Abstract
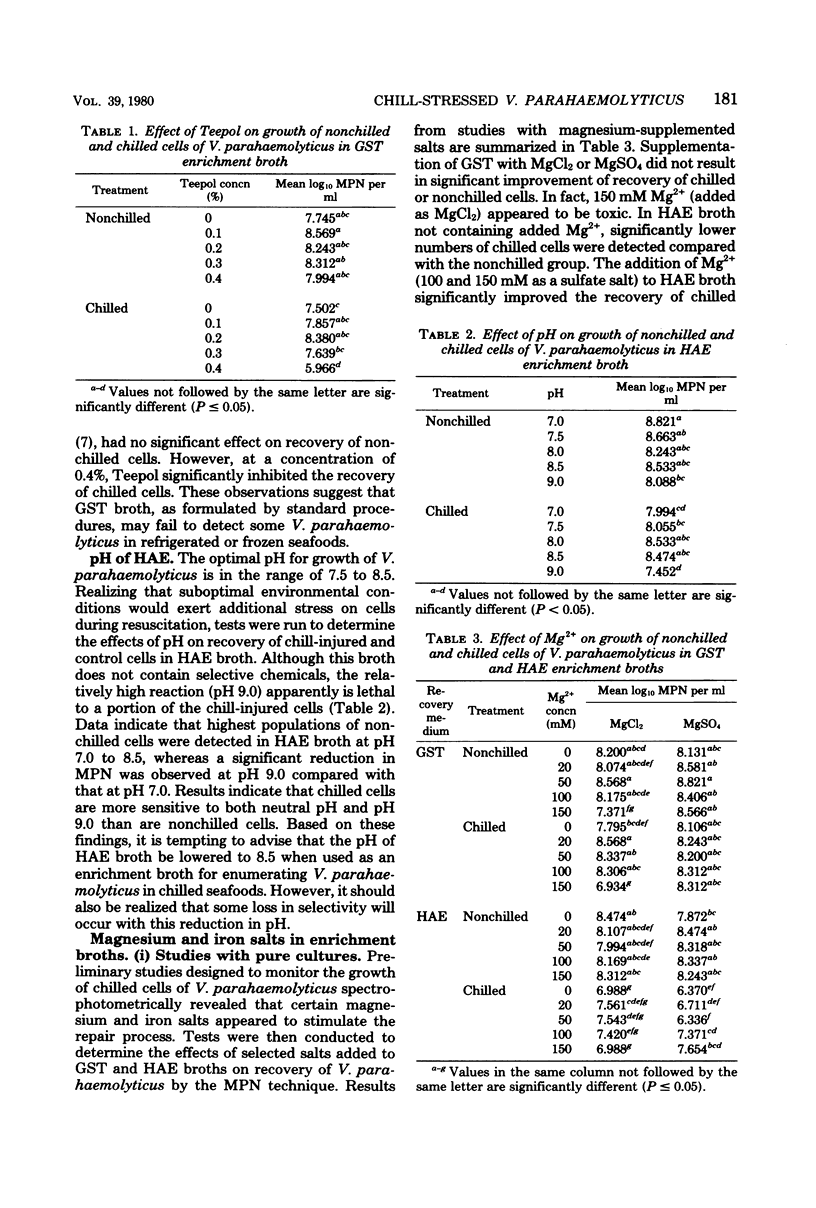
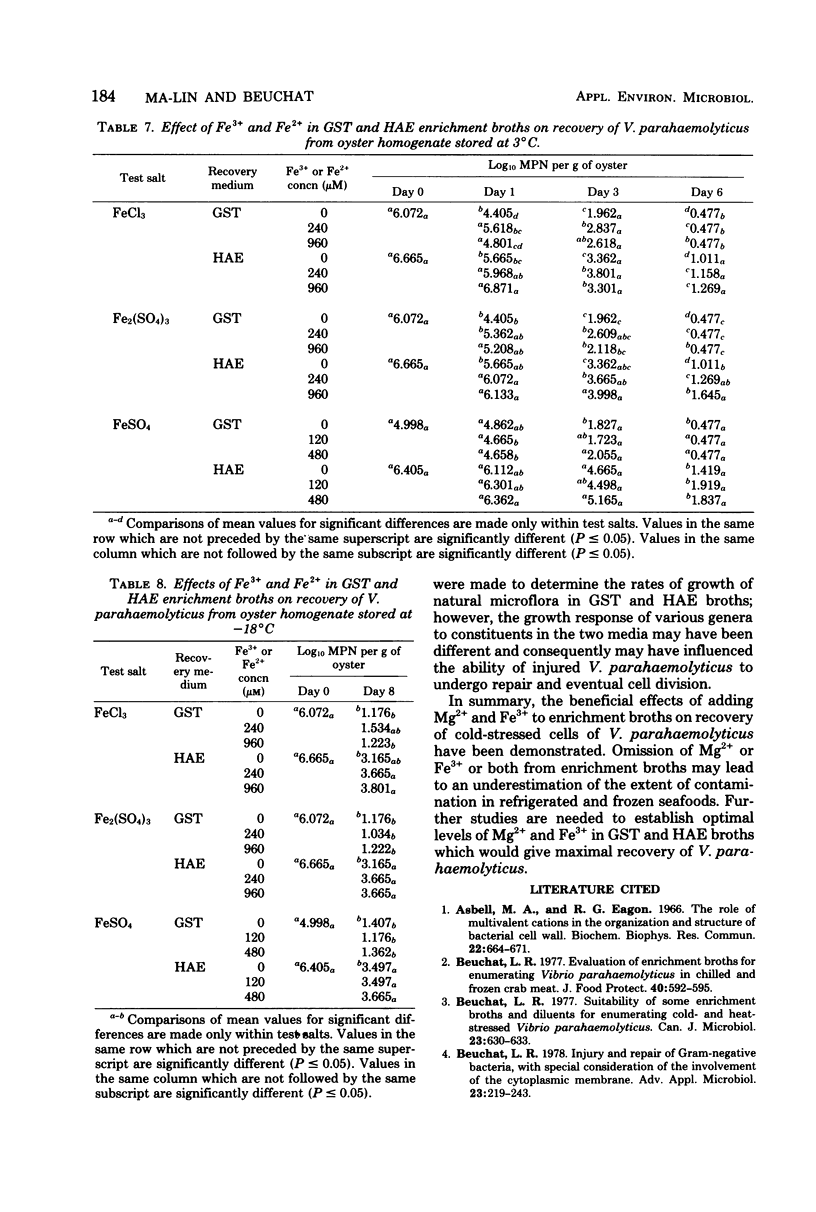
The effects of magnesium and iron salts on the recovery and growth of chill-stressed cells of Vibrio parahaemolyticus were studied. Supplementation of glucose salt Teepol (GST) broth with 20 to 100 mM of Mg2+ significantly (P less than or equal to 0.05) increased the number of cells recovered from oyster homogenate stored at 3 degrees C. Populations detected with supplemented GST were comparable to those obtained with Horie arabinose ethyl violet (HAE) broth, with or without Mg2+. Recovery of V. parahaemolyticus from homogenates stored at -18 degrees C was also improved when enrichment broths supplemented with Mg2+ were used. Ferric iron (added as FeCl3) at 240 microM in GST and 240 or 960 microM in HAE significantly enhanced the extent of recovery of chilled cells. Ferrous iron was generally less effective. Teepol did not influence the growth of nonchilled cells, but significantly reduced the viable population in suspensions of chilled cells when used at a level of 0.4% in GST. The relatively high pH (9.0) of HAE caused a significant reduction in the number of viable, chill-stressed cells of V. parahaemolyticus. The overall results indicated that HAE broth is superior to GST for recovering V. parahaemolyticus from refrigerated and frozen oyster homogenates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. The role of multivalent cations in the organization and structure of bacterial cell walls. Biochem Biophys Res Commun. 1966 Mar 22;22(6):664–671. doi: 10.1016/0006-291x(66)90198-7. [DOI] [PubMed] [Google Scholar]
- Beuchat L. R. Injury and repair of gram-negative bacteria, with special consideration of the involvement of the cytoplasmic membrane. Adv Appl Microbiol. 1978;23:219–243. doi: 10.1016/s0065-2164(08)70071-6. [DOI] [PubMed] [Google Scholar]
- Beuchat L. R. Suitability of some enrichment broths and diluents for enumerating cold- and heat-stressed Vibrio parahaemolyticus. Can J Microbiol. 1977 May;23(5):630–633. doi: 10.1139/m77-092. [DOI] [PubMed] [Google Scholar]
- Covert D., Woodburn M. Relationships of temperature and sodium chloride concentration to the survival of Vibrio parahaemolyticus in broth and fish homogenate. Appl Microbiol. 1972 Feb;23(2):321–325. doi: 10.1128/am.23.2.321-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinis J. J., Beuchat L. R., Boswell F. C. Antimetabolite sensitivity and magnesium uptake by thermally stressed Vibrio parahaemolyticus. Appl Environ Microbiol. 1978 Jun;35(6):1035–1040. doi: 10.1128/aem.35.6.1035-1040.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinis J. J., Beuchat L. R., Jones W. K. Growth of heat-injured Vibrio parahaemolyticus in media supplemented with various cations. Appl Environ Microbiol. 1977 May;33(5):1079–1084. doi: 10.1128/aem.33.5.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A., Hurst A. Magnesium requirement of Staphylococcus aureus for repair from sublethal heat injury. Can J Microbiol. 1976 Aug;22(8):1202–1205. doi: 10.1139/m76-177. [DOI] [PubMed] [Google Scholar]
- Hurst A. Bacterial injury: a review. Can J Microbiol. 1977 Aug;23(8):935–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- Hurst A., Hughes A., Duckworth M., Baddiley J. Loss of D-alanine during sublethal heating of Staphylococcus aureus S6 and magnesium binding during repair. J Gen Microbiol. 1975 Aug;89(2):277–284. doi: 10.1099/00221287-89-2-277. [DOI] [PubMed] [Google Scholar]
- Matches J. R., Liston J., Daneault L. P. Survival of Vibrio parahaemolyticus in fish homogenate during storage at low temperatures. Appl Microbiol. 1971 May;21(5):951–952. doi: 10.1128/am.21.5.951-952.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson M. D., Gomez R. F., Martin S. E. The involvement of nucleic acids in bacterial injury. Adv Appl Microbiol. 1978;23:263–285. doi: 10.1016/s0065-2164(08)70073-x. [DOI] [PubMed] [Google Scholar]
- Ray B., Speck M. L. Freeze-injury in bacteria. CRC Crit Rev Clin Lab Sci. 1973 Aug;4(2):161–213. doi: 10.3109/10408367309151556. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E. EFFECT OF MAGNESIUM ON PERMEABILITY CONTROL IN CHILLED BACTERIA. Nature. 1964 Sep 19;203:1304–1305. doi: 10.1038/2031304a0. [DOI] [PubMed] [Google Scholar]
- Unemoto T., Tsuruoka T., Hayashi M. Role of Na + and K + in preventing lysis of a slightly halophilic Vibrio alginolyticus. Can J Microbiol. 1973 May;19(5):563–571. doi: 10.1139/m73-093. [DOI] [PubMed] [Google Scholar]
- Vanderzant C., Nickelson R. Survival of Vibrio parahaemolyticus in shrimp tissue under various environmental conditions. Appl Microbiol. 1972 Jan;23(1):34–37. doi: 10.1128/am.23.1.34-37.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M. J., Mossel D. A. Sublethal cold shock in Vibrio parahaemolyticus. Appl Environ Microbiol. 1977 Jul;34(1):97–98. doi: 10.1128/aem.34.1.97-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


