channel expression or growth factor dependence (see for references Montaño et al. 2009). Each type of DRG neuron has different endings in both the spinal cord and in the target organs and transmits information of different sensory modalities to the central nervous system (Perl, 1992). It is currently accepted that the largest DRG neurons are proprioceptive and innervate sensory organs in muscle and joints; the neurons in the mid-size range innervate peripheral mechanoreceptors; and the small-diameter neurons are related to different types of nociceptors (Lawson, 1992; Perl, 1992; Koltzenburg et al. 1997).
The IVD is primarily innervated by small DRG neurons, which are classified based on their stimulus–response function and also based on their neurochemistry and connectivity (Lawson, 1992). In fact, small DRG neurons are classified into peptidergic NGF-dependent and non-peptidergic glial cell-line derived neurotrophic factor (GDNF)-dependent neurons. They consistently express the high-affinity NGF and brain-derived neurotrophic factor (BDNF) receptors TrkA and TrkB or the GDNF receptor Ret, respectively (Silverman & Kruger, 1990; Snider & McMahon, 1998; Salio et al. 2005; Merighi et al. 2008; see also Ernsberger, 2009; Valdes-Sanchez et al. 2010). The characteristics of these two types of neurons are summarized in Fig. 2 (Averill et al. 1995; Molliver et al. 1997; Bennett et al. 1998). The NGF-dependent population represents roughly 40% of DRG neurons, whereas the GDNF-dependent population represents roughly 30%; the percentage of BDNF-dependent neurons has not yet been established (Silverman & Kruger, 1990; Averill et al. 1995; Valdes-Sanchez et al. 2010). There are also electrophysiological differences between these two types of neurons that demonstrate that they are functionally different (Stucky & Lewin, 1999) and, whereas NGF-dependent neurons are critical to hyperalgesic responses induced by inflammation (Woolf et al. 1994; Mantyh et al. 1997; Koltzenburg et al. 1999), the GDNF-dependent neurons are important in neuropathic pain (Malmberg et al. 1997). The nociceptive DRG neurons form free nerve endings in the tissues that are their targets of innervation and project to lamina I and the outer part of lamina II (Perl, 1992; see for a recent review Merighi et al. 2008).
Both types of small DRG neurons innervate the IVD (Fig. 2, top). Most of them are small, NGF-dependent nociceptive neurons. Thus, they are positive for SP and CGRP, the vanilloid receptor 1 (TRPV1) and P2X(3), all of which are proteins associated with sensing pain, and express the high-affinity (TrkA) and low-affinity (p75NTR) receptors for NGF. In contrast, the percentage of Ret-positive/TrkA-negative/NGF-independent neurons innervating the IVD is very low (Fig. 2, top).
A remarkable finding in the field of the sensory innervation of IVDs was the demonstration that in normal conditions some NGF-dependent DRG neurons express BDNF (8–24%; Ohtori et al. 2003) as this protein participates in nociceptive transmission (see Merighi et al. 2008).
Nerve supply and characterization of nerve fibers innervating degenerating and painful intervertebral discs
In human and animal models of degenerating IVDs, especially in painful IVDs, it has been observed that innervation is increased and that the nociceptive nerve fibers grow into what are usually aneural inner parts of the AF and even into the NP, sometimes together with blood vessels (Fig. 2, bottom). The immunohistochemical profile of nerve fibers and neurons innervating the pathological IVDs is identical to that reported in normal conditions; thus, the differences in the pattern of innervation of the IVD in normal conditions in comparison with degenerated conditions are quantitative rather than qualitative (Coppes et al. 1990; Ashton et al. 1994; Brown et al. 1997; Freemont et al., 1997; Johnson et al. 2002; Melrose et al. 2002; see Takahasi et al. 2009). Interestingly, there is increased expression of NGF in parallel with the hyperinnervation of the IVD (Kokubo et al. 2008).
However, Roberts et al. (1995) and Coppens et al. (1997) observed in degenerated IVDs an increase in the number of Golgi-tendon organ-like structures such as Ruffini’s and Pacinian corpuscles.
In addition to the sensory nerve fibers, there is growing evidence that sympathetic afferents are also increased in degenerating IVDs and that they play a significant role in lower back pain (see for a review Takebayashi et al. 2006).
Neurotrophins and their receptors
The family of NTs consists of four members: NGF, BDNF, NT-3 and NT-4/5 (Barbacid, 1994, 1995; Lewin & Barde, 1996). Except for NT-4/5, which is absent in avian species, the NT sequences are highly conserved phylogenetically in vertebrates (Hallböök et al. 2006). Two additional NTs named NT-6 (Götz et al. 1994) and NT-7 (Lai et al. 1998; Nilsson et al. 1998), both with NGF-like activity, have been isolated and cloned in fish.
The NTs are synthesized as immature precursors that are proteolytically cleaved inside the cell to release C-terminal mature products (Teng & Hempstead, 2004). Nevertheless, peptides generated from pro-NGF and pro-NT-3 also seem to be active as they are able to bind to and activate NT receptors, especially a complex of p75NTR and sortilin (Barrett, 2000; Lee et al. 2001; Chao & Bothwell, 2002; Dicou, 2006, 2007, 2008). NGF and NT-3 are released through a constitutive secretory pathway, whereas BDNF is secreted through regulated pathways (see Hempstead, 2006; Schweigreiter, 2006).
The NTs activate two different types of receptors, the Trk family of receptor tyrosine kinase and p75NTR, a member of the tumor necrosis factor (TNF) receptor super-family, which serves as a pan-NT receptor (Lu et al. 2005; Reichardt, 2006). The Trk receptors are a family of transmembrane glycoproteins, which includes three members, TrkA, TrkB and TrkC (Fig. 3). They have similar organization: an intracellular tyrosine kinase domain, a short transmembrane sequence and an extracellular region containing a signal peptide, two cysteine-rich domains, a cluster of three leucine-rich motifs and two Ig-like domains. The full-length TrkA, TrkB and TrkC have estimated molecular weights of 140, 145 and 145 kDa, respectively. Each Trk preferentially binds a single NT. TrkA is the receptor for NGF, both BDNF and NT-4/5 bind to TrkB, and NT-3 is the ligand for TrkC (Lewin & Barde, 1996; Chao, 2003; Huang & Reichardt, 2003; Segal, 2003; Reichardt, 2006). In addition to the three full-length Trk proteins, all three trk genes originate other protein isoforms by alternative splicing; they have variations both in the extracellular domain (TrkA) and in the intracellular domain (TrkB and TrkC) (Vega et al. 2004; Tacconelli et al. 2005; Reichardt, 2006).
Fig. 3.
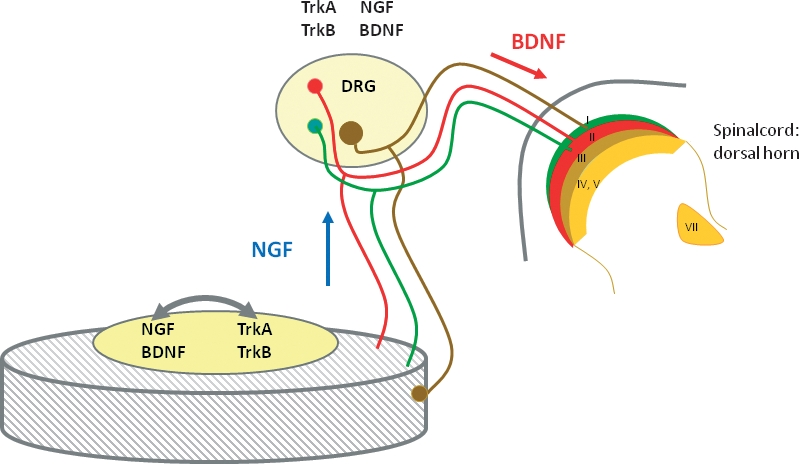
The cells of the intervertebral disc (IVD) can be both a source and a target for neurotrophins (NTs). According to the classic neurotrophic theory, NTs produced in the IVD, especially nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), can be retrogradely transported to the soma of sensory neurons (blue diamond) where they potentiate nerve growth and expression of specific neurotransmitters like substance P or calcitonin gene-related peptide, as well as other molecules related to nociception. Interestingly, the dorsal root ganglion (DRG) neurons innervating IVDs express receptors for these NTs. However, DRG neurons that innervate the IVD also produce NTs, which can be transported anterogradely to the IVD (especially NGF) and interact with NTRs expressed in the nucleus pulposus cells stimulating the production of several molecules. Furthermore, they can reach the dorsal horn of the spinal cord (especially BDNF; red diamond) to modulate synaptic transmission. Finally, expression of both NTs and NTRs in cells of the IVD strongly suggests autocrinia and/or paracrinia.
The low-affinity receptor p75NTR is a member of the TNF receptor super-family that binds all NTs with similar affinity but different kinetics. The p75NTR gene generates two isoforms, full-length and short-p75NTR (von Schack et al. 2001). Structurally, p75NTR is a transmembrane glycoprotein that lacks enzymatic activity but has a death domain sequence (Dechant & Barde, 2002; Roux & Barker, 2002) (Fig. 4). The short isoform of p75NTR lacks NT-binding activity but retains the ability to interact with Trk receptors (Roux & Barker, 2002; Nykjaer et al. 2005). Finally, there is evidence that sortilin and the Nogo receptor complex interact with precursor forms of NGF and BDNF (Nykjaer et al. 2004; Jansen et al. 2007).
Fig. 4.
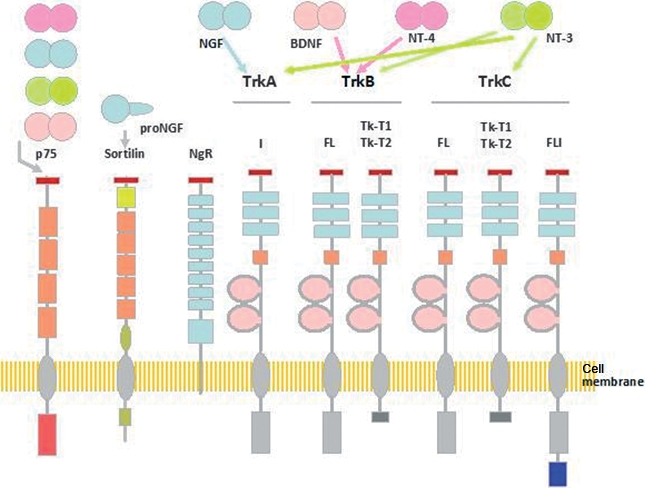
Schematic representation of the structure of p75NTR, sortilin, Nogo receptor (NgR) and Trk receptors, and of the neurotrophins (NTs) and pro-NTs that bind each of them. Arrows represent NT binding possibilities to neurotrophin receptors (NTRs). Five different isoforms of TrkA have been isolated, some of them representing tissue-specific isoforms. Isoforms of TrkB and TrkC include full-length, tyrosine kinase truncated (TK-T1 and TK-T2) and tyrosine kinase inserted isoforms. The low-affinity pan-NT receptor p75NTR has two isoforms, the full-length and the short p75NTR. Sortilin and the NgR complex interact with p75NTR in mediating some actions of NTs. BDNF, brain-derived neurotrophic factor; DRG, dorsal root ganglion; NGF, nerve growth factor.
Upon binding to membrane receptors, NTs initiate a variety of signals that lead to regulation of cell proliferation, differentiation and survival (Segal, 2003; Reichardt, 2006; Skaper, 2008). Trk and p75NTR initiate different signals depending on whether they are expressed alone or co-expressed. NTs acting through Trks promote survival via phosphorylation and inactivation of several proapoptotic molecules, including Bad, and promote differentiation via activation of the Ras/Raf/extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase cascade (Segal, 2003). Binding to p75NTR alone, NTs are capable of inducing apoptosis or promote survival depending on the intracellular adaptor molecules available (Yaar et al. 2002; Teng & Hempstead, 2004) or acting through the NF-κB pathway (Roux & Barker, 2002). Co-expression of Trks and p75NTR increases high-affinity NT binding, enhances Trk ability to discriminate a preferred ligand from the other NTs and promotes the survival effects of the NTs (Dechant & Barde, 2002; Roux & Barker, 2002; Teng & Hempstead, 2004). Finally, pro-NTs interacting with the p75NTR-sortilin complex induce apoptotic death (reviewed in Nykjaer et al. 2005) and, in the case of co-expression of p75 NTR with the Nogo receptor complex, Nogo induces growth inhibition (reviewed in Teng & Hempstead, 2004).
The intervertebral disc cells as a source of and a target for neurotrophins in health and disease
Extrapolating the neurotrophic theory to IVD innervation, and therefore assuming that the normal pattern of innervation of a tissue is related to the basal levels of NTs, a source of NTs must exist in normal and pathological IVDs. Originally, it was accepted that NTs are produced by the tissues that are targets of NT-dependent neurons and the NTs are transported retrogradely to the neuron body where they support neuronal survival (Levi-Montalcini, 1987; Delcroix et al. 2003; Zweifel et al. 2005). Subsequently it was discovered that signaling by NTs may include anterograde trophic signaling, local trophic interactions and autocrine signaling (see Schecterson & Bothwell, 1992; Acheson et al. 1995; Bothwell, 1995; Robinson et al. 1996). Sensory neurons express NTs (especially NGF and BDNF; Wright & Snider, 1995). BDNF can be anterogradely transported to the peripheral target organs or to the dorsal horn of the spinal cord, thus affecting their central and peripheral targets (Fig. 4) (Salio et al. 2005, 2007; see for a review Obata & Noguchi, 2006) but there is not convincing evidence for anterograde transport of other NTs.
Neurotrophins and neurotrophin receptors in normal intervertebral discs (Fig. 4, top)
NGF, which supports the survival of at least a subpopulation of sensory neurons innervating IVDs, is expressed by cells in the NP and AF, at both mRNA and protein levels, although the constitutive expression is low in the NP and not detectable in the AF cells in vitro (Abe et al. 2007). Furthermore, using immunohistochemistry and in-situ hybridization, both NGF and their receptors, TrkA and p75NTR, can be detected in the AF cells (Gigante et al. 2003) and TrkA has been found in AF and NP cells (Abe et al. 2007; Sugiura et al. 2008). In humans, NGF and TrkA are expressed by cells of the normal IVD in all regions (Purmessur et al. 2008). Nevertheless, Freemont et al. (2002a,b); never detected NGF, TrkA or p75NTR in normal or degenerated IVDs.
Both BDNF and TrkB are expressed in healthy IVD cells. The percentage of positive cells is significantly higher in the outer AF compared with either the inner AF or the NP. Interestingly, BDNF expression in the NP cells is upregulated under conditions of increased osmolarity (Boyd et al. 2005; Gruber et al. 2008).
Thus, NTs synthesized in the IVD can be transported retrogradely to the DRG sensory neuron bodies where they can regulate the expression of some neuropeptides such as SP or CGRP. Furthermore, it is possible to speculate that NTs other than BDNF could be transported anterogradely from the DRG to the IVD and activate NTRs in the AF and NP cells. When NTs produced in DRG neurons are anterogradely transported to the superficial layers of the dorsal horn of the spinal cord they can also influence synaptic transmission at this level (Fig. 5). Finally, the expression of both NTs and NTRs in the AF and NP cells suggests, in addition to the neurotrophic function, an autocrine or paracrine role for these molecules in regulating the biology of the IVD.
Fig. 5.
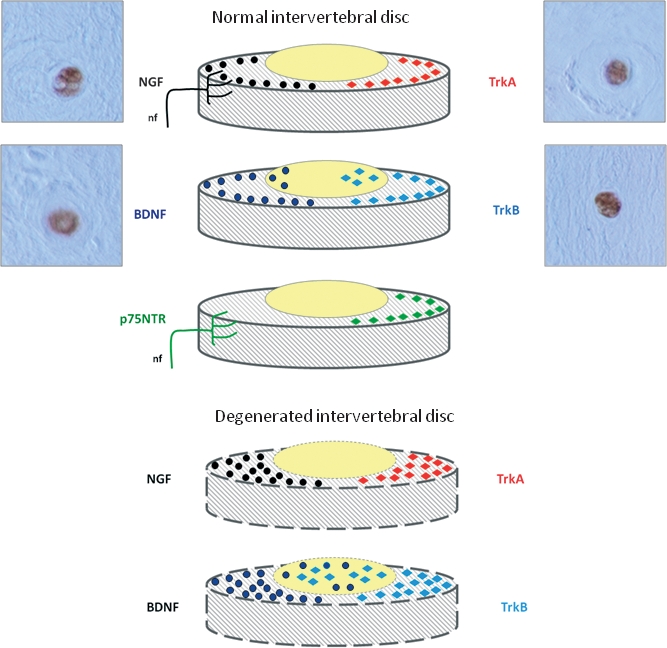
Detectable levels of both neurotrophins (NTs) (left) and NTRs (right) can be found in healthy intervertebral discs (IVDs) (top). Nerve growth factor (NGF) has been detected in the outer layers of the annulus fibrosus (AF) as well as in nerve fibers reaching it; a similar pattern of distribution in the AF has also been reported for TrkA. Cultured nucleus pulposus (NP) cells also express NGF and TrkA. The more external layers of the AF express brain-derived neurotrophic factor (BDNF) and TrkB, and BDNF is also present in NP cells. p75NTR was present in the superficial layers of the AF as well as in the nerve fibers supplying the IVD. Degeneration of the IVD (bottom) occurs in parallel with increased levels of expression of NTs and NTRs. NGF and TrkA are overexpressed and detected in the AF; expression of BDNF and TrkB is also increased in both the AF and NP. Micrographs are examples of the immunohistochemical detection of NGF, BDNF, TrkA and TrkB in cells from herniated (not degenerated) human IVDs. Positive immunostaining was also found for NT-3 (data not shown), whereas results of immunolabeling for TrkC and p75NTR were always negative. FL, full-length; nf, nerve fibres; NgR, Nogo receptor.
Neurotrophins and neurotrophin receptors in degenerated and painful intervertebral discs (Fig. 4, bottom)
In contrast with the lack of expression of any component of the NT system (Freemont et al. 2002a,b;) or expression at basal levels in the normal IVD (Gigante et al. 2003; Abe et al. 2007; Gruber et al. 2007; Purmessur et al. 2008), there is general consensus that NGF, TrkA and p75NTR are always expressed at increased levels in painful IVDs (Freemont et al. 2002a,b;). NGF, BDNF, TrkA and TrkB are expressed by IVD cells at every stage of degeneration, although only BDNF is significantly increased in parallel with the degree of degeneration (Purmessur et al. 2008). In-vitro BDNF gene expression in cultured AF cells has a significant positive correlation with IVD degeneration (Gruber et al. 2008). Recently, Yamauchi et al. (2009) observed that NGF from degenerative NP promoted axonal growth and induced expression of SP in cultures of DRG.
In addition to the IVD, in pathological conditions, additional sources of NTs are the inflammatory cells. NGF is produced, secreted and released from eosinophils, mast cells, lymphocytes and synovial fibroblasts, chondrocytes and macrophages (Bonini et al. 2003; Vega et al. 2003). A recent study reported that herniated discs contain granulation tissue, newly developed blood vessels and massive infiltration of CD68-positive macrophages, which are an important source of NTs (Kokubo et al. 2008). In other degenerating joints, Barthel et al. (2009) have found that synovial cells and CD14+ cells (macrophages) from patients with rheumatoid arthritis and spondyloarthrosis express NGF and BDNF mRNAs.
Cytokines, neurotrophins and pain: a complex network in intervertebral discs
The importance of NGF and TrkA in nociception has been highlighted by observations in mice lacking expression of these molecules (see Fariñas, 1999) and humans suffering congenital insensitivity to pain with anhydrosis, which is caused by TrkA mutations (Indo et al. 1996). In both mouse and human there is unresponsiveness to painful stimuli. In addition to the role of NGF-TrkA in the development of nociceptors, NGF continues to play a major role in pain processing after the primary nociceptive DRG neurons lose their absolute dependence on NGF in the postnatal period (Pezet & McMahon, 2006).
NGF is a peripheral pain mediator in different conditions. Inflammation occurs simultaneously with the upregulation and release of inflammatory cytokines and peptides, including NGF, by the inflamed tissues (Woolf et al. 1994; O’Connor et al. 2004) and expression of TrkA in DRG neurons (Pezet et al. 2001). In addition to these direct actions, NGF also modulates pain by altering the effectiveness of the central nociceptor signals through BDNF. Thus, to understand the role of NTs in discogenic pain, four main aspects may be considered: (i) the proinflammatory cytokines as regulators of NGF synthesis; (ii) the role of NTs, especially NGF, in regulating nerve ingrowth in IVDs in inflammation or degenerating pathologies; (iii) the regulation of the synthesis of pain-related peptides (SP and CGRP) and pain neuromodulators (BDNF) by NGF, and the anterograde transport and release of pain-related molecules from DRGs to inflamed tissues; and (iv) the role of SP, CGRP and BDNF in the transmission of pain in the dorsal horn of the spinal cord. Thus, the neurotrophic and neurotropic activities, as well as the sensitizing effects of NGF, lead us to believe that NGF expressed during IVD degeneration contributes to both the nerve ingrowth into the IVD and the changes in gene expression of DRG neurons.
Proinflammatory cytokines regulate the expression and synthesis of NGF
The NGF levels of expression increase in a variety of inflammatory conditions (see Pezet & McMahon, 2006). In these circumstances, constitutive NGF expression can be upregulated and ex-novo NGF production can be triggered in a variety of cells including mast cells and macrophages. The stimuli for this increased NGF production include interleukin (IL)-1β, TNF-α, platelet-derived growth factor, IL-4 and transforming growth factor-β (Safieh-Garabedian et al. 1995; Woolf et al. 1997; see for references Lee et al. 2009) (Fig. 6A).
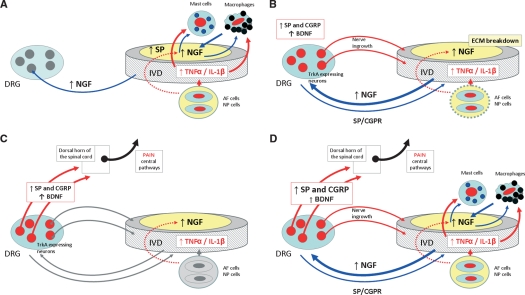
Fig. 6.
Schematic representation of the possible mechanisms involved in the genesis of the discogenic pain. (A) Inflammation causes release of proinflammatory cytokines in the intervertebral disc (IVD), which act on mast cells and macrophages to trigger secretion of NGF. Cells in the IVD upregulate expression of NGF and substance P (SP) during inflammation. Increased levels of NGF can be retrogradely transported to dorsal root ganglia (DRGs) or stimulate mastocytes and macrophages locally initiating a positive feedback loop. (B) Increased levels of NGF reaching the DRGs act on TrkA-expressing neurons inducing expression of peptides that mediate pain [SP and calcitonin gene-related peptide (CGRP)]. The increased levels of NGF in the IVD, as well as the breakdown of the IVD aggrecans, result in ingrowth of nociceptive nerve fibers and, presumably, in anterograde transport to the IVD, which maintains pain. (C) Synaptic transmission in lamina I and II of the dorsal horn of the spinal cord is mediated by SP and CGRP. In addition, brain-derived neurotrophic factor (BDNF) produced in DRGs projects to the same spinal cord laminas and modulates pain transmission. (D) These complex networks are able to originate and maintain pain of IVD origin.
In painful IVDs the levels of proinflammatory mediators are higher than in the asymptomatic IVDs, suggesting that an inflammatory state is present (Burke et al. 2002). Cells from herniated and degenerated IVDs produce and release TNF-α, IL-1β and IL-8 (Takahashi et al. 1996; Rand et al. 1997; Ahn et al. 2002; Lee et al. 2009), and both IL-1β and TNF-α, in turn, upregulate NGF mRNA expression and the secretion of NGF protein in cultured human IVD cells (Abe et al. 2007). Also, cells isolated from human NP in the presence of IL-1β significantly increase NGF and BDNF gene expression, whereas treatment with TNF-α was associated with upregulation of SP in these cells (Purmessur et al. 2008). Recently, it was demonstrated that levels of NGF and SP are increased in human painful IVDs (Richardson et al. 2009).
The NGF released in inflamed IVDs may act directly on nociceptive fibers and neurons to trigger its hyperalgesic effects but it also appears to have a number of indirect actions in mast cells and macrophages to stimulate the release of mediators. The binding of NGF to Trk and p75 expressed by nociceptors initiates complex signal transduction pathways that result in the activation of transcription factors such as NF-κB, which induces the expression of a large number of proinflammatory cytokines (Wallach et al. 2002). Therefore, the inter-relationships between NGF and inflammatory cytokines are complex and reciprocal (Cunha & Ferreira, 2003; Thacker et al. 2007; Spedding & Gressens, 2008; Uçeyler et al. 2009).
Upregulation of NGF expression occurs simultaneously with nerve ingrowth in degenerating intervertebral discs
As indicated above, increased levels of NTs and NTRs in IVDs undergoing signs of degeneration or in IVDs from patients suffering discogenic pain occur in parallel with increased innervation (Freemont et al. 2002b; Coppes et al. 1990, 1997; Palmgren et al. 1996, 1999; Freemont et al. 1997; Brown et al. 1997; see Johnson et al. 2001, 2007; Holm et al. 2002; Kokubo et al. 2008; Purmessur et al. 2008). These data strongly suggest that there is a positive correlation between the levels of NTs and density of innervation in the IVD (Yamauchi et al. 2007). However, TNF-α could also influence nerve ingrowth because in-vitro experiments demonstrate that it is more effective in inducing the expression of growth axonic protein 43 in NGF-sensitive neurons than in GDNF-sensitive neurons (Aoki et al. 2007).
As hyperinnervation of the IVD was observed almost exclusively in painful discs, ‘nerve ingrowth’ into the inner parts of IVDs may be one of the causes of chronic discogenic pain. Nevertheless, this hypothesis cannot be accepted while it is not supported by experimental data. In fact, a rabbit annular-puncture model of IVD degeneration demonstrated that, in the degenerated IVD, nerves are only occasionally observed in the outermost part of the AF and no nerves can be found in scar tissues formed outside the discs, only in the scar tissues on the surface of the puncture (Aoki et al. 2006), suggesting that nerve ingrowth is associated with the process of tissue repair rather than the degenerative process itself.
Although it is evident that a positive correlation exists between the NT levels and the density of innervation in IVDs, other factors in addition to NTs must be considered to explain the hyperinnervation observed in degenerating IVDs. The extracellular matrix of both the AF and NP contains large amounts of proteoglycans, principally aggrecans (see for a review Feng et al. 2006). One of the most significant biochemical changes that occur in IVD degeneration is the loss of large proteoglycans and increase of small proteoglycans (for a review see Urban & Roberts, 2003). The normal aggrecans from human IVDs are able to inhibit nerve growth (Johnson et al. 2002) and therefore may explain in part why IVDs are not innervated or are poorly innervated. Nevertheless, when IVDs degenerate, the absence of normal amounts and proportions of aggrecans may result in nerve ingrowth. However, behind this simple explanation there is a high degree of complexity as more factors are involved; for example, the activation of matrix metalloproteases responsible for breakdown of the extracellular matrix in the degenerating IVD is simultaneous with elevated levels of NGF (Richardson et al. 2009).
Finally, it is still unclear whether such nerve ingrowth in IVDs is a part of the normal tissue repair and wound healing, and thus accompanies neo-angiogenesis (see Olmarker, 2005). Both the neo-vascularization and neo-innervation are induced by bioactive molecules produced in pathological IVDs (Melrose et al. 2002; Olmarker, 2005; Lee et al. 2009) and NGF itself has also been identified as a proangiogenic factor (Szekanecz & Koch, 2007).
NGF regulates the synthesis of nociceptive neuropeptides and brain-derived neurotrophic factor
NGF regulates gene expression in small-size TrkA-expressing DRG neurons. In the presence of NGF, the expression of SP and CGRP increase at both mRNA and protein levels (Malcangio et al. 1997;Malcangio et al. 2000; Pezet et al. 2001) (Fig. 6B). In addition, a number of receptors and proteins, such as calcium and potassium voltage-gated ion channels, TRPV1, P2X3, acid-sensing ion channel proteins and G-protein-coupled receptors, are also regulated by NGF (Park et al. 2003). All of these molecules are related to acute pain and some of them, like SP and CGRP, are used as neurotransmitters in the dorsal horn of the spinal cord. In the case of chronic pain, the activation of afferent nerve fibers induces the release of these neurotransmitters at the nerve terminal after anterograde transport and stimulates mast cells to induce inflammation. This inflammatory response caused by neurotransmitters is termed neurogenic inflammation (see Seidel et al. 2009). NGF may also have long-term effects on nociceptors via regulation of receptor proteins such as TRPV1, bradykinin receptors and sodium channels (Ramer et al. 2001; Priestley et al. 2002).
Similarly, the treatment with NGF induces BDNF expression in virtually all TrkA-positive DRG neurons, whereas normally the percentage is ∼ 10% (Mannion et al. 1999; Priestley et al. 2002). Importantly, there is experimental evidence that peripheral inflammation results in an increased content of BDNF in the DRGs, which is associated with increased and widespread BDNF release in the dorsal horn of the spinal cord (Lever et al. 2001; Walker et al. 2001).
Interestingly, it has been observed that some unknown components of the IVD can directly induce the expression of NTs in DRGs. Thus, the application of SP induced a marked increase of BDNF-immunoreactive neurons within the superficial layer of the dorsal horn (Onda et al. 2003, 2004).
Brain-derived neurotrophic factor controls pain transmission in the spinal cord
The induction of BDNF expression by NGF occurs in parallel with increased release of BDNF from central nociceptive terminals, i.e. at the dorsal horn of the spinal cord. BDNF produced in DRG sensory neurons is transported anterogradely to the spinal cord where it is located in large dense-cored vesicles in terminals of primary afferent fibers in group I and II glomeruli in the spinal cord. BDNF modulates fast excitatory and inhibitory signals, as well as slow peptidergic neurotransmission in the spinal cord, and the vesicles containing BDNF are the same as those that contain SP (Salio et al. 2007). All together, this suggests that, in addition to the roles in the spinal cord, BDNF can modulate the functions mediated by small-diameter sensory neurons (see Merighi et al. 2008). What are the implications of these changes in BDNF levels for nociceptive processing? The dorsal horn is an important point of integration and processing of nociceptive information, and BDNF appears to regulate this system (Bennet, 2001; Pezet & McMahon, 2006; Merighi et al. 2008).
Concluding remarks
Low back pain is a major clinical problem that results in an important socioeconomic burden, which appears to be increasing despite technical and therapeutic advances in its diagnosis and treatment (see Van der Roer et al. 2005; Dagenais et al. 2008). A common source of low back pain might be the IVD (Deyo & Weinstein, 2001).
The biological evidence summarized in this article strongly suggests that NTs play a role in the genesis and maintenance of painful stimuli from degenerating IVDs. NTs, together with extracellular matrix modifications and some cytokines, regulate the nerve ingrowth into the IVD, the synthesis of pain-related peptides in the IVD itself but especially in DRGs, the synapses related to pain transmission in the dorsal horn of the spinal cord, and the biology of the proinflammatory cytokines. A complex network centered on NTs, mainly NGF and BDNF, can rationally explain in part the cellular and molecular mechanisms of discogenic pain. Nevertheless, the pathophysiological and molecular mechanisms underlying the origin of the discogenic pain must be investigated in depth because other factors need to be considered.
Future studies based on the roles of NTs in the IVD, and the proposed roles for NTs regulating discogenic pain, must be stimulated. NTs can be the targets for therapeutic strategies for discogenic pain and the disruption of the pathological processes in which NTs are involved could benefit the control of pain and prevention of the degeneration of the IVD (see Masuda & An, 2004, 2006).
However, animal models for IVD degeneration (see Singh et al. 2005) must be analyzed, focusing the research on animal strains with mutations in genes encoding molecules involved in discogenic pain. Currently experiments are in progress in our laboratory to analyze the structure of IVDs in mouse carrying mutations in genes for NTs and their receptors.
References
- Abe Y, Akeda K, An HS, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- Acheson A, Conover JC, Fandl JP, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Ohtori S, Takahashi K, et al. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine. 2004a;29:1077–1081. doi: 10.1097/00007632-200405150-00005. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Takahashi Y, Takahashi K, et al. Sensory innervation of the lateral portion of the lumbar intervertebral discs in rats. Spine J. 2004b;4:275–280. doi: 10.1016/j.spinee.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Ohtori S, Takahashi K, et al. Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Akeda K, An H, et al. Nerve fiber ingrowth into scar tissue formed following nucleus pulposus extrusion in the rabbit anular-puncture disc degeneration model: effects of depth of puncture. Spine. 2006;31:E774–E780. doi: 10.1097/01.brs.0000238681.71537.41. [DOI] [PubMed] [Google Scholar]
- Aoki Y, An HS, Takahashi K, et al. Axonal growth potential of lumbar dorsal root ganglion neurons in an organ culture system: response of nerve growth factor-sensitive neurons to neuronal injury and an inflammatory cytokine. Spine. 2007;32:857–863. doi: 10.1097/01.brs.0000259810.48681.90. [DOI] [PubMed] [Google Scholar]
- Ashton IK, Robert S, Jaffray DC, et al. Neuropeptides in the human intervertebral disc. J Orthop Res. 1994;12:186–192. doi: 10.1002/jor.1100120206. [DOI] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, et al. Immunocyto-chemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61:205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- Barthel C, Yeremenko N, Jacobs R, et al. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res Ther. 2009;11:R82. doi: 10.1186/ar2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet GJ. Are the complex regional pain syndromes due to neurogenic inflammation? Neurology. 2001;57:2161–2162. doi: 10.1212/wnl.57.12.2161. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Michael GJ, Ramachandran N, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286–293. doi: 10.1097/00007632-198304000-00009. [DOI] [PubMed] [Google Scholar]
- Bonini S, Rasi G, Bracci-Laudiero ML, et al. Nerve growth factor: neurotrophin or cytokine? Int Arch Allergy Immunol. 2003;131:80–84. doi: 10.1159/000070922. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Boyd LM, Richardson WJ, Chen J, et al. Osmolarity regulates gene expression in intervertebral disc cells determined by gene array and real-time quantitative RT-PCR. Ann Biomed Eng. 2005;33:1071–1077. doi: 10.1007/s10439-005-5775-y. [DOI] [PubMed] [Google Scholar]
- Brown MF, Hukkanen MV, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147–153. doi: 10.1302/0301-620x.79b1.6814. [DOI] [PubMed] [Google Scholar]
- Burke JG, Watson WG, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg. 2002;84-B:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Colombini A, Lombardi G, Corsi MM, et al. Pathophysiology of the human intervertebral disc. Int J Biochem Cell Biol. 2008;40:837–842. doi: 10.1016/j.biocel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Connally GH, Sanders SH. Predicting low back pain patients’ response to lumbar sympathetic nerve blocks and interdisciplinary rehabilitation: the role of pretreatment overt pain behavior and cognitive coping strategies. Pain. 1991;44:139–146. doi: 10.1016/0304-3959(91)90127-J. [DOI] [PubMed] [Google Scholar]
- Coppes MH, Marani E, Thomeer RT, et al. Innervation of annulus fibrosus in low back pain. Lancet. 1990;336:189–190. doi: 10.1016/0140-6736(90)91723-n. [DOI] [PubMed] [Google Scholar]
- Coppes MH, Marani E, Thomeer RT, et al. Innervation of “painful” lumbar discs. Spine. 1997;22:2342–2349. doi: 10.1097/00007632-199710150-00005. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Ferreira SH. Peripheral hyperalgesic cytokines. Adv Exp Med Biol. 2003;521:22–39. [PubMed] [Google Scholar]
- Dagenais S, Caro J, Heldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, et al. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;34:363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- Dicou E. Multiple biological activities for two peptides derived from the nerve growth factor precursor. Biochem Biophys Res Commun. 2006;347:833–837. doi: 10.1016/j.bbrc.2006.06.171. [DOI] [PubMed] [Google Scholar]
- Dicou E. Peptides other than the neurotrophins that can be cleaved from proneurotrophins: a neglected story. Arch Physiol Biochem. 2007;113:228–233. doi: 10.1080/13813450701531250. [DOI] [PubMed] [Google Scholar]
- Dicou E. High levels of the proNGF peptides LIP1 and LIP2 in the serum and synovial fluid of rheumatoid arthritis patients: evidence for two new cytokines. J Neuroimmunol. 2008;194:143–146. doi: 10.1016/j.jneuroim.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135–1139. doi: 10.1302/0301-620X.89B9.18939. [DOI] [PubMed] [Google Scholar]
- Ehrenhaft JC. Development of the vertebral column as related to certain congenital and pathological changes. Surg Gynecol Obstet. 1943;76:282–292. [Google Scholar]
- Ernsberger U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell Tissue Res. 2009;336:349–384. doi: 10.1007/s00441-009-0784-z. [DOI] [PubMed] [Google Scholar]
- Fariñas I. Neurotrophin actions during the development of the peripheral nervous system. Microsc Res Tech. 1999;45:233–242. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<233::AID-JEMT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Feng H, Danfelter M, Strömqvist B, et al. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- Freemont AJ. The cellular pathobiology of the degenerated intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Jeziorska M, Hoyland JA, et al. Mast cells in the pathogenesis of chronic back pain: a hypothesis. J Pathol. 2002a;197:281–285. doi: 10.1002/path.1107. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Watkins A, Maitre CL, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002b;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- Gigante A, Bevilacqua C, Pagnotta A, et al. Expression of NGF, Trka and p75 in human cartilage. Eur J Histochem. 2003;47:339–344. [PubMed] [Google Scholar]
- Götz R, Köster R, Winkler C, et al. Neurotrophin-6 is a new member of the nerve growth factor family. Nature. 1994;372:266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- Groen GJ, Baljet B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282–296. doi: 10.1002/aja.1001880307. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Ingram JA, Hoelscher G, et al. Brain-derived neurotrophic factor and its receptor in the human and the sand rat intervertebral disc. Arthritis Res Ther. 2008;10:R82. doi: 10.1186/ar2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipavlou AG, Tzermiadianos MN, Bogduk N, et al. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;90:1261–1270. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Wilson K, Thorndyke M, et al. Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav Evol. 2006;68:133–144. doi: 10.1159/000094083. [DOI] [PubMed] [Google Scholar]
- Hempstead BL. Dissecting the diverse actions of pro- and mature neurotrophins. Curr Alzheimer Res. 2006;3:19–24. doi: 10.2174/156720506775697061. [DOI] [PubMed] [Google Scholar]
- Holm S, Indahl A, Solomonow M. Sensorimotor control of the spine. J Electromyogr Kinesiol. 2002;12:219–234. doi: 10.1016/s1050-6411(02)00028-7. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuro-nal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Hurri K, Karppinen J. Discogenic pain. Pain. 2004;112:225–228. doi: 10.1016/j.pain.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Hyodo H, Sato T, Sasaki H, et al. Discogenic pain in acute nonspecific low-back pain. Eur Spine J. 2005;14:573–577. doi: 10.1007/s00586-004-0844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–1457. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Evans H, Menage J, et al. Immuno-histochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine. 2001;26:2550–2557. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Caterson B, Eisenstein SM, et al. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Patterson AM, Eisenstein SM, et al. The presence of pleiotrophin in the human intervertebral disc is associated with increased vascularization: an immuno-histologic study. Spine (Phila Pa 1976) 2007;32:1295–1302. doi: 10.1097/BRS.0b013e31805b835d. [DOI] [PubMed] [Google Scholar]
- Kokubo Y, Uchida K, Kobayashi S, et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008;9:285–295. doi: 10.3171/SPI/2008/9/9/285. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Bennett DLH, Shelton DL, et al. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11:1698–1704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Grönblad M, Antti-Poika I, et al. Neuroimmunohistochemical analysis of peridiscal nociceptive neural elements. Spine. 1990;15:383–386. doi: 10.1097/00007632-199005000-00008. [DOI] [PubMed] [Google Scholar]
- Lai KO, Fu WY, Ip FC, et al. Cloning and expression of a novel neurotrophin, NT-7, from carp. Mol Cell Neurosci. 1998;11:64–76. doi: 10.1006/mcne.1998.0666. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Morphological and biochemical cell types of sensory neurons. In: Scott SA, editor. Sensory Neurons: Diversity, Development and Plasticity. New York: Oxford University Press; 1992. pp. 27–59. [Google Scholar]
- Lee R, Kermani P, Teng KK, et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lee S, Moon CS, Sul D, et al. Comparison of nerve growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42:1504–1511. doi: 10.1016/j.clinbiochem.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, et al. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Garrett NE, Tomlinson DR. Nerve growth factor treatment increases stimulus-evoked release of sensory neuropeptides in the rat spinal cord. Eur J Neurosci. 1997;9:1101–1104. doi: 10.1111/j.1460-9568.1997.tb01462.x. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Boucher TJ, McMahon SB. Intrathecally injected neurotrophins and the release of susbtance P from the rat isolated spinal cord. Eur J Neurosci. 2000;12:139–144. doi: 10.1046/j.1460-9568.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, et al. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, et al. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci USA. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Masuda K, An HS. Growth factors and the intervertebral disc. Spine J. 2004;4:330S–340S. doi: 10.1016/j.spinee.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy PW. Sparse substance P-like immunoreactivity in intervertebral discs. Acta Orthop Scand. 1993;64:664–668. doi: 10.3109/17453679308994593. [DOI] [PubMed] [Google Scholar]
- McCarthy PW, Carruthers B, Martin D, et al. Immunohistochemical demonstration of sensory nerve fibers and endings in lumbar intervertebral discs of the rat. Spine. 1991;16:653–655. doi: 10.1097/00007632-199106000-00010. [DOI] [PubMed] [Google Scholar]
- McCarthy PW, Petts P, Hamilton A. RT97-, calcitonin gene-related peptide-like immunoreactivity in lumbar intervertebral discs and adjacent tissue from the rat. J Anat. 1992;180:15–24. [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Roberts S, Smith S, et al. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine. 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, et al. IB-4 binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Montaño JA, Pérez-Piñera P, García-Suárez O, et al. Development and neuronal dependence of cutaneous sensory nerve formations: lessons from neurotrophins. Microsc Res Tech. 2009 doi: 10.1002/jemt.20790. (in press). PMID: 19839059. [DOI] [PubMed] [Google Scholar]
- Morinaga T, Takahashi K, Yamagata M, et al. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine. 1996;21:1848–1851. doi: 10.1097/00007632-199608150-00002. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Takahashi K, Takahashi Y, et al. The afferent pathways of discogenic low-back pain: evaluation of L2 spinal nerve infiltration. J Bone Joint Surg. 1996a;78-B:606–612. [PubMed] [Google Scholar]
- Nakamura S, Takahashi K, Takahashi Y, et al. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine. 1996b;21:917–924. doi: 10.1097/00007632-199604150-00003. [DOI] [PubMed] [Google Scholar]
- Nilsson AS, Fainzilber M, Falck P, et al. Neurotrophin-7: a novel member of the neurotrophin family from the zebrafish. FEBS Lett. 1998;424:285–290. doi: 10.1016/s0014-5793(98)00192-6. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE, Petersen CM. p75NTR – live or let die. Curr Opin Neurobiol. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- O’Connor TM, O’Connor J, O’Brian DI, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi Y, Takahashi K, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24:2295–2299. doi: 10.1097/00007632-199911150-00002. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Yamagata M, et al. Neurons in the dorsal root ganglia of T13, L1 and L2 innervate the dorsal portion of lower lumbar discs in rats. J Bone Joint Surg. 2001a;83-B:1191–1194. doi: 10.1302/0301-620x.83b8.11012. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Chiba T, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral discs in rats. Spine. 2001b;26:946–950. doi: 10.1097/00007632-200104150-00020. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Chiba T, et al. Substance P and calcitonin gene-related peptide immunoreactive sensory DRG neurons innervating the lumbar intervertebral discs in rats. Ann Anat. 2002;184:235–240. doi: 10.1016/S0940-9602(02)80113-3. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Moriya H. Existence of brain-derived neurotrophic factor and vanilloid receptor subtype 1 immunoreactive sensory DRG neurons innervating L5/6 intervertebral disc in rats. J Orthop Sci. 2003;8:84–87. doi: 10.1007/s007760300014. [DOI] [PubMed] [Google Scholar]
- Olmarker K. Neovascularization and neoinnervation of subcutaneously placed nucleus pulposus and the inhibitory effects of certain drugs. Spine. 2005;30:1501–1504. doi: 10.1097/01.brs.0000167823.17687.ec. [DOI] [PubMed] [Google Scholar]
- Onda A, Murata Y, Rydevik B, et al. Immunoreactivity of brain-derived neurotrophic factor in rat dorsal root ganglion and spinal cord dorsal horn following exposure to herniated nucleus pulposus. Neurosci Lett. 2003;352:49–52. doi: 10.1016/j.neulet.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Onda A, Murata Y, Rydevik B, et al. Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine. 2004;29:1857–1861. doi: 10.1097/01.brs.0000137054.08788.b2. [DOI] [PubMed] [Google Scholar]
- Palmgren T, Grönblad M, Virri J, et al. Immunohistochemical demonstration of sensory and autonomic nerve terminals in herniated lumbar disc tissue. Spine (Phila Pa 1976) 1996;21:1301–1306. doi: 10.1097/00007632-199606010-00004. [DOI] [PubMed] [Google Scholar]
- Palmgren T, Grönblad M, Virri J, et al. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine. 1999;24:2075–2079. doi: 10.1097/00007632-199910150-00002. [DOI] [PubMed] [Google Scholar]
- Park SY, Choi JY, Kim RU, et al. Downregulation of voltage-gated potassium channel alpha gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol Cells. 2003;16:256–259. [PubMed] [Google Scholar]
- Pederson HE, Blunck CFJ, Gardner E. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerves (sinu-vertebral nerves): with an experimental study of their functions. J Bone Joint Surg Am. 1956;38-A:377–391. [PubMed] [Google Scholar]
- Perl ER. Function dorsal root ganglion neurons: an overview. In: Scott SA, editor. Sensory Neurons: Diversity, Development and Plasticity. New York: Oxford University Press; 1992. pp. 3–23. [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Pezet S, Onteniente B, Jullien J, et al. Differential regulation of NGF receptors in primary sensory neurons by adjuvant-induced arthritis in the rat. Pain. 2001;90:113–125. doi: 10.1016/s0304-3959(00)00393-6. [DOI] [PubMed] [Google Scholar]
- Priestley JV, Michael GJ, Averill S, et al. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. doi: 10.1139/y02-034. [DOI] [PubMed] [Google Scholar]
- Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degen-erate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X3 expression in sensory neurons. J Neurochem. 2001;77:864–875. doi: 10.1046/j.1471-4159.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- Rand N, Reichert F, Floman Y, et al. Murine nucleus pulposus-derived cells secrete interleukin-1-β, -6, and -10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine. 1997;22:2598–2602. doi: 10.1097/00007632-199711150-00002. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SM, Dyle P, Minogue BM, et al. Increased expression of matrix metalloproteinase-10. Nerve growth factor and substance P in the painful degenerated intervertebral disc. Arthritis Res Ther. 2009;11:R126. doi: 10.1186/ar2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Eisenstein SM, Menage J, et al. Mechanoreceptors in intervertebral discs. Spine. 1995;20:2645–2651. doi: 10.1097/00007632-199512150-00005. [DOI] [PubMed] [Google Scholar]
- Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- Robinson M, Buj-Bello A, Davies AM. Paracrine interactions of BDNF involving NGF-dependent embryonic sensory neurons. Mol Cell Neurosci. 1996;7:143–151. doi: 10.1006/mcne.1996.0011. [DOI] [PubMed] [Google Scholar]
- Roofe PG. Innervation of annulus fibrosus and posterior longitudinal ligament. Arch Neurol Psychiatry. 1940;44:100–103. [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, et al. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, et al. Ultrastructural evidence for a pre- and postsynaptic localization of full-length trkB receptors in substantia gelatinosa (lamina II) of rat and mouse spinal cord. Eur J Neurosci. 2005;22:1951–1966. doi: 10.1111/j.1460-9568.2005.04392.x. [DOI] [PubMed] [Google Scholar]
- Salio C, Averill S, Priestley JV, et al. Costorage of BDNF and neuropeptides within individual dense-core vesicles in central and peripheral neurons. Dev Neurobiol. 2007;67:326–338. doi: 10.1002/dneu.20358. [DOI] [PubMed] [Google Scholar]
- Sameda H, Takahashi Y, Takahashi K, et al. Dorsal root ganglion neurons with dichotomizing afferent fibers to both the lumbar intervertebral disc and the groin skin. J Bone Joint Surg. 2003;85-B:600–603. doi: 10.1302/0301-620x.85b4.13306. [DOI] [PubMed] [Google Scholar]
- Sariola H. The neurotrophic factors in non-neuronal tissues. Cell Mol Life Sci. 2001;58:1061–1066. doi: 10.1007/PL00000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, et al. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schweigreiter R. The dual nature of neurotrophins. Bioessays. 2006;28:583–594. doi: 10.1002/bies.20419. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Seidel MF, Herguijuela M, Forkert R, et al. Nerve growth factor in rheumatic diseases. Semin Arthritis Rheum. 2009 doi: 10.1016/j.semarthrit.2009.03.002. (in press) [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Singh K, Masuda K, An HS. Animal models for human disc degeneration. Spine J. 2005;5:267S–279S. doi: 10.1016/j.spinee.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Spedding M, Gressens P. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp. 2008;289:222–233. doi: 10.1002/9780470751251.ch18. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;9:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Ohtori S, Yamashita M, et al. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral disc in rats. Spine. 2008;33:2047–2051. doi: 10.1097/BRS.0b013e31817f8d58. [DOI] [PubMed] [Google Scholar]
- Suseki K, Takahashi Y, Takahashi K, et al. Sensory nerve fibers from lumbar intervertebral discs pass through rami communicantes. J Bone Joint Surg. 1998;80-B:737–742. doi: 10.1302/0301-620x.80b4.8239. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Koch AE. Mechanisms of disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- Tacconelli A, Farina AR, Cappabianca L, et al. Alternative TrkAIII splicing: a potential regulated tumor-promoting switch and therapeutic target in neuroblastoma. Future Oncol. 2005;1:689–698. doi: 10.2217/14796694.1.5.689. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakajima Y, Sakamoto T, et al. Capsaicin applied to rat lumbar intervertebral disc causes extravasation in the groin skin: a possible mechanism of referred pain of the intervertebral disc. Neurosci Lett. 1993;161:1–3. doi: 10.1016/0304-3940(93)90125-5. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Suguro H, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Sato A, Nakamura SI, et al. Regional correspondence between the ventral portion of the lumbar intervertebral disc and the groin mediated by a spinal reflex. A possible basis of discogenic referred pain. Spine (Phila Pa 1976) 1998;23:1853–1858. doi: 10.1097/00007632-199809010-00010. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Chiba T, Kurokawa M, et al. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine. 2003;28(9):871–880. doi: 10.1097/01.BRS.0000058717.43888.B9. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Aoki A, Ohtori S. Resolving discogenic pain. Eur Spine J. 2008;17(Suppl 4):S428–S431. doi: 10.1007/s00586-008-0752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahasi Y, Ohtori S, Takahashi K. Peripheral nerve pathways of afferent fibers innervating the lumbar spine in rats. J Pain. 2009;10:416–425. doi: 10.1016/j.jpain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Takebayashi T, Cavanaugh JM, Kallakuri S, et al. Sympathetic afferent units from lumbar intervertebral discs. J Bone Joint Surg Br. 2006;88:554–557. doi: 10.1302/0301-620X.88B4.17194. [DOI] [PubMed] [Google Scholar]
- Teng KK, Hempstead BL. Neurotrophins and their receptors: signaling trios in complex biological systems. Cell Mol Life Sci. 2004;61:35–48. doi: 10.1007/s00018-003-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125–137. doi: 10.1016/s1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Marchand F, et al. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- Uçeyler N, Schäfers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Sanchez T, Kirstein M, Perez-Vaillalba A, et al. BDNF is essentially required for the early postnatal survival of nociceptors. Dev Biol. 2010;339:465–476. doi: 10.1016/j.ydbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Van der Roer N, Goossens MEJB, Evers SMAA, et al. What is the most-cost-effective treatment for patients with low back pain. A systematic review. Best Pract Res Clin Rheumatol. 2005;19:671–684. doi: 10.1016/j.berh.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Vega JA, García-Suárez O, Hannestad J, et al. Neurotrophins and the immune system. J Anat. 2003;203:1–19. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega JA, García-Suárez O, Germanà A. Vertebrate thymus and the neurotrophin system. Int Rev Cytol. 2004;237:155–204. doi: 10.1016/S0074-7696(04)37004-X. [DOI] [PubMed] [Google Scholar]
- Walker SM, Mitchell VA, White DM, et al. Release of immunoreactive brain-derived neurotrophic factor in the spinal cord of the rat following sciatic nerve transection. Brain Res. 2001;899:240–247. doi: 10.1016/s0006-8993(01)02259-4. [DOI] [PubMed] [Google Scholar]
- Wallach D, Arumugam TU, Boldin MP, et al. How are the regulators regulated? The search for mechanisms that impose specificity on induction of cell death and NF-kappaB activation by members of the TNF/NGF receptor family. Arthritis Res. 2002;4(Suppl 3):S189–S196. doi: 10.1186/ar585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weistein J, Claverie W, Gibson S. The pain of discography. Spine. 1988;13:1344–1348. doi: 10.1097/00007632-198812000-00002. [DOI] [PubMed] [Google Scholar]
- Wiberg G. Back pain in relation to the nerve supply of the intervertebral discs. Acta Orthop Scand. 1947;19:211–221. doi: 10.3109/17453674908991094. [DOI] [PubMed] [Google Scholar]
- Willenegger S, Friess AE, Lang J, et al. Immunohistochemical demonstration of lumbar intervertebral disc innervation in the dog. Anat Histol Embryol. 2005;34:123–128. doi: 10.1111/j.1439-0264.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma QP, et al. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, et al. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351:329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Fine RE, et al. Amyloid β binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J Biol Chem. 2002;277:7720–7725. doi: 10.1074/jbc.M110929200. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Murabe M, et al. Gadd45a, the gene induced by the mood stabilizer valproic acid, regulates neurite outgrowth through JNK and the substrate paxillin in N1E-115 neuroblastoma cells. Exp Cell Res. 2007;313:1886–1896. doi: 10.1016/j.yexcr.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Inoue E, Koshi T, et al. Nerve growth factor of cultured medium extracted from human degenerative nucleus promotes sensory nerve growth and induces substance P in vitro. Spine (Phila Pa 1976) 2009;34:2263–2269. doi: 10.1097/BRS.0b013e3181a5521d. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]