Abstract
The aim of the present investigation, which represents an extension of a previous study, was to investigate the effect of ferutinin in recovering severe osteoporosis due to estrogen deficiency after rat ovariectomy and to compare phytoestrogen effects with those of estrogens commonly used in hormone replacement therapy (HRT) by women with postmenopausal osteoporosis. The animal model used was the Sprague–Dawley ovariectomized rat. Ferutinin was orally administered (2 mg kg−1 per day) for 30 or 60 days starting from 2 months after ovariectomy (i.e. when osteoporosis was clearly evident) and its effects were compared with those of estradiol benzoate (1.5 μg per rat twice a week, subcutaneously injected) vs. vehicle-treated ovariectomized (OVX) and sham-operated (SHAM) rats. Histomorphometric analyses were performed on trabecular bone of lumbar vertebrae (4th and 5th) and distal femoral epiphysis, as well as on cortical bone of femoral diaphysis. Bone histomorphometric analyses showed that ferutinin seems to display the same effects on bone mass recorded with estradiol benzoate, thus suggesting that it could enhance the recovery of bone loss due to severe estrogen deficiency in OVX rats. On this basis, the authors propose listing ferutinin among the substances representing a potential alternative for the treatment of postmenopausal osteoporosis, which occurs as a result of estrogen deficiency.
Keywords: bone mass, ferutinin, osteoporosis, ovariectomized rat, phytoestrogen
Introduction
The present study represents the completion of our previous investigations published in 2009 (Palumbo et al. 2009); they both, as a whole, have the aim to evaluate the effects of ferutinin (a phytoestrogen extracted from the Ferula hermonis root) in preventing and/or recovering the loss of bone mass due to estrogen deficiency, using ovariectomized rats as the experimental animal model, and to compare phytoestrogen effects with those of estrogens commonly used in hormone replacement therapy (HRT) by women with postmenopausal osteoporosis.
Osteoporosis is a chronic bone disease, in which the skeleton becomes fragile, and leads to an increased risk of fractures. In osteoporosis the bone mineral density is rapidly reduced, the bone microarchitecture is disrupted and the amount/variety of non-collagenous bone proteins is altered (Chestnut, 1995; Paschalis et al. 1997). In menopausal women, the rapid decrease of estrogens is the predominant cause of the imbalance between bone formation and bone resorption, which results, in turn, in severe bone loss (Riggs & Melton, 1986). HRT is the traditional method for recovering both bone loss and incidence of skeletal fractures in postmenopausal women (Turner et al. 1994), but, as is well known, it has negative side effects, increasing the occurrence of cardiovascular diseases and endometrial/breast/ovarian malignant cancers (Genant et al. 1998; Termine & Wong, 1998; Lacey et al. 2002; Beral, 2003).
In this context, recent studies have shown that phytoestrogens (a group of plant-derived substances) mime estrogens as osteoprotective substances (Albertazzi, 2002; Wang et al. 2006), without displaying the negative side effects on the etiogenesis of some types of cancer (Lian et al. 2001; Wu et al. 2002; Murray et al. 2003; Limer & Speirs, 2004; Eason et al. 2005; Gallo et al. 2006; Garcia-Perez et al. 2006; Duffy et al. 2007). They are structurally similar to estradiol and their estrogenic-like activity may also depend on their affinity to some estrogen receptors (ERs). Phytoestrogens appear to bind preferentially to the ERβ and have sometimes been classified as selective estrogen receptor modulators (SERMs) (Brzezinski & Debi, 1999; An et al. 2001; Messina et al. 2006). ERβ may play a protective role in breast cancer development by reducing mammary cell growth, as well as inhibiting the stimulatory effects of ERα (An et al. 2001; Strom et al. 2004). Considering the properties of such natural compounds, phytoestrogens could be employed as complementary/alternative medicine (CAM) instead of HRT, to recover menopausal symptoms (Lee et al. 2000; Morris et al. 2000).
The estrogenic-like effects of the phytoestrogen ferutinin have been demonstrated in in vitro and in vivo experiments, particularly in relation to its apoptotic properties, as well as to its influence on calcium mobilization and mitochondrial permeability (Ignatkov et al. 1990; Appendino et al. 2002; Ikeda et al. 2002; Halestrap & Brennerb, 2003; Macho et al. 2004). Despite the huge amount of data published on the effect of ferutinin on calcium-related cellular processes, few observations are reported in literature concerning the effect of ferutinin on the skeleton, particularly on bone metabolism in both the prevention and curative treatment of osteoporosis. Recently, our investigations on the influence of ferutinin on bone metabolism in ovariectomized rats indicated that ferutinin could prevent osteoporosis due to severe estrogen deficiency; in particular, our findings suggest that ferutinin seems to be more effective in preventing bone loss compared with estradiol benzoate (Palumbo et al. 2009).
The present study, which represents an extension of the one quoted above, investigates the effect of ferutinin on the recovery of severe osteoporosis due to estrogen deficiency which occurred after rat ovariectomy.
Materials and methods
Experimental animals and treatments
Forty female Sprague–Dawley rats weighing 170–200 g were purchased from Harlan Italy (Udine, Italy). They were 7 week old according to the general age-models used by Kalu (1991) and Fanti et al. (1998). All rats were housed two per cage and maintained under controlled conditions (22 ± 1 °C, 55–60% humidity, 12-h light/12-h dark). Commercial rat pellets free of estrogenic substances (Global Diet 2018; Mucedola s.r.l., Milan, Italy) and drinking water were available ad libitum throughout the whole experimental period. After 7 days of acclimation to housing conditions, the rats were randomized into four groups of 10 animals each: one group of rats were sham-operated, whereas the rats of the other three groups were ovariectomized. The rats were previously anesthetized with ketamine hydrochloride (Ketavet 100®; Farmaceutici Gellini S.p.a., Aprilia, Italy) plus xylazine hydrochloride (Rompun®; Bayer, Leverkusen, Germany) and the ovaries were bilaterally removed; sham-operation was performed in the same way as ovariectomy, but only exposing the ovaries. Two months after ovariectomy, namely when osteoporosis was caused by the consequent estrogen deficiency, half of the rats of each group underwent the following treatments for 30 days and the remaining ones for 60 days.
Group 1 (SHAM): Sham-operated controls receiving vehicle (5% Tween 80 in water)
Group 2 (C-OVX): Ovariectomized controls receiving vehicle (5% Tween 80 in water)
Group 3 (F-OVX): Ovariectomized treated with ferutinin 2 mg kg−1 day−1
Group 4 (EB-OVX): Ovariectomized treated with estradiol benzoate 1.5 μg per rat twice a week.
Ferutinin was supplied by Indena S.p.a. (Milan, Italy), solubilized in Tween 80 (5%) and deionized water and administered in a volume of 5 mL kg−1 by oral gavage. The dose was selected on the basis of our earlier studies of ferutinin effect on rat sexual behavior (Zanoli et al. 2005; Zavatti et al. 2006) as well as on preventing osteoporosis (Palumbo et al. 2009). Estradiol benzoate (Estradiolo AMSA®, Rome, Italy), used as a reference compound, was dissolved in peanut oil and subcutaneously injected in a volume of 0.3 mL per rat.
The body weight of each animal was recorded at four different times: before ovariectomy (i.e. at the start of the experiment), 2 months after ovariectomy (namely, at the beginning of treatment), and after 30 and 60 days of treatment. At the end of the treatments, all the rats were sacrificed.
All experiments were carried out according to the Bioethical Committee of the Italian National Institute of Health. Animal care, maintenance and surgery were conducted in accordance with Italian law (D.L. no. 116/1992) and European legislation (EEC no. 86/609).
Histology and histomorphometric evaluations
The lumbar vertebrae (4th and 5th) and the right femur were removed from each animal, deprived of soft tissues, fixed in sodium phosphate-buffered (PBS) 4% paraformaldehyde pH 7.4, dehydrated in graded ethanol and embedded in methylmethacrylate resin (Sigma Aldrich, Milan, Italy). The 4th and the 5th lumbar vertebrae were sectioned according to sagittal and transversal planes, respectively, whereas the femurs were sagittally cut at the distal epiphyseal level and transversally cut at the mid-diaphyseal region by means of a Leica SP 1600 diamond saw microtome cutting system (Leica SpA, Milan, Italy) to obtain 50-μm-thick sections for histomorphometric analysis.
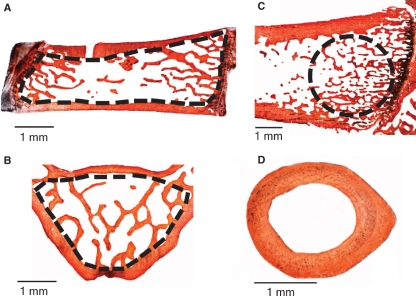
Histomorphometric analysis was performed, as previously reported (Palumbo et al. 2009), on Alizarin-Red stained sections using a light microscope (Zeiss Axiophot; Jena, West Germany) equipped with an image analysis system (Nikon DS-5Mc camera connected to a personal computer – NIS Elements AR 2.20 Nikon software). In histomorphometric evaluations of vertebral bodies, only trabecular bone was taken into account: it was manually selected, outlining the internal surface of the cortical bone (broken lines in Fig. 1A,B). In femoral sagittal sections, a constant area (3.5 mm²) of trabecular bone was selected by drawing a circular line adjacent to the cartilagineous plate (broken line in Fig. 1C). In transversal mid-diaphyseal femoral sections, the cortical bone area was measured (Fig. 1D).
Fig. 1.
Histological sections (50 μm thick) taken from SHAM group showing the regions in which the histomorphometrical analyses were performed: (A) sagittal section of the 4th lumbar vertebra; (B) transversal section of the 5th lumbar vertebra; (C) sagittal section of the distal epiphysis of femur; (D) transversal section at the mid-diaphyseal level of femur. The broken lines indicate the areas in which evaluations were recorded.
The following parameters were calculated: (i) trabecular bone volume (BV/TV) expressed in percent values, i.e. the ratio between the trabecular bone area (BV) and the total area (TV) in trabecular bone, and (ii) cortical bone area (Ct-B-Ar), i.e. the difference between the total cross- section area and the medullary canal area in cortical bone.
Serum measurement
After sacrifice, blood samples were collected in tubes and the sera were immediately separated by centrifugation (4 °C) at 1500 g for 15 min. Sera were than aliquoted into small volumes and stored at −20 °C for successive analyses. The serum levels of total magnesium, calcium, inorganic phosphorus and alkaline phosphatase (ALP) were determined by colorimetry, using commercial test kits (Roche Diagnostic, Milan, Italy) on a Roche Automated Analyzer Cobas 6000 (Roche Diagnostic).
Statistics
The data were analyzed using GraphPad Prism Software, version 4.0 for Windows (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (anova) with Newman–Keuls test for post-hoc comparisons was performed between individual treatment groups and controls. Values of P< 0.05 indicate significant differences among groups.
Results
Body weights
Body weight histograms of all animal groups are shown in Fig. 2. At the start of the experiment, before ovariectomy, all animal body weights were similar. Two months after ovariectomy (namely, at the beginning of treatment) the body weight of ovariectomized rats (C-OVX, F-OVX and EB-OVX) was significantly higher, as expected, with respect to SHAM (P< 0.001). After 30 and after 60 days of chronic administration of ferutinin as well as of estradiol benzoate the body weight of treated animals (F-OVX and EB-OVX) decreased significantly (P< 0.001) in comparison with C-OVX and similar to that of SHAM.
Fig. 2.
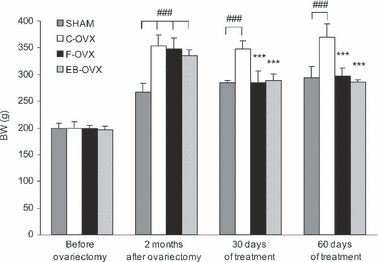
Histograms showing the mean values of body weights (BW, g) recorded from all animal groups at four different times: (1) at the start of the experiment (before ovariectomy), (2) 2 months after ovariectomy, (3) after 30 days of treatment and (4) after 60 days of treatment. Values are expressed as mean ± SEM. ***P< 0.001 vs. C-OVX; ###P< 0.001 vs. SHAM (anova followed by Newman–Keuls test). SHAM, sham-operated controls receiving vehicle; C-OVX, ovariectomized controls receiving vehicle; F-OVX, ovariectomized treated with ferutinin; EB-OVX, ovariectomized treated with estradiol benzoate; BW, body weight.
Histology and histomorphometric evaluations
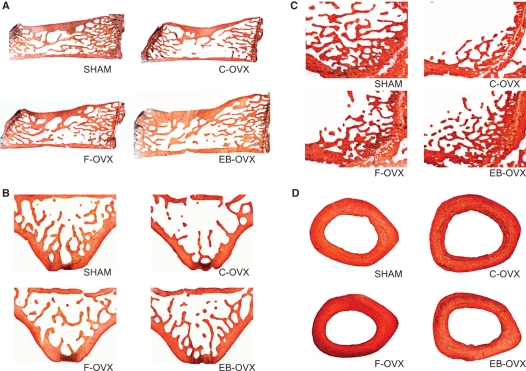
Regarding the morphological observations, histological sections of vertebrae and femurs from treated and control animal groups are shown in Fig. 3. As regards the 30-day treatment, sections taken from both SHAM and treated (F-OVX and EB-OVX) groups clearly showed a higher amount of trabecular bone (Fig. 3A–C) with respect to that observed in the C-OVX group. Considering the SHAM and treated groups, the trabecular bone appeared more abundant in the SHAM sections than in the F-OVX and EB-OVX ones, whereas it did not differ between the two treated groups. No differences in the amount of cortical bone in the mid-diaphyseal femoral sections were seen among the four groups (Fig. 3D). The same results were found for the 60-day treatment (images not included).
Fig. 3.
LM micrographs (50 μm thick) showing the bone histology from the four experimental animal groups after 30 days of treatment. (A) Sagittal sections of the 4th lumbar vertebra; (B) transversal sections of the 5th lumbar vertebra; (C) sagittal sections of the distal epiphysis of femur; (D) transversal sections at the mid-diaphyseal level of femur.
The histomorphometric results obtained after 30 and 60 days of treatment are shown in Figs 4 and 5, respectively. The mean values of BV/TV reported in the (A), (B) and (C) histograms of both figures were recorded from trabecular bone of the 4th (A) and the 5th (B) vertebral bodies and of femur distal epiphysis (C), whereas the Ct-B-Ar mean values reported in (D) were recorded from femur cortical bone at the mid-diaphyseal level. The quantitative analyses showed different results in trabecular and cortical bone, as described below.
Fig. 4.
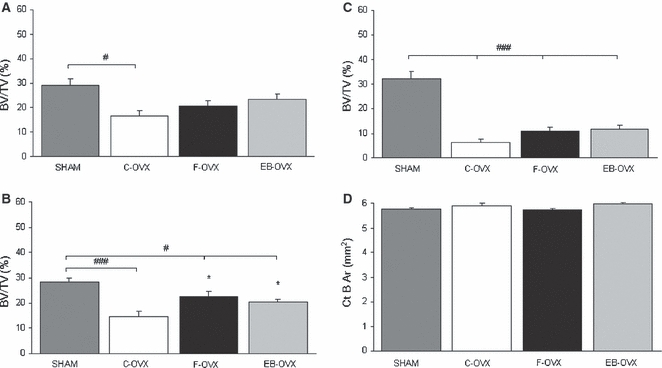
Mean values of histomorphometric parameters, expressed as BV/TV (%) and CT-B-Ar (mm2), in both trabecular and cortical bone of the four animal groups after 30 days of treatment. (A) Sagittal section of the 4th lumbar vertebra; (B) transversal section of the 5th lumbar vertebra; (C) sagittal section of the distal epiphysis of femur; (D) transversal section at the mid-diaphyseal level of femur. Values are expressed as mean ± SEM. *P< 0.05 vs. C-OVX; #P< 0.05, ###P< 0.001 vs. SHAM (anova followed by Newman–Keuls test). SHAM, sham-operated controls receiving vehicle; C-OVX, ovariectomized controls receiving vehicle; F-OVX, ovariectomized treated with ferutinin; EB-OVX, ovariectomized treated with estradiol benzoate.
Fig. 5.
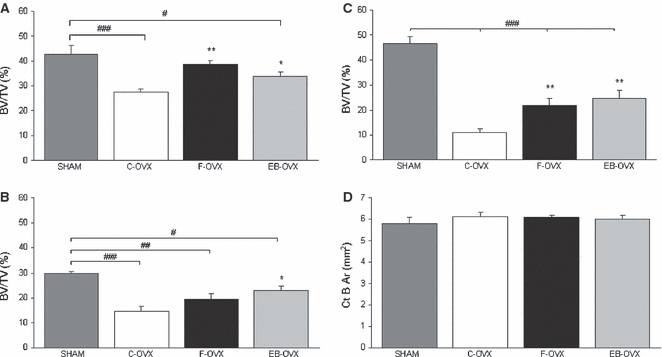
Mean values of histomorphometric parameters, expressed as BV/TV (%) and Ct-B-Ar (mm2), in both trabecular and cortical bone of the four animal groups after 60 days of treatment. (A) Sagittal section of the 4th lumbar vertebra; (B) transversal section of the 5th lumbar vertebra; (C) sagittal section of the distal epiphysis of femur; (D) transversal section at the mid-diaphyseal level of femur. Values are expressed as mean ± SEM. *P< 0.05, **P< 0.01 vs. C-OVX; #P< 0.05, ##P< 0.01, ###P< 0.001 vs. SHAM (anova followed by Newman–Keuls test). SHAM, sham-operated controls receiving vehicle; C-OVX, ovariectomized controls receiving vehicle; F-OVX, ovariectomized treated with ferutinin; EB-OVX, ovariectomized treated with estradiol benzoate.
Trabecular bone
After both 30 and 60 days of treatment (Figs 4 and 5, Tables 1 and 2) the BV/TV mean values in the C-OVX group were always statistically lower, as expected, with respect to the SHAM-operated animals (A,B,C). The values of BV/TV in F-OVX and EB-OVX animals were always higher, but were statistically significant only after 60 days of treatment with respect to the C-OVX ones, although they did not reach the values of SHAM animals. The values of bone mass of the two groups of treated animals (F-OVX and EB-OVX) were always similar; it is also to be noted that in the 4th vertebral bodies as well as in femur distal epiphyses, the values of bone mass were higher after 60 days than after 30 days of treatment (compare Fig. 4A,C with Fig. 5A and C).
Table 1.
Mean values of histomorphometric parameters, expressed as BV/TV (%) and Ct-B-Ar (mm2), in both trabecular and cortical bone of the four animal groups, 2 months after OVX and at the end of 30 days of treatment.
| 4th lumbar vertebra sagittal sections BV/TV (%) | 5th lumbar vertebra transversal sections BV/TV (%) | Femur sagittal sections of distal epiphysis BV/TV (%) | Femur transversal sections of mid-diaphysis Ct-B-Ar (mm2) | |
|---|---|---|---|---|
| SHAM | 29.2 ± 2.6 | 28.4 ± 1.6 | 32.1 ± 3.1 | 5.740 ± 0.066 |
| C-OVX | 16.8 ± 2.0# | 14.7 ± 2.0### | 6.4 ± 1.3### | 5.888 ± 0.114 |
| F-OVX | 20.5 ± 2.2 | 22.5 ± 2.0#,* | 10.6 ± 1.7### | 5.718 ± 0.105 |
| EB-OVX | 23.3 ± 2.2 | 20.5 ± 0.9#,* | 11.6 ± 1.6### | 5.965 ± 0.050 |
Values are expressed as mean ± SEM.
P< 0.05 vs. C-OVX;
P< 0.05,
P< 0.001 vs. SHAM (anova followed by Newman–Keuls test).
SHAM, sham-operated controls receiving vehicle; C-OVX, ovariectomized controls receiving vehicle; F-OVX, ovariectomized treated with ferutinin; EB-OVX, ovariectomized treated with estradiol benzoate.
Mean values of histomorphometric parameters, expressed as BV/TV (%) and Ct-B-Ar (mm2), in both trabecular and cortical bone of the four animal groups, 2 months after OVX and at the end of 60 days of treatment.
| 4th lumbar vertebra sagittal sections BV/TV (%) | 5th lumbar vertebra transversal sections BV/TV (%) | Femur sagittal sections of distal epiphysis BV/TV (%) | Femur transversal sections of mid-diaphysis Ct-B-Ar (mm2) | |
|---|---|---|---|---|
| SHAM | 42.8 ± 3.5 | 29.7 ± 1.0 | 46.6 ± 2.6 | 5.798 ± 0.311 |
| C-OVX | 27.5 ± 1.1### | 14.6 ± 2.0### | 11.1 ± 1.5### | 6.135 ± 0.188 |
| F-OVX | 38.7 ± 1.6** | 19.5 ± 2.3## | 21.8 ± 2.8###,** | 6.087 ± 0.105 |
| EB-OVX | 33.8 ± 1.6#,* | 22.9 ± 1.7#,* | 24.6 ± 3.2###,** | 6.002 ± 0.179 |
Values are expressed as mean ± SEM.
P< 0.05
P < 0.01 vs. C-OVX;
P< 0.05
P< 0.01
P< 0.001 vs. SHAM (anova followed by Newman–Keuls test).
SHAM, sham-operated controls receiving vehicle; C-OVX, ovariectomized controls receiving vehicle; F-OVX, ovariectomized treated with ferutinin; EB-OVX, ovariectomized treated with estradiol benzoate.
Cortical bone
No statistically significant differences were found in cortical bone area (Ct-B-Ar) at femoral mid-diaphyseal level among the four groups after 30 and 60 days of treatments (Figs 4D and 5D,Tables 1 and 2).
Serum measurement
The rat serum parameters of the four groups are reported in Table 3. After 30 and 60 days of treatment, the serum magnesium and calcium mean values did not differ among the four groups, whereas only after 60 days of treatment were the inorganic phosphorus mean values of all ovariectomized animals (both control and treated) lower than SHAM animals (P< 0.05). Serum ALP levels of F-OVX were always higher than all the other groups, with statistically significant differences compared with all the other groups after 30 days of treatment, but only compared with EB-OVX group after 60 days of treatment.
Table 3.
Mean values of rat serum parameters in the four animal groups (30 and 60 days of treatment).
| Treatment group | Mg (mg dL−1) | Ca (mg dL−1) | P inorganic (mg dL−1) | ALP (UI L−1) |
|---|---|---|---|---|
| 30-day treatment | ||||
| SHAM | 2.43 ± 0.06 | 10.6 ± 0.01 | 6.29 ± 0.43 | 103 ± 13.65 |
| C-OVX | 2.41 ± 0.07 | 10.18 ± 0.18 | 7.18 ± 0.3 | 81.6 ± 3.98 |
| F-OVX | 2.43 ± 0.04 | 10.56 ± 0.1 | 6.09 ± 0.22 | 144.6 ± 15.4**,#,++ |
| EB-OVX | 2.48 ± 0.05 | 10.52 ± 0.1 | 6.57 ± 0.25 | 75 ± 12.5 |
| 60-day treatment | ||||
| SHAM | 2.57 ± 0.06 | 10.25 ± 0.16 | 7.52 ± 0.46 | 89.5 ± 9.24 |
| C-OVX | 2.86 ± 0.4 | 10.28 ± 0.02 | 6.2 ± 0.22# | 90.2 ± 8.61 |
| F-OVX | 2.55 ± 0.07 | 10.58 ± 0.12 | 6.37 ± 0.27# | 109.2 ± 7.19+ |
| EB-OVX | 2.47 ± 0.04 | 10.58 ± 0.14 | 6.02 ± 0.17# | 72.8 ± 5.91 |
All values are expressed as mean ± SEM.
anova followed by Newman–Keuls test:
P< 0.01 vs. C-OVX;
P< 0.05,
P< 0.01 vs. EB-OVX;
P< 0.05 vs. SHAM.
SHAM, sham-operated controls receiving vehicle; C-OVX, ovariectomized controls receiving vehicle; F-OVX, ovariectomized treated with ferutinin; EB-OVX, ovariectomized treated with estradiol benzoate.
Discussion
The aim of the present investigation, based on the rat model, was to study the effects of ferutinin administration in recovering severe osteoporosis due to estrogen deficiency which occurs after ovariectomy, and to compare these effects with those of estradiol benzoate treatment, in the search for a suitable alternative to the long-term hormone replacement therapy (HRT) commonly used by osteoporotic women. The OVX rat model has been used because of their ability to simulate the various clinical human syndromes deriving from osteoporosis (Kalu, 1991; Wronski & Yen, 1991; Xv et al. 2002; Comelekoglu et al. 2006; Wang et al. 2006).
The first point to discuss is the difference between this and our previous study (Palumbo et al. 2009) concerning the time that elapses between ovariectomy and the start of the treatment. In the previous investigation the chronic treatment with both ferutinin and estradiol benzoate started the day after ovariectomy, whereas in the present work the same treatment was performed 2 months after ovariectomy; this explains some of the circumstances outlined below. According to the ponderal gain it is to be noted that, in the present work, 2 months after ovariectomy the body weights of all OVX animals (C-OVX, F-OVX, EB-OVX) were, as expected, all similar but higher with respect to the SHAM group; after both 30 and 60 days of treatment the F-OVX and EB-OVX animal body weights decreased, reaching those of the SHAM group but, unlike what occurred in the previous study, they never fell below the values of SHAM ones. This difference between the two studies is due to the period of time (2 months) in the present work that elapses between the day of ovariectomy and the starting day of the treatment. For this reason, in the present paper, on the basis that differences do not exist among the weights of treated (F-OVX and EB-OVX) and SHAM animals, histomorphometric parameters (BV/TV and Ct-B-Ar) were not normalized (i.e. corrected) with respect to body weight (such normalization was necessary in the previous work to eliminate the recorded effects of different body weights). It is confirmed, by the data here recorded, that ferutinin significantly inhibited the ponderal increase, indicating estrogen-like effects of ferutinin on body weight.
The second point to discuss is that despite the recovery of osteoporotic bone that occurred, the values of bone mass of treated animals do not reach those of the SHAM group; this is due to the fact that the treatment started after the occurrence of a severe osteoporosis that was established as a consequence of estrogen deficiency over a period of 2 months following the ovariectomy. It is also to be noted that BV/TV values recorded in the 4th vertebral bodies as well as in femur distal epiphyses increased after 60 days compared with 30 days of treatment, suggesting that if the administration of ferutinin were extended for a period longer than 60 days the loss of bone mass could be completely recovered, probably reaching the BV/TV values of the SHAM group.
Another finding to underline is that ovariectomy affects trabecular and cortical bone in different ways, as in OVX animals the bone mass loss observed in trabecular bone was not equally observed in cortical bone; in fact, the values of femur Ct-B-Ar are similar in the OVX and SHAM groups (Figs 4 and 5). These data are in line with a previous paper (Lozupone & Favia, 1988) showing that the decrease in bone mass starts earlier and is more extensive in the spongiosa than in the compacta of rats fed with a low-calcium diet. This fact is a consequence of the different pattern of distribution of mechanical stresses acting on the two different bony architectures and is probably related to the different metabolism of the various skeletal regions, which, in turn, affect the bone turnover rate of the different regions, viz. metaphysis compared with diaphyses (Canè et al. 1982). Moreover, other authors have shown that cortical bone is not very susceptible to bone loss due to ovariectomy standing the increased endosteal osteoblasts (Turner et al. 1987; Jee et al. 1990; Liu & Kalu, 1990).
The only difference observed in serum Mg, Ca and P inorganic levels, related to P inorganic levels after 60 days of treatment in all ovariectomized animal groups vs. the SHAM ones. It is possible to argue that homeostatic mechanisms are able to maintain serum levels of these minerals despite ovariectomy. The values of ALP, the most widely recognized biochemical marker for osteoblastic activity (Evans et al. 1990; Nian et al. 2006), were higher in F-OVX than in all the other groups, with statistical significance mostly after 30 days of treatment. This suggests that the process of osteogenesis is triggered more in the F-OVX group. This result notwithstanding, the bone mass values were not significantly higher in F-OVX animals than EB-OVX animals, but it is possible to explain this apparent incongruity by the occurrence of a lower osteoclastic activity in EB-OVX. In line with this interpretation are the observations of some authors concerning the E2 stimulatory effect by estrogens on the induction of osteoclast apoptosis in vitro and in vivo and, consequently, their direct effect on the decrease of osteoclast activity (Hughes et al. 1996; Venken et al. 2008). As is well known, mature osteoclasts reabsorb bone, and an increase in their number implies increased bone destruction; because estrogens facilitate the expression of the FasL gene in osteoclasts via ERα and control the life span of osteoclasts by inducing apoptosis, they act to inhibit bone resorption by controlling the number of osteoclasts (Nakamura et al. 2007; Imai et al. 2009).
The same considerations explain the similar levels of serum calcium in F-OVX and EB-OVX animals, based on the positive modulation of bone formation in F-OVX and the negative effect on osteoclastic activity in EB-OVX. Both serum and urinary indexes of osteoclast activity will be considered in the near future to clarify the implications of this.
In the light of the observations reported in the present paper on the effect of ferutinin in recovering severe osteoporosis secondary to ovariectomy in rats, the authors suggest listing ferutinin among the substances representing a potential alternative for the recovery of bone mass in postmenopausal osteoporosis which occurs in women as a result of estrogen deficiency. This fact acquires much greater importance in the light of recent tenable evidence, as cited above, reported by some authors concerning the absence of negative side effects caused by phytoestrogens, particularly genistein, 8-prenylnaringenin, resveratrol and red clover extract, on the tropism of various organs commonly targeted by estrogens (Lian et al. 2001; Burdette et al. 2002; Wu et al. 2002; Murray et al. 2003; Limer & Speirs, 2004; Eason et al. 2005; Hümpel et al. 2005; Gallo et al. 2006; Garcia-Perez et al. 2006; Whitsett & Lamartiniere, 2006; Duffy et al. 2007). The authors are aware that additional studies are required to characterize the mechanism by which ferutinin acts in improving/resolving severe degrees of bone mass loss.
Acknowledgments
This study was supported by 2009-2010 ‘Fondazione di Vignola’ funds and by ‘Banca Popolare dell’Emilia Romagna’.
References
- Table 2.Albertazzi P. Purified phytoestrogens in postmenopausal bone health: is there a role for genistein? Climacteric. 2002;5:190–196. [PubMed] [Google Scholar]
- An J, Tzagarakis-Foster C, Scharschmidt TC, et al. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276:17808–17814. doi: 10.1074/jbc.M100953200. [DOI] [PubMed] [Google Scholar]
- Appendino G, Spagliardi P, Cravotto G, et al. Daucane phytoestrogens: a structure-activity study. J Nat Prod. 2002;65:1612–1615. doi: 10.1021/np0201671. [DOI] [PubMed] [Google Scholar]
- Beral V. Million Women Study collaborators. Breast cancer and hormone replacement therapy in the Million Women Study. Lancet. 2003;362:1160. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Brzezinski A, Debi A. Phytoestrogens: the “natural” selective receptor modulators? Eur J Obstet Gynecol Reprod Biol. 1999;85:47–51. doi: 10.1016/s0301-2115(98)00281-4. [DOI] [PubMed] [Google Scholar]
- Burdette JE, Liu J, Lantvit D, et al. Trifolium pratense (red clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J Nutr. 2002;132:27–30. doi: 10.1093/jn/132.1.27. [DOI] [PubMed] [Google Scholar]
- Canè V, Marotti G, Volpi G, et al. Size and density of osteocyte lacunae in different regions of long bones. Calcif Tissue Int. 1982;34:558–563. doi: 10.1007/BF02411304. [DOI] [PubMed] [Google Scholar]
- Chestnut CH., III . Drug therapy: calcitonin, bisphosphonates, and anabolic steroids. In: Riggs BL, Melton LJ III, editors. Osteoporosis: Etiology, Diagnosis, and Management. Philadelphia: Lippincott-Raven; 1995. [Google Scholar]
- Comelekoglu U, Bagis S, Yalin S, et al. Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin Rheumatol. 2006;26:380–384. doi: 10.1007/s10067-006-0367-2. [DOI] [PubMed] [Google Scholar]
- Duffy C, Perez K, Partridge A. Implications of phytoestrogen intake for breast cancer. CA Cancer J Clin. 2007;57:260–277. doi: 10.3322/CA.57.5.260. [DOI] [PubMed] [Google Scholar]
- Eason RR, Till SR, Velarde MC, et al. Uterine phenotype of young adult rats exposed to dietary soy or genistein during development. J Nutr Biochem. 2005;16:625–632. doi: 10.1016/j.jnutbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Evans DB, Bunning RAD, Russell RGG. The effect of recombinant human interleukine-1β on cellular proliferation and the production of prostaglandin E2, plasminogen activator, ostecalcin and alkaline phosphatase by osteoblast-like cells derived from human bone. Biochem Biophys Res Commun. 1990;166:208–216. doi: 10.1016/0006-291x(90)91932-i. [DOI] [PubMed] [Google Scholar]
- Fanti P, Monier-Faugere MC, Geng Z, et al. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos Int. 1998;8:274–281. doi: 10.1007/s001980050065. [DOI] [PubMed] [Google Scholar]
- Gallo D, Zannoni GF, Martinelli E, et al. Estradiol and phytoestrogens differently influence the rodent postmenopausal mammary gland. Menopause. 2006;13:72–79. doi: 10.1097/01.gme.0000191208.05491.94. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez MA, Noguera R, del Val R, et al. Comparative effects of estradiol, raloxifene, and genistein on the uterus of ovariectomized mice. Fertil Steril. 2006;86:1003–1005. doi: 10.1016/j.fertnstert.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Genant HK, Baylink DJ, Gallagher JC. Estrogens in the prevention of osteoporosis in postmenopausal women. Am J Obstet Gynecol. 1998;161:1842–1846. doi: 10.1016/s0002-9378(89)80004-3. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Brennerb C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Dai A, Tiffee JC, et al. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- Hümpel M, Isaksson P, Schaefer O, et al. Tissue specificity of 8-prenylnaringenin: protection from ovariectomy induced bone loss with minimal trophic effects on the uterus. J Steroid Biochem. 2005;97:299–305. doi: 10.1016/j.jsbmb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ignatkov V, Akhmedkhodzhaeva KhS, Babichev VN. The effect of tefestrol on the secretion of luteinizing hormone from the hypophysis. Farmakol Toksikol. 1990;53:37–38. [PubMed] [Google Scholar]
- Ikeda K, Arao Y, Otsuka H, et al. Terpenoids found in the Umbelliferae family act as agonists/antagonists for ER(alpha) and ER(beta): differential transcription activity between ferutinine-liganded ER(alpha) and ER(beta) Biochem Biophys Res Commun. 2002;291:354–360. doi: 10.1006/bbrc.2002.6446. [DOI] [PubMed] [Google Scholar]
- Imai Y, Nakamura T, Matsumoto T, et al. Molecular mechanisms underlying the effects of sex steroids on bone and mineral metabolism. J Bone Miner Metab. 2009;27:127–130. doi: 10.1007/s00774-008-0021-y. [DOI] [PubMed] [Google Scholar]
- Jee WSS, Mori S, Lee XJ, et al. Prostaglandin E2 enhances cortical bone mass and activates intracortical bone remodeling in intact and ovariectomized female rats. Bone. 1990;11:253–266. doi: 10.1016/8756-3282(90)90078-d. [DOI] [PubMed] [Google Scholar]
- Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- Lacey JV, Jr, Mink PJ, Lubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- Lee MM, Lin SS, Wrensch MR, et al. Alternative therapies used by women with breast cancer in four ethnic populations. J Natl Cancer Inst. 2000;92:42–47. doi: 10.1093/jnci/92.1.42. [DOI] [PubMed] [Google Scholar]
- Lian Z, Niwa K, Tagami K, et al. Preventive effects of isoflavones, genistein and daidzein, on estradiol-17β- related endometrial carcinogenesis in mice. Jpn J Cancer Res. 2001;92:726–734. doi: 10.1111/j.1349-7006.2001.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limer JL, Speirs V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. 2004;6:119–127. doi: 10.1186/bcr781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kalu DN. Human parathyroid hormone-(1-34) prevents bone loss and augments bone formation in sexually mature ovariectomized rats. J Bone Miner Res. 1990;5:973–982. doi: 10.1002/jbmr.5650050911. [DOI] [PubMed] [Google Scholar]
- Lozupone E, Favia A. Distribution of resorption processes in the compacta and spongiosa of bones from lactating rats fed a low-calcium diet. Bone. 1988;9:215–224. doi: 10.1016/8756-3282(88)90034-8. [DOI] [PubMed] [Google Scholar]
- Macho A, Blanco-Molina M, Spagliardi P, et al. Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem Pharmacol. 2004;68:875–883. doi: 10.1016/j.bcp.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- Morris KT, Johnson N, Homer L, et al. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am J Surg. 2000;179:407–411. doi: 10.1016/s0002-9610(00)00358-5. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Meyer WR, Lessey BA, et al. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: a pilot trial. Menopause. 2003;10:456–464. doi: 10.1097/01.GME.0000063567.84134.D1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Nian H, Qin L-P, Zhang Q-Y, et al. Antiosteoporotic activity of Er-Xian Decoction, a traditional Chinese herbal formula, in ovariectomized rats. J Ethnopharmacol. 2006;108:96–102. doi: 10.1016/j.jep.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Palumbo C, Ferretti M, Bertoni L, et al. Influence of ferutinin on bone metabolism in ovariectomized rats. I: Role in preventing osteoporosis. J Bone Miner Metab. 2009;27:538–545. doi: 10.1007/s00774-009-0070-x. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Betts F, Di Carlo E, et al. FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton W., III Involutional osteoporosis. N Engl J Med. 1986;314:1676. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Strom A, Hartman J, Foster JS, et al. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine JD, Wong M. Post-menopausal women and osteoporosis: available choices for maintenance of skeletal health. Maturitas. 1998;30:241–245. doi: 10.1016/s0378-5122(98)00092-9. [DOI] [PubMed] [Google Scholar]
- Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17β-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987;2:115–122. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- Turner RT, Riggs BL, Spelsberg TC. Skeletal effects of estrogen. Endocr Rev. 1994;15:275–300. doi: 10.1210/edrv-15-3-275. [DOI] [PubMed] [Google Scholar]
- Venken K, Callewaert F, Boonen S, et al. Sex hormones, their receptors and bone health. Osteoporos Int. 2008;19:1517–1525. doi: 10.1007/s00198-008-0609-z. [DOI] [PubMed] [Google Scholar]
- Wang ZL, Sun JY, Wang DN, et al. Pharmacological studies of the large-scaled purified genistein from Huaijiao (Sophora japonica-Leguminosae) on anti-osteoporosis. Phytomedicine. 2006;13:718–723. doi: 10.1016/j.phymed.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Whitsett TG, Jr, Lamartiniere CA. Genistein and resveratrol: mammary cancer chemoprevention and mechanisms of action in the rat. Expert Rev Anticancer Ther. 2006;6:1699–1706. doi: 10.1586/14737140.6.12.1699. [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Yen CF. The ovariectomized rat as an animal model for postmenopausal bone loss. Cell Mater. 1991;Suppl 1:69–74. [Google Scholar]
- Wu AH, Wan P, Hankin J, et al. Adolescent and adult soy intake and risk of breast cancers in Asian Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- Xv SY, Bian RL, Chen X. Methods in Pharmacological Sciences. Beijing: People’s Medical Publishing House; 2002. pp. 1560–1568. [Google Scholar]
- Zanoli P, Rivasi M, Zavatti M, et al. Activity of single components of Ferula hermonis on male rat sexual behavior. Int J Impot Res. 2005;17:513–518. doi: 10.1038/sj.ijir.3901346. [DOI] [PubMed] [Google Scholar]
- Zavatti M, Montanari C, Zanoli P. Role of ferutinin in the impairment of female sexual function by Ferula hermonis. Physiol Behav. 2006;89:656–661. doi: 10.1016/j.physbeh.2006.08.002. [DOI] [PubMed] [Google Scholar]