Abstract
Increasing evidence for a cerebellar role in human cognition has accrued with respect to anatomically and functionally distinct lobules. Questions of laterality, however, have been largely overlooked. This study therefore introduced and applied a novel measurement protocol for comparatively bias-free analysis of cerebellar asymmetries. Volumetric measurements were performed on magnetic resonance images from a single pair of monozygotic handedness-discordant twins. Against a background of functional cortical asymmetry for verbal and visuo-spatial functional magnetic resonance imaging activation, which was mirrored in the left-handed twin (Lux et al. 2008), between-twin differences in cerebellar asymmetry are described. Interestingly, asymmetry measures for the whole cerebellum did not correspond to either the direction of hand preference or to the weaker (functional magnetic resonance imaging) lateralization of the left-handed twin. The twins both showed clockwise cerebellar torques. This mirrored a counter-clockwise cerebral torque in the right-handed twin only. Selected single cerebellar lobules V and VII displayed between-twin laterality differences that partially reflected their discrepant handedness. Whole cerebellum anatomical measures appeared to be unrelated to single functional cortical asymmetries. These analyses contribute further anatomical evidence pertaining to the existence of multiple structurally and functionally distinct cortico-cerebellar networks of the healthy human brain in vivo.
Keywords: asymmetry, cerebellar lobule V, cerebellar lobule VII, cerebellum, lateralization, left-handedness, torque, twins
Introduction
Classically the cerebellum has been considered part of the motor control system (Glickstein, 1993; Brodal & Bjaalie, 1997), in which its principal role is the rapid integration of temporal with sensory and cognitive information (Huang, 2008). This is essential for motor programs, such as motor sequencing and visual saccades, which require rapid processing (Stein & Glickstein, 1992). Recently, cerebellar participation in ‘higher cognitive function’ has been a topic of renewed interest (Leiner et al. 1989; Silveri & Misciagna, 2000; Ramnani, 2006; Walter & Joanette, 2007; Baillieux et al. 2008). Functional imaging of the cerebellum in vivo has been performed with cognitive tasks of verbal fluency, visuo-spatial attention, emotion processing and working memory (amongst others) (cf. Stoodley & Schmahmann, 2009). Clinical reports of acquired cerebellar lesion have described a ‘cerebellar cognitive affective syndrome’ (Schmahmann & Sherman, 1998; Mariën et al. 2008), as well as diverse patterns of neuropsychological deficits (Silveri et al. 1998; Ackermann et al. 2007). The functional cerebro-cerebellar mechanisms underpinning such effects remain to be fully explained (cf. Mariën et al. 2000).
Unlike other cerebral sites, the cerebellum principally represents ipsilateral space, hence most cerebro-cerebellar connections are crossed. There is a functional correlation between the contralateral hemispheres of the cerebrum and cerebellum, which has been substantiated by functional imaging data (Stoodley & Schmahmann, 2009) and anatomical analyses (Ramnani, 2006). On imaging, several cognitive functions associated with cerebellar activation show clear cerebral hemispheric lateralization. These functions tend to cause crossed cerebellar activation (Hu et al. 2008). For example, cross-lateralized activation in cerebral and cerebellar hemispheres during verbal fluency task performance has been reported across subjects with differences in cerebral lateralization for language (Hubrich-Ungureanu et al. 2002; Jansen et al. 2005). Poststroke switches in cortical language lateralization (i.e. from left to right) have also been accompanied by changes in cerebellar laterality (i.e. from right to left) (Connor et al. 2006). This indicates the functional salience of lateralized cerebro-cerebellar circuits to cognition.
Despite its limited volume, there are more neurons in the cerebellum than in the remainder of the brain (Andersen et al. 1992). However, its functional microcircuitry is better understood than that of the cerebral cortex (Ito, 2006; Ausim Azizi, 2007; Huang, 2008). The principal reason for this is its uniform crystalline structure. The functional units of Purkinje cells, climbing fibres and parallel fibres are repeated in the same ultrastructure throughout the entire cerebellar cortex (Ramnani, 2006), in spite of the functional differences that exist between the different lobules and areas. The functional implications are twofold. Firstly, differences between cerebellar areas are largely dictated by their respective input and output connections. Thus, anatomically distinct pathways suggestive of different functions have been described for the cerebella of non-human primates (Kelly & Strick, 2003). Their functional relevance is further substantiated by functional connectivity magnetic resonance imaging studies delineating at least four functionally and anatomically distinct networks in human subjects (Krienen & Buckner, 2009). In all of these studies, lobule V is exclusively associated with frontal motor areas. By contrast, lobule VII shows extensive connectivity with prefrontal cortical areas, hence pointing to some involvement with ‘higher cognitive processing’ (Ramnani, 2006). Secondly, there is a direct correlation between grey matter volume and the availability of functionally uniform processing units for the cerebellar cortex, pointing to a potential causal link between the volume of a cerebellar structure and its function. Empirically this has been supported by documenting the cerebellar volume of male musicians, as correlating with practice intensity (the results in female subjects were less straightforward) (Hutchinson et al. 2003). Similar effects were previously reported in association with the hippocampus and spatial memory (Maguire et al. 2000). Unlike the hippocampus, however, adult neurogenesis does not occur in the cerebellum (Lai et al. 2003), therefore leaving the aetiology of the cerebellar volume changes unexplained.
Investigations of cerebellar asymmetries in structure and function are essential adjuncts to cortical studies for characterizing the role of laterality and its associated processing mechanisms in the human brain. The key role played by the cerebellum in motor dexterity, attention, verbal and visuo-spatial processing places it in a unique position for determining the factors influencing lateralization. Identifying the correlations (and/or dissociations) between cerebellar and cerebral hemispheric specializations is integral to resolving outstanding questions such as: the aetiology and evolution of hemispheric specialization, the advantages (and disadvantages) of a lateralized brain, and the genetic or developmental biases in populations.
As indicated in earlier studies (Sommer et al. 1999; Lux et al. 2008), a possible starting point is the analysis of single twin pairs. Monozygotic twins constitute an ideal pair of matched subjects, due to their virtually identical genetic makeup and typically well-matched environmental backgrounds. There are, however, important constraints regarding this approach: even monozygotic twins exhibit differences with respect to epigenetic and other changes undergone during development (Singh et al. 2002; Dubois et al. 2008; Reichenberg et al. 2009). Furthermore, the effects observed by this approach do not allow for statistically validated generalizations. Single twin pair assessments remain a useful tool for describing unusual and rare cases, however, and this includes the presentation of neuropsychological features. Such qualitative findings can be employed to evaluate the validity of appropriate predictive hypotheses. Single case studies can also aid in the development of new measurement protocols that serve as a reference for future research on similar or contrasting populations, respectively.
As an initial step towards documenting the nature of cerebellar laterality, the current study aimed to assess structural asymmetries by employing magnetic resonance imaging (MRI)-based volumetric morphometry in a pair of monozygotic handedness-discordant female twins (reared together) for whom various cerebral measures of functional and structural lateralization have been previously published (Lux et al. 2008). Cerebellar asymmetries were analysed at two levels: whole cerebellum and individual lobules possessing potential functional relevance to handedness, language and visuo-spatial processing (lobules V and VII). Three open questions pertain to: (i) whether functional lateralization is reflected by anatomical volume asymmetries in the cerebellum; (ii) whether this mirrors known cortical asymmetries; and (iii) whether the cerebella of these particular twins are mismatched with respect to cerebellar lateralization.
Materials and methods
Subjects
The participants were 61-year-old female monozygotic twins from the adult twin registry (Twins UK) (Spector & Williams, 2006). As indicated in Lux et al. (2008), they were of similar birth weight within the normal range [right-handed (RH) twin, 2.47 kg; left-handed (LH) twin, 2.35 kg] and there were no indications of abnormal development. The LH twin was born with a left arm fracture, showed a mild left-hand tremor and was wearing a right leg brace at testing (due to an unrelated orthopaedic injury in adulthood). This twin pair belonged to a series of 26 pairs, which had been selected on the basis of strongly discordant hand preference for writing (Gurd et al. 2006). The RH twin had a hand preference index of +0.91 and the LH twin’s was −0.72 (modified Edinburgh Handedness Inventory). Further selection was based on their exceptional mismatch for functional cerebral lateralization of both left and right hemisphere-sensitive tasks; namely atypical lateralization of verbal and visuo-spatial functions in the LH twin (Lux et al. 2008). Although not included in the cortical functional MRI (fMRI) region of interest (ROI) analyses, cerebellar activation was present on verbal (all conditions summed vs. baseline) as well as visuo-spatial attention tasks (line bisection vs. line crossing) (Lux et al. 2008).
There was a reporting error in our earlier study (Lux et al. 2008), which we would like to correct here, namely that there was actually a cerebral torque difference between the twins: RH = −2.1, counter-clockwise and LH = +1.6, clockwise (contrary to the previous report).
Magnetic resonance imaging
The structural magnetic resonance images were acquired on a 1.5-T Magnetom SONATA (Siemens, Erlangen, Germany) MRI Scanner. The anatomical whole-brain images were obtained using a T1-weighted, 3D gradient echo-pulse sequence (FLASH, fast low-angle shot) with the following parameters: Repetition time, 1200ms; echo time, 5.6ms; inversion time, 19° flip angle; matrix size, 160*256*208; voxel size, 1 mm isotropic; acquisition, coronal; averages, 3.
Volumetry
The method for volumetric assessment of structural asymmetries in the cerebellum has to satisfy two requirements. The technique has to be sensitive to the small hemispheric spatial differences in the cerebellum and its lobules, particularly within the limits of MRI spatial resolution (in this study, 1*1*1 mm voxel size). It also has to be spatially unbiased in order to accurately reflect the asymmetries. Automated methods map volumes with a probabilistic preference for a predetermined averaged shape (Diedrichsen et al. 2009). This approach can be particularly problematic for measures (such as laterality) that show a clear population bias or reflect pathological conditions where structures deviate from the underlying probabilistic model shape (cf. Pardoe et al. 2009). This does not pertain to manual methods, which do not show an inherent shape bias (cf. Roberts et al. 2000; Ronan et al. 2006). A manual voxel-counting approach was therefore chosen here to obtain an unbiased measurement at the required levels of precision. To the extent that there is error in this technique, it would be due to intra-rater error and would be reflected in potential variability between repeated measures per structure (which may be compounded by measurement of partial volumes).
Quantitative analysis
All quantitative measurements were performed using ‘measure’ software (developed at Johns Hopkins University) (Barta et al. 1995). Images were first analysed and then converted, rotated and resampled using the free DICOM viewer ‘Osirix’ (Rosset et al. 2004), the free tool ‘MRIcro’ (Rorden & Brett, 2000) and the free set of tools ‘Analysis of Functional Neuroimages (AFNI)’ (Cox, 1996). For consistency of analysis, images were rotated according to the major cerebellar landmarks; in the axial plane the midline of the brainstem and the vermis were used as vertical reference points, in the coronal plane the vermis was used as the vertical reference, and in the sagittal plane the roof of the fourth ventricle was used as the vertical reference. Following this rotation, the image files were resampled using the AFNI toolset to maintain voxel orientation perpendicular to the major axes (an essential prerequisite for the comparability of different structures measured using the Cavalieri method). The prepared image files were then analysed using two procedures. The measurement of inter-hemispheric volume differences within the coronal plane (along the anterior/posterior axis) was performed to establish the 1D asymmetry profile (cf. Mackay et al. 2003). Subsequently, the overall hemispheric differences between gross structures (i.e. total hemispheres and specific lobules) were determined. For the whole cerebellum, measurements of the superior cerebellar peduncles were excluded on the more anterior slices. For measurement of the single lobules, distinguishable white matter tracts were excluded.
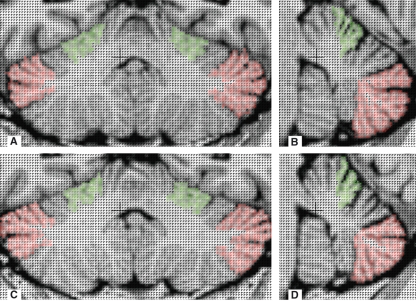
The rater was blind to the identity of the datasets (vis-à-vis handedness of the twins) and the laterality of each image (i.e. which side of the image represents which side of the person). Measurements were performed manually by marking the probes located on the structures of interest (i.e. whole cerebellum, lobule V or lobule VII) in the sagittal plane. One measure consisted of the sum of all of the probes across the different planes examined. Only for the 1D asymmetry profile were these whole measure analyses performed on a slice-by-slice basis (comparing left vs. right hemispheric volumes in the horizontal plane). Both the blinded analysis procedure and the sagittal orientation of original image acquisition aided in ensuring that no left/right bias was introduced into the measurement process. Each complete measurement per structure included five single measures (acquired as described above). If any of these measures exceeded a 10% range about the group mean, all five measures were dismissed and the procedure repeated. For the 1D asymmetry profile, measurements were dismissed if more than 10% of the analysed slices from any of the five measures deviated by more than 10% from the group mean (excluding the most anterior seven and most posterior five slices per measure, due to their very limited volume). The anatomical delineation of the structures was further adjudicated by a local expert with specialist knowledge of the cerebellum (J.F. Stein). This was then substantiated by reference to Schmahmann’s nomenclature and to the MRI atlas of cerebellar anatomy (Schmahmann et al. 1999) (Fig. 1).
Fig. 1.
Examples of Cavalieri sections indicating a sample of 'probes' included in the measurement of cerebellar lobules V (green) and VII (red). (A) right-hander, coronal (B) right-hander, sagittal (C) left-hander, coronal (D) left-hander, sagittal.
This study included comparison of the lateralized volume distributions of lobule VII (Crus I, Crus II and VIIB), which has extensive prefrontal connections (Kelly & Strick, 2003) as well as functional participation in selected aspects of verbal and visuo-spatial processing (Ramnani, 2006; Stoodley & Schmahmann, 2009). It also included analyses of lobule V, which is functionally related to dexterity and hand movements (Ramnani, 2006; Stoodley & Schmahmann, 2009).
Results
Statistical considerations
Volume measurements per structure are presented as medians derived from multiple individual measurements. The purpose of the multiple measurements was to assess internal, intra-rater consistency as well as random error. That is, considering that two individual biological structures are never perfectly identical, the statistical procedures were adopted to determine whether the direction and magnitude of observed differences reflected actual between-twin differences vs. random measurement error.
The error analyses employed intra-class correlation coefficients (ICCs) of the measurement calculated using the PASW Statistics Package Version 18.0. The 1D asymmetry profile measurements performed were that each individual measure contains approximately 50 data points (the inter-hemispheric volume difference at each of the coronal slices where cerebellar matter was present). For each of the twins, the ICC was calculated using a one-way random effects model, which was adopted to assess the proportion of variance between the five different measurements that was associated with the variance between actual target values (McGraw & Wong, 1996). All P-values were derived from the Mann–Whitney test as its non-parametricity provided a better fit to the Cavalieri samples (which tended not to be normally distributed) (García-Fiñana et al. 2009).
Whole cerebellum
Whole (i.e. total) cerebellar volumes differed significantly between the twins; all values reported are medians derived from the five individual measurements: RH twin, 118.32 mL; LH twin, 112.16 mL [Mann–Whitney point estimate for ETA1–ETA2, 6.26; 96.3% confidence interval (CI) for ETA1–ETA2, (5.31, 6.90); W = 40.0; test of ETA1 = ETA2 vs. ETA1 ≠ ETA2 was significant at P = 0.01] (Fig. 2).
Fig. 2.
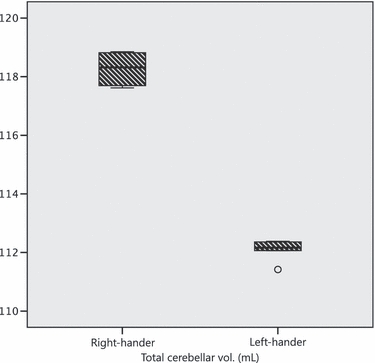
Total cerebellar volume for the two twins. The boxplot indicates the overall spread of independently measured values.
1D asymmetry slice profiles
The asymmetries are reported as percent of total cerebellar volume (per twin). The inter-hemispheric volume differences (% cerebellar asymmetry) were calculated as [(median right – left hemisphere)/median total cerebellar volume × 100%]. The results showed an opposing directionality of percent volume asymmetry between twins. This was more pronounced in the LH twin, for whom the right hemisphere was larger [RH twin, −0.57%; LH twin, 2.15%; Mann–Whitney point estimate for ETA1–ETA2, −2.5900; 96.3% CI for ETA1–ETA2, (−2.9799, −2.3101); W = 15.0; test of ETA1 = ETA2 vs. ETA1 ≠ ETA2 was significant at P = 0.01] (Fig. 3). 1D asymmetry profiles were calculated on a slice-by-slice basis from the inter-hemispheric volume differences: [(right – left hemisphere)/total cerebellar volume*100%]. A comparison of each combination of two individual measures of the same structure was carried out using a Pearson correlation coefficient, which showed that for all comparisons, r > 0.9. The ICC for the set of five measurements was 0.90 for the RH twin and 0.93 for the LH twin. The five independently measured profiles per twin are shown in Fig. 4.
Fig. 3.
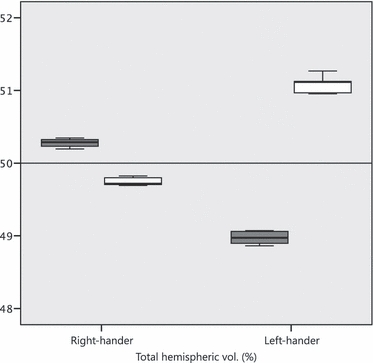
Total hemispheric volume in % of total cerebellar volume. The boxplot indicates the overall spread of independently measured values. (dark boxes: left hemisphere; light boxes: right hemisphere).
Fig. 4.
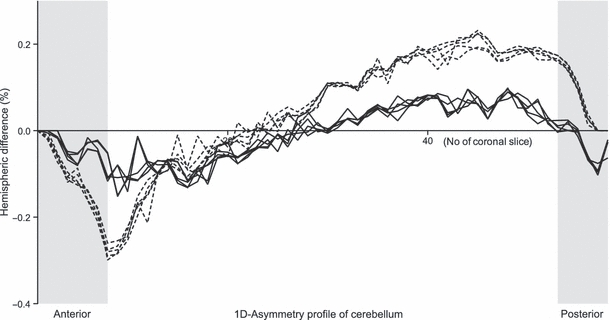
Asymmetry profile along the anterior-posterior axis. Each point represents the difference between right and left hemispheric volume at a single coronal slice, relative to total cerebellar volume in percent (ie. [slice volume (right hemisphere) - slice volume (left hemisphere)] / total cerebellar volume * 100. The gray areas refer to the slices that were excluded from the imposed variability requirement. (continuous line: right-hander, broken line: left-hander).
Cerebellar torque
A basic shape profile similarity was observed between the twins. The left hemisphere volume was greater in the anterior direction and the right hemisphere volume was greater in the posterior direction, which is indicative of clockwise cerebellar torque. There was a significant difference between twins in the minimal and maximal values [median across five measurements: RH twin minimal, −0.14%; RH twin maximal, 0.10%; LH twin minimal, −0.28%; LH twin maximal, 0.22%; minimum: Mann–Whitney point estimate for ETA1–ETA2, 0.14000; 96.3% CI for ETA1–ETA2, (0.12000, 0.16999); W = 40.0; test of ETA1 = ETA2 vs. ETA1 ≠ ETA2 was significant at P = 0.01; maximum: Mann–Whitney point estimate for ETA1–ETA2, −0.12000; 96.3% CI for ETA1–ETA2, (−0.13000, −0.09001); W = 15.0; test of ETA1 = ETA2 vs. ETA1 ≠ ETA2 was significant at P< 0.01], indicating that the LH twin had a greater extent of clockwise cerebellar torque.
Cerebellar lobule asymmetries
Selected lobule asymmetry values were calculated as [(median right − median left volume)/median total volume*100%] per lobule (V and VII). (i) The lobules were of similar size relative to the total cerebellar volume in the twins (RH twin lobule V, 7.14%; RH twin lobule VII, 34.49%; LH twin lobule V, 6.90%; LH twin lobule VII, 33.91%). (ii) The lobular volume asymmetries did not match the overall cerebellar hemispheric asymmetries and nor were they consistent within or between twins (Table 1).
Table 1.
Summary of cortical and cerebellar asymmetries of the left-handed (LH) and right-handed (RH) twin.
| LH twin | RH twin | |
|---|---|---|
| Cerebral cortical fMRI activation | ||
| Verbal | Right > left* | Left > right |
| Visuo-spatial attention | Left > right* | Right > left |
| Anatomy | ||
| Cortical torque | Clockwise* | Counter-clockwise |
| Cerebellar fMRI activation | ||
| Verbal | Left > right* | Right > left |
| Visuo-spatial attention | Left > right | – |
| Anatomy | ||
| Cerebellar torque | Clockwise | Clockwise |
| Volume | ||
| Hemispheric | Right > left | Left > right |
| Lobule V | Left > right | Right > left |
| Lobule VII | Left ≥ right | Left > right |
Atypical.
fMRI, functional magnetic resonance imaging.
In the RH twin, both lobules were more strongly asymmetric than in the LH twin. The values for lobule V were RH twin, 2.44 and LH twin, −0.86 [Mann–Whitney point estimate for ETA1–ETA2, 3.30; 95% CI for ETA1–ETA2, (2.36, 4.13); W = 40; test of ETA1 = ETA2 vs. ETA1 ≠ ETA2 was significant at P = 0.012]. The values for lobule VII were RH twin, −2.91 and LH twin, −0.09 [Mann–Whitney point estimate for ETA1–ETA2, −2.94; 95% CI for ETA1–ETA2, (−3.57, −2.12); W = 15; test of ETA1 = ETA2 vs. ETA1 ≠ ETA2 was significant at P = 0.012] (Fig. 5).
Fig. 5.
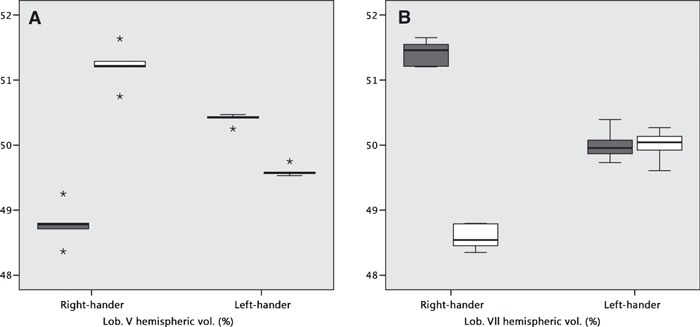
Volumes of hemispheric components of (A) lobule V and (B) lobule VII. Reported in % of total lobular volume. The boxplot indicates the overall spread of independently measured values. (dark boxes: left hemisphere; light boxes: right hemisphere).
Discussion
As indicated in the summary Table 1 and consistent with the limited literature on the cerebellum (Hayter et al. 2007; Hu et al. 2008; Stoodley & Schmahmann, 2009), the asymmetry measures that we report for the whole cerebellum do not differ in direction between twins (Figs 3 and 4). Although non-dextral singletons are in general assumed to show less consistent and pronounced asymmetry than dextrals (Knecht et al. 2000, 2002; Annett, 2003; Gurd et al. 2006), this phenomenon is not particularly reflected in this twin pair. A between-twin difference between cerebellar lobules is, however, observed.
Relation between cerebellar functional lateralization and anatomical asymmetries
Between twins, there tended to be a difference between cortical functional and behavioural laterality measures. The RH twin showed typical left hemisphere dominance for verbal and right hemisphere dominance for visuo-spatial attentional processes, whereas the LH twin showed the opposite, atypical dominance for both. Hand preference index values were +90 for the RH twin and −50 for the LH twin. Cerebellar volume differences also varied (Table 1). Table 1 summarizes the directionality of cortical and cerebellar functional and anatomical volume differences (displayed independent of sign conventions). The published fMRI laterality values refer to fMRI (1.5T) ROI analyses for percent activated voxels per region (expressed as a laterality quotient, wherein the difference between hemispheres is divided by the sum of the two). For the verbal task (verbal fluency), the cortical ROI was medial and inferior frontal (i.e. Broca’s area and its right hemisphere analogue). For the visuo-spatial attention task (landmark), the ROI was the entire parietal lobe bilaterally. On the verbal task both twins were strongly lateralized but in opposite directions; the RH twin showed a predicted left hemisphere asymmetry (+0.91) in contrast to the LH twin’s right hemisphere asymmetry (−0.92). These were accompanied by activations in the contralateral cerebella for both twins (i.e. RH twin right cerebellum and LH twin left cerebellum, although not included in the ROI). Likewise, for the visuo-spatial attention task, the RH twin showed a predictable right hemisphere asymmetry (+0.50) in contrast to the LH twin’s left hemisphere asymmetry (−0.72). The anatomical values refer selectively to whole cerebellar volume asymmetries, as well as to those of the two functionally relevant lobules, V and VII (as indicated above) (Lux et al. 2008).
With respect to lobule V, which is involved in motor control and sensory feedback of the hand and arm, there is rightward asymmetry in the RH twin but leftward asymmetry in the LH twin (albeit less pronounced in the latter) (Fig. 5A). This is congruent with the directionality of cerebellar activation associated with the verbal fluency fMRI results (Lux et al. 2008). In lobule VII, by contrast, there is leftward volume asymmetry in the RH twin but no significant asymmetry in the LH twin. It is difficult to interpret the functional significance of this, although Stoodley & Schmahmann (2009) claim that visuo-spatial and verbal functions are represented in different hemispheres of lobule VII.
Results such as these highlight some general problems with respect to global asymmetry measurement. Despite reflecting potential functional roles, they sum across several opposing structural features, hence potentially obscuring other relevant correlations. Global measures such as torque should ideally be accompanied by comparisons between functional regions – both cortical and cerebellar. In the light of such phenomena, the notion that functional laterality measures would be directly reflected in cerebellar structural asymmetries receives only qualified support specific to lobule V here. This effect appears to be congruent with hand dominance differences between our twins (cf. Hubrich-Ungureanu et al. 2002; Jansen et al. 2005).
Cerebellar vs. cortical asymmetries
Previous studies on singletons have emphasized a match between the directionality of cerebral and cerebellar torque within individuals (Snyder et al. 1995; Szeszko et al. 2003). Figure 4 shows the same clockwise direction of cerebellar torque in both twins. Because of the within-twin pair difference in cerebral torques (RH twin, counter-clockwise; LH twin, clockwise; Table 1), the directionality of cerebellar torque is not matched with that of cerebral torque for the RH twin. It is possible that some discrepancies in the published accounts of whole cerebellar asymmetries are the result of measurement differences caused by a failure to reorient the acquired images according to cerebellar landmarks (Snyder et al. 1995; Szeszko et al. 2003). This is an essential step which was introduced into our study. Asymmetries were therefore measured intrinsic to the cerebellum (but not relative to those due to rotation of the whole cerebellum within the posterior fossa). It is those intrinsic structural asymmetries that are most likely to have functional correlates and therefore it is important that they be taken into account in future studies.
The particular cerebellar lobules described here have different neocortical connections. Most notable are the extensive connections between lobule VII and the prefrontal cortex (Brodmann area 46) (cf. Kelly & Strick, 2003, macaque; Krienen & Buckner, 2009, human). During human evolution, lobule VII and its connections underwent extensive expansion, in concert with the increasing role played by the cerebellum (especially lobule VII) in ‘higher cognitive function’. However, in the brains described here, the gross volume asymmetries of cerebellar lobule VII (Fig. 5B) do not predict frontal lobe asymmetries (RH twin: lobule VII, −2.83%; frontal lobe, −0.22%; LH twin: lobule VII, −0.01%; frontal lobe, 2.12%). Thus, the suggestion that cerebellar anatomy mirrors cerebral asymmetries can only be supported with respect to limited aspects of cerebellar/cerebral torque and only in the RH twin.
Within-twin pair differences in cerebellar anatomy and lateralization
The measurements detailed here revealed substantial differences in total cerebellar volume as well as in asymmetry measures between twins. This is analogous to published accounts of MRI-based volumetric studies of diverse cerebral structures in twins (e.g. Lohmann et al. 1999). Monozygotic twins can show marked differences in cerebellar anatomy despite their shared genetic determinism. This would appear to indicate that anatomical laterality is influenced by a combination of environmental, developmental and epigenetic factors even in monozygotic twins (cf. Singh et al. 2002; Haque et al. 2009). Such findings are consistent with a more general observation that twin pair variations can be present in behavioural, structural and functional laterality measures (Sommer et al. 2002; Annett, 2003; Gurd et al. 2006).
Concluding remarks
Our most striking finding is the disparate direction of cerebral (counter-clockwise) vs. cerebellar (clockwise) torque in the RH twin. The LH twin showed clockwise directionality of torque in cerebral and cerebellar regions. Her cortical torque was atypical. Despite understanding the cytoarchitecture of the cerebellum, major limitations in knowledge of its principal processing abilities vis-à-vis‘higher cognitive processing’ remain. Even with respect to simple motor sequence learning, functional imaging studies have produced inconsistent results concerning the role of the cerebellum. For example, both increases and decreases of activation have been described in various regions during learning (Toni et al. 1998; van Mier et al. 2004; Ramnani, 2006). The probability is that this relates to limitations of the method of in-vivo functional imaging (cf. Hardcastle, 2007). Currently it is not possible to discriminate experimentally between the different signals of cerebellar processing resulting from simple vs. complex spikes from Purkinje cells with in-vivo functional imaging.
Thus, correlations between anatomical and functional asymmetries are, of necessity, bounded by the paradigms used to assess them (Hardcastle, 2007). Probabilistic mapping based on populations (cf. Scheperjans et al. 2008) needs to be augmented with thorough analysis of individual cases. This applies particularly in instances where the populations are not necessarily representative (as with structural asymmetries, laterality and twins) or where there are two or more subpopulations to consider (e.g. handedness discordant pairs). Parallel assessment of different lateralized features (e.g. different tasks of verbal memory: acquisition, maintenance, rehearsal, visuo-spatial tasks, manual tasks and analysis of structural asymmetries) using contrasting methods within individuals is therefore essential.
Within this context the current study introduced a novel protocol for the measurement of cerebellar asymmetries. The protocol was applied and novel evidence was thereby derived. Despite its limits (e.g. single twin pair, single gender and older age) (cf. Hutchinson et al. 2003), this new evidence contributes to the overall understanding of cerebral and cerebellar laterality effects, and the limits of normal variation, at different functional levels in healthy adult brains. The cerebellum constitutes a comparatively under-investigated neuroanatomical domain with respect to the development and evolution of human cerebral asymmetries. Chimpanzee studies have established correlations between handedness and cerebellar asymmetry without a population bias, despite the bias for cerebral torque (Cantalupo et al. 2008). Is it possible, we ask, that the participation of specific cerebellar lobules in cognitive task performance has led to an adaptation of cerebellar asymmetry, which in turn has facilitated the handedness bias characteristic of humans? Or is cerebellar asymmetry hierarchically secondary to handedness, which is itself related to cortical development?
Monozygotic twins offer a genetically unique comparison group. Further identifying the correlation and, more importantly, the dissociation between different laterality measures within individual twin pairs and comparing such findings with appropriate comparison groups (e.g. dizygotic twins, singletons) is the next step in determining the characteristics of this specific population. It is important that structural asymmetries be considered within an inter-disciplinary framework, if function is to be inferred from their structure. This extends to the assessment of lateralized subcortical structures such as the basal ganglia (cf. Tomer et al. 2008), potential interactions between brain asymmetries and other bodily systems such as the immune system (cf. Quaranta et al. 2008), large-scale genetic studies (e.g. Francks et al. 2007), and use of new hypothesis-generating genomic and proteomic analysis techniques (Geschwind & Konopka, 2009). Undoubtedly, the relevance of integrating between different levels of description has been highlighted by the cerebellar asymmetry data presented here.
Acknowledgments
We are grateful to the participants, N. Ramnani, J. F. Stein, M. García-Fiñana, S. Keller, U. Kischka, C. MacKay, S. Lux, H. Burnham, H. Osborne, J. C. Marshall and I. Wartenburger for their contributions to this work. We especially thank two anonymous reviewers for their helpful comments on the manuscript. This study was funded by the British Academy, Critchley Charitable Trust, Joan Critchley, Medical Research Council, UK, University of Oxford John Fell Fund, German Academic Foundation and Magdalen College, Oxford.
References
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6:202–213. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol. 1992;326:549–560. doi: 10.1002/cne.903260405. [DOI] [PubMed] [Google Scholar]
- Annett M. Cerebral asymmetry in twins: predictions of the right shift theory. Neuropsychologia. 2003;41:469–479. doi: 10.1016/s0028-3932(02)00137-9. [DOI] [PubMed] [Google Scholar]
- Ausim Azizi S. …And the olive said to the cerebellum: organization and functional significance of the olivo-cerebellar system. Neuroscientist. 2007;13:616–625. doi: 10.1177/1073858407299286. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, et al. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Barta PE, Petty RG, McGilchrist I, et al. Asymmetry of the planum temporale: methodological considerations and clinical associations. Psychiatry Res. 1995;61:137–150. doi: 10.1016/0925-4927(95)02650-m. [DOI] [PubMed] [Google Scholar]
- Brodal P, Bjaalie JG. Salient anatomic features of the cortico-ponto-cerebellar pathway. Prog Brain Res. 1997;114:227–249. doi: 10.1016/s0079-6123(08)63367-1. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Freeman H, Rodes W, et al. Handedness for tool use correlates with cerebellar asymmetries in chimpanzees (Pan troglodytes) Behav Neurosci. 2008;122:191–198. doi: 10.1037/0735-7044.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor LT, DeShazo Braby T, Snyder AZ, et al. Cerebellar activity switches hemispheres with cerebral recovery in aphasia. Neuropsychologia. 2006;44:171–177. doi: 10.1016/j.neuropsychologia.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, et al. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori-Tolsa C, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131:2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francks C, Maegawa S, Laurén J, et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol Psychiatry. 2007;12:1129–1139. doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fiñana M, Keller SS, Roberts N. Confidence intervals for the volume of brain structures in Cavalieri sampling with local errors. J Neurosci Methods. 2009;179:71–77. doi: 10.1016/j.jneumeth.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Konopka G. Neuroscience in the era of functional genomics and systems biology. Nature. 2009;461:908–915. doi: 10.1038/nature08537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M. Motor skills but not cognitive tasks. Trends Neurosci. 1993;16:450–451. doi: 10.1016/0166-2236(93)90074-v. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Schulz J, Cherkas L, et al. Hand preference and performance in 20 pairs of monozygotic twins with discordant handedness. Cortex. 2006;42:934–945. doi: 10.1016/s0010-9452(08)70438-6. [DOI] [PubMed] [Google Scholar]
- Haque FN, Gottesman II, Wong AHC. Not really identical: epigenetic differences in monozygotic twins and implications for twin studies in psychiatry. Am J Med Genet C Semin Med Genet. 2009;151:136–141. doi: 10.1002/ajmg.c.30206. [DOI] [PubMed] [Google Scholar]
- Hardcastle VG. Neurobiology. In: Hull DL, Ruse M, editors. The Cambridge Companion to the Philosophy of Biology. Cambridge: Cambridge University Press; 2007. pp. 275–290. [Google Scholar]
- Hayter AL, Langdon DW, Ramnani N. Cerebellar contributions to working memory. NeuroImage. 2007;36:943–954. doi: 10.1016/j.neuroimage.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Hu D, Shen H, Zhou Z. Functional asymmetry in the cerebellum: a brief review. Cerebellum. 2008;7:304–313. doi: 10.1007/s12311-008-0031-2. [DOI] [PubMed] [Google Scholar]
- Huang C. Implications on cerebellar function from information coding. Cerebellum. 2008;7:314–331. doi: 10.1007/s12311-008-0032-1. [DOI] [PubMed] [Google Scholar]
- Hubrich-Ungureanu P, Kaemmerer N, Henn FA, et al. Lateralized organization of the cerebellum in a silent verbal fluency task: a functional magnetic resonance imaging study in healthy volunteers. Neurosci Lett. 2002;319:91–94. doi: 10.1016/s0304-3940(01)02566-6. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Lee LH, Gaab N, et al. Cerebellar volume of musicians. Cereb Cortex. 2003;13:943–949. doi: 10.1093/cercor/13.9.943. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Jansen A, Flöel A, Van Randenborgh J, et al. Crossed cerebro-cerebellar language dominance. Hum Brain Mapp. 2005;24:165–172. doi: 10.1002/hbm.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;3:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Knecht S, Flöel A, Dräger B, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci. 2002;5:695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Reappraising the cerebellum: what does the hindbrain contribute to the forebrain? Behav Neurosci. 1989;103:998–1008. doi: 10.1037//0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cereb Cortex. 1999;9:754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Lux S, Keller S, Mackay C, et al. Crossed cerebral lateralization for verbal and visuo-spatial function in a pair of handedness discordant monozygotic twins: MRI and fMRI brain imaging. J Anat. 2008;212:235–248. doi: 10.1111/j.1469-7580.2008.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C, Barrick T, Roberts N, et al. Application of a new image analysis technique to study brain asymmetry in schizophrenia. Psychiatry Res. 2003;124:25–35. doi: 10.1016/s0925-4927(03)00088-x. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P, Engelborghs S, Pickut BA, De Deyn PP. Aphasia following cerebellar damage: fact or fallacy? J Neurolinguistics. 2000;13:145–171. [Google Scholar]
- Mariën P, Baillieux H, De Smet HJ, et al. Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study. Cortex. 2008;45:527–536. doi: 10.1016/j.cortex.2007.12.010. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- van Mier H, Perlmutter J, Petersen S. Functional changes in brain activity during acquisition and practice of movement sequences. Motor Control. 2004;4:500–520. doi: 10.1123/mcj.8.4.500. [DOI] [PubMed] [Google Scholar]
- Pardoe HR, Pell GS, Abbott DF, et al. Hippocampal volume assessment in temporal lobe epilepsy: how good is automated segmentation? Epilepsia. 2009;12:2586–2592. doi: 10.1111/j.1528-1167.2009.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta A, Siniscalchi M, Albrizio M, et al. Influence of behavioural lateralization on interleukin-2 and interleukin-6 gene expression in dogs before and after immunization with rabies vaccine. Behav Brain Res. 2008;186:256–260. doi: 10.1016/j.bbr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Mill J, MacCabe JH. Epigenetics, genomic mutations and cognitive function. Cogn Neuropsychiatry. 2009;14:377–390. doi: 10.1080/13546800902978417. [DOI] [PubMed] [Google Scholar]
- Roberts N, Puddephat MJ, McNulty V. The benefit of stereology for quantitative radiology. Br J Radiol. 2000;73:679–697. doi: 10.1259/bjr.73.871.11089458. [DOI] [PubMed] [Google Scholar]
- Ronan L, Doherty CP, Delanty N, et al. Quantitative MRI: a reliable protocol for measurement of cerebral gyrification using stereology. Magn Reson Imaging. 2006;24:265–272. doi: 10.1016/j.mri.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff EB, Hömke L, et al. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex. 2008;18:2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Misciagna S. Language, memory, and the cerebellum. J Neurolinguistics. 2000;13:129–143. [Google Scholar]
- Silveri MC, Di Betta AM, Filippini V, et al. Verbal short-term store-rehearsal system and the cerebellum. Evidence from a patient with a right cerebellar lesion. Brain. 1998;121:2175–2187. doi: 10.1093/brain/121.11.2175. [DOI] [PubMed] [Google Scholar]
- Singh SM, Murphy B, O’Reilly R. Epigenetic contributors to the discordance of monozygotic twins. Clin Genet. 2002;62:97–103. doi: 10.1034/j.1399-0004.2002.620201.x. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bilder RM, Wu H, et al. Cerebellar volume asymmetries are related to handedness: a quantitative MRI study. Neuropsychologia. 1995;33:407–419. doi: 10.1016/0028-3932(94)00125-9. [DOI] [PubMed] [Google Scholar]
- Sommer EC, Ramsey NF, Bouma A, et al. Cerebral mirror-imaging in a monozygotic twin. Lancet. 1999;354:1445–1446. doi: 10.1016/s0140-6736(99)04130-6. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandi RC, et al. Language lateralization in monozygotic twin pairs concordant and discordant for handedness. Brain. 2002;125:2710–2718. doi: 10.1093/brain/awf284. [DOI] [PubMed] [Google Scholar]
- Spector TD, Williams FM. The Adult Twin Registry (TwinsUK) Twin Res Hum Genet. 2006;9:899–906. doi: 10.1375/183242706779462462. [DOI] [PubMed] [Google Scholar]
- Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev. 1992;72:967–1017. doi: 10.1152/physrev.1992.72.4.967. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Gunning-Dixon F, Ashtari M, et al. Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biol Psychiatry. 2003;53:450–459. doi: 10.1016/s0006-3223(02)01529-9. [DOI] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, et al. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R. The time course of changes during motor sequence learning: a whole-brain fMRI study. NeuroImage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- Walter N, Joanette Y. The unnoticed contributions of the cerebellum to language. Folia Phoniatr Logop. 2007;59:171–176. doi: 10.1159/000102928. [DOI] [PubMed] [Google Scholar]