Abstract
Using light microscopy, we examined Hunter-Schreger Band (HSB) patterns on the axial and occlusal/incisal surfaces of 160 human teeth, sectioned in both the buccolingual and mesiodistal planes. We found regional variations in HSB packing densities (number of HSBs per mm of amelodentinal junction length) and patterns throughout the crown of each class of tooth (maxillary and mandibular: incisor, canine, premolar, and molar) examined. HSB packing densities were greatest in areas where functional and occlusal loads are greatest, such as the occlusal surfaces of posterior teeth and the incisal regions of incisors and canines. From this it is possible to infer that the behaviour of ameloblasts forming enamel prisms during amelogenesis is guided by genetic/evolutionary controls that act to increase the fracture and wear resistance of human tooth enamel. It is suggested that HSB packing densities and patterns are important in modern clinical dental treatments, such as the bonding of adhesive restorations to enamel, and in the development of conditions, such as abfraction and cracked tooth syndrome.
Keywords: enamel, histology, Hunter-Schreger Bands, prisms, tooth, tooth wear
Introduction
Enamel is the hardest tissue in the vertebrate body and is found covering the crowns of teeth in mammals, reptiles, and amphibians (Boyde, 1997; Koenigswald & Sander, 1997a; Rensberger, 1997). It is insensitive to external stimuli (Boyde, 1997). The internal microstructure of enamel is complex, reflecting the nature of the mechanism behind its formation and the biomechanical demands to which it is exposed (Rensberger, 1997).
At its simplest level, enamel is composed of inorganic and organic phases with the former predominating in mature enamel where it comprises 91% of the volume and 98% of the weight (Boyde, 1964, 1997). The basic structural unit of enamel is the ‘prism’ or ‘rod’, which is composed of crystallites of hydroxyapatite orientated to optimize the mechanical properties of the material (Osborn, 1973; Boyde, 1997; Rensberger, 1997). Although a ‘prism sheath’ has traditionally been described as separating adjacent prisms, it is now thought that this ‘sheath’ is at least in part an artefact of microscopy techniques and the discontinuity between adjacent prisms is, in reality, more subtle. Taken as a whole, the ‘inter-prismatic material’ is a kerithroid or honeycomb-like tracery characterized by the change in crystallite orientation between adjacent prisms that affects the behaviour of transmitted or reflected light, along with a local increase in protein content that alters the refractive index of enamel in this region (Osborn & Roberts, 1971; Koenigswald & Sander, 1997b). Each prism runs in a sinuous course from near the amelodentinal junction (ADJ) to near the external enamel surface (EES) (Osborn, 1973; Berkovitz et al. 1995) and the paths undertaken by enamel prisms, including any decussations or bending, reflect the movements of the ameloblasts that form them during amelogenesis (Boyde, 1964; Osborn, 1973).
It has previously been described that, when light is reflected off an enamel surface that has been created by longitudinally sectioning or fracturing a tooth, an alternating series of dark and light bands may be seen (Hunter, 1778; Schreger, 1800) (Fig. 1). These features are called Hunter-Schreger Bands (HSBs) in recognition of the first observers credited with reporting this curious phenomenon (Hoffman-Axthelm, 1981; Homma, 1990). Neither of those investigators could suggest a reason for the presence of the bands. Until the 1960s the accepted explanation was that the appearance of HSBs was caused by differences in calcification and hardness throughout the enamel, demonstrated by acid-etching, silver-staining, and microradiography techniques (Hollander et al. 1935; Gustafson, 1945; Baud & Held, 1956; Mortell & Peyton, 1956). It was then realized that such theories failed to take into account the significance of varying prism directions in the preparation of specimens included in previous studies (Osborn, 1965). Osborn (1965) postulated that the appearance of HSBs was an optical phenomenon related to the changes in the directions of enamel prisms as they pass through the body of enamel from the ADJ to the EES. Current opinion suggests that the appearance of HSBs is related to the synchronous decussation of enamel prisms in the horizontal plane and is probably caused by reflection of light by inter-prismatic material (Osborn, 1990).
Fig. 1.

The appearance of Hunter-Schreger Bands (alternate dark and light bands), viewed on the labial surface of a maxillary canine using reflected light.
Very few empirical data are available regarding the quantity and distribution of HSBs. Such information would provide useful insights into the behaviour of ameloblasts during amelogenesis. Comparative studies of animal enamel have related HSB packing density with consistency of diet and wear resistance (Rensberger, 1997). There is little doubt that the irregularity of etched enamel surfaces, as occurs during the placement of a dental restoration, is due in part to the variable width and packing density of HSBs and this in turn may affect the bond strength of adhesive restorations to enamel (Shimada & Tagami, 2003). Therefore, the aim of this study was to quantify the HSB packing density in longitudinally-sectioned axial and occlusal surfaces in a representative sample of teeth from the human permanent dentition.
Materials and methods
Sample
To facilitate this study, a privately-owned collection of teeth was made available. All of the teeth in the collection had been extracted in the course of routine dental treatment by some general dental practitioners in Cork City, Ireland during the years 1970–1985. All of the patients had been asked and had given permission for the extracted teeth to be used for the purposes of teaching and/or research. Every patient was of Irish ancestry.
One hundred and sixty permanent teeth were selected. The teeth that were chosen were the first in each series (incisor, canine, premolar, and molar) from each jaw, as representing the most characteristic examples of their type. Selected teeth had intact crowns with minimal or no evidence of attrition, abrasion or erosion, were visually free from defects such as hypoplasia, caries or fractures, had normal root and crown morphology and were unrestored. The numbers of individual tooth types chosen were limited by the overall size of the collection. The only teeth present in relative abundance were maxillary and mandibular first premolars, and the best 30 of these were selected. It was not possible to match those numbers within the remaining tooth types. The best 20 specimens were chosen for the anterior categories and the best 10 for the molars. Thus, the distribution of tooth types included was:
Maxillary central incisors 20 teeth
Maxillary canines 20 teeth
Maxillary first premolars 30 teeth
Maxillary first molars 10 teeth
Mandibular central incisors 20 teeth
Mandibular canines 20 teeth
Mandibular first premolars 30 teeth
Mandibular first molars 10 teeth
Preparation of specimens
Each incisor, canine, and premolar tooth was sectioned once in the mid-buccolingual plane using an ISOMET low-speed saw (Beuhler Ltd, Lake Bluff, IL, USA) fitted with a 4″ diameter diamond-coated wafering blade. The plane of section on canines passed through the cusp tip, whereas those on premolars passed through the buccal and lingual cusps. The molar teeth were sectioned twice in the long axis of their crowns in a buccopalatal or buccolingual direction; the first section passed through both mesial cusps and the second passed through both distal cusps. The sectioned teeth were cleaned of the lubricant fluid by being immersed in a bath of Histolene (CellPath Ltd, Powys, Wales, UK) for 3 h and dried with Velin tissue (Koch-Light Laboratories Ltd, Buckinghamshire, UK). These sectioned specimens were imaged and analysed as outlined below. Following imaging of the specimens in the buccolingual plane, the specimens were then sectioned in the mesiodistal plane, again in the long axis of the crown. Incisor and canine teeth were orientated to allow the plane of section to pass through their incisal edges and cusp tips, respectively. The mesiodistal sectioning plane through the premolar and molar specimens passed midway between their buccal and lingual surfaces.
Imaging
Each specimen was mounted on a glass slide using Plasticine (Flair Leisure Products PLC, Surrey, UK) with care being taken to ensure that the plane of the cut surface of the tooth was parallel to the surface of the glass. The specimens were examined under reflected light using a photomicroscope (Nikon Inc., Instrument Group, Garden City, NY, USA) with a ×4 objective lens. The reflected light was provided by a fibre optic light source (Schott AG, Mainz, Germany), adjusted to give an optimal image. The photomicroscope was fitted with a Panasonic F15HS video camera (Matsushita Electric Industrial Co., Berkshire, UK) that transmitted the image to a Trinitron television monitor (Sony, Hampshire, UK). The magnification factor for the image was calculated.
A tracing was made of the specimen directly from the screen of the television monitor onto a transparent acetate sheet (Folex AG, Switzerland), showing the full extent of the ADJ and EES. The acetate sheet was then removed from the monitor and placed on a horizontal work surface. For each axial surface (i.e. labial, buccal, palatal, lingual, mesial, and distal), the ADJ and EES of each specimen were divided into four segments of equal length. These were named cervical quarter, lower middle quarter, upper middle quarter, and incisal quarter (Fig. 2). The ADJ along the incisal edge of each anterior tooth examined in the mesiodistal plane was divided into two equal segments, as was the ADJ on the occlusal surface of each cusp on posterior teeth sectioned in the buccolingual plane (Fig. 3). The only exception was the occlusal surface of the diminutive lingual cusp of mandibular premolars, which was not sub-divided due to its small size. The ADJ of the occlusal surfaces of premolars and maxillary molars sectioned in the mesiodistal plane mesial to the crest of the transverse or oblique occlusal ridge was divided into two segments of equal length, as was the ADJ distal to the crest of the ridge. As the mid-mesiodistal plane of section for the mandibular molars passed along the occlusal fissure, the ADJ in this region was divided into four segments of equal length.
Fig. 2.
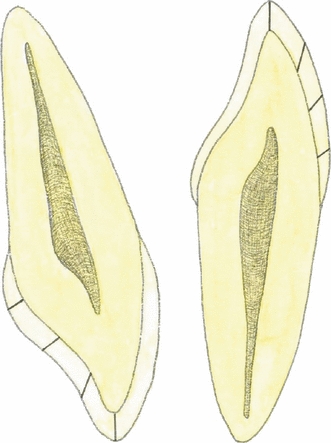
The illustration on the left shows the division into four equal segments of the lingual enamel on the mesial half of a maxillary left central incisor sectioned longitudinally in the mid-labiopalatal plane. The illustration on the right shows the same division into quarters on the mesial half of a mandibular left central incisor sectioned longitudinally in the mid-labiolingual plane. From the incisal edge and moving cervically, these segments were named incisive quarter, upper middle quarter, lower middle quarter, and cervical quarter, respectively.
Fig. 3.
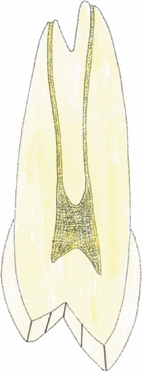
This illustration shows the division into two equal segments of the occlusal enamel on the buccal and palatal cusps on the mesial half of a maxillary right first premolar sectioned longitudinally in the mid-buccopalatal plane.
To transfer the divisions between the segments to the specimens, the tracing of each specimen was replaced on the monitor screen and superimposed on the image and the divisions were marked in the plasticine used for mounting each specimen with a sharp dental explorer (Ash Probe No. 9; Ash Instruments, Surrey, UK). Using ×10 magnification and reflected light, the numbers of HSBs occurring in each segment were counted directly from the monitor screen. A single HSB was defined as a single light or dark band. HSBs were counted in a cervico-occlusal direction. ‘Borderline HSBs’ (those that were intercepted by the division), were included in the more cervical segment. The packing density of the HSBs within each segment was calculated by dividing the total number of HSBs by the length of the ADJ within that segment.
Statistical analysis
The observed HSB packing densities were investigated for differences between tooth groups, tooth surfaces, and tooth segments using a commercially available statistical analytical statistical software package (spss® for Windows® v.13.0, Chicago, IL, USA).
As the distribution of the observed HSB packing densities varied minimally from the normal distribution, parametric tests were considered to be reasonably robust and appropriate for analysing the outcome data. anova models were set up to look for significant differences between the HSB packing density means, adjusting for tooth group, tooth surface, and tooth segment, as appropriate. The P-value was assumed to be 0.05; this was adjusted for multiple testing, using either the Bonferroni or Tamhane corrections, for the t-tests carried out between pairs of surfaces/segments when the anova was significant.
Results
Intra-tooth group comparison of mean Hunter-Schreger Band packing densities: buccolingual plane
The mean HSB packing density in the buccolingual plane for the different types of teeth examined is given in Table 1 and two representative examples are displayed as bar charts in Fig. 4. The data show that the overall pattern of HSB packing density is very similar, but not identical, on the labial and palatal sides within each tooth group. The HSB packing density is least in the cervical quarter and rises through the lower and upper middle quarters to reach a maximum in the incisal quarter. For maxillary incisors, maxillary canines, mandibular canines, and maxillary first premolars, the mean HSB packing density in the incisal quarter is approximately twice that of the cervical quarter but this difference increases to threefold or thereabouts in the remaining teeth.
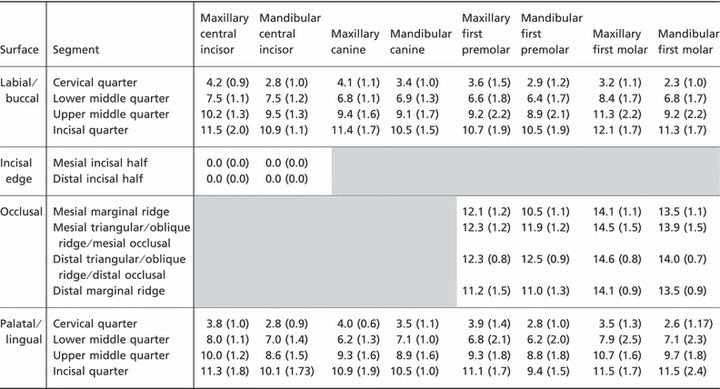
Table 1.
Mean Hunter-Schreger Band (HSB) packing density (HSBs mm−1) in each tooth segment on the buccal, occlusal and lingual surfaces of teeth examined (SDs in parentheses).
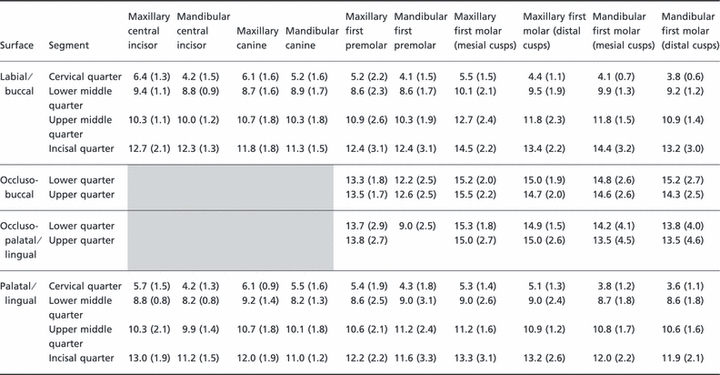 |
Fig. 4.
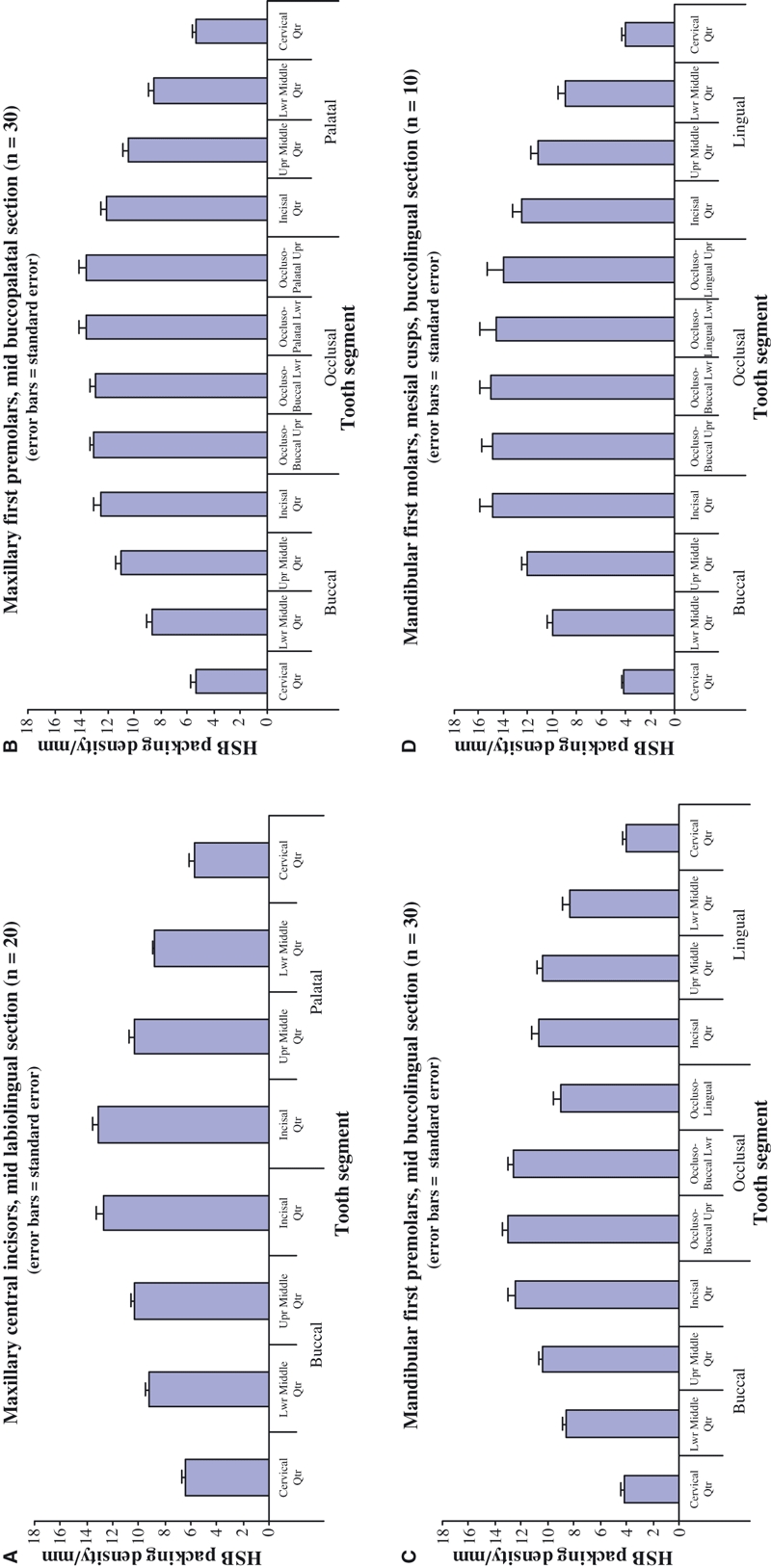
Mean Hunter-Schreger Band (HSB) packing densities: (A) maxillary central incisors, (B) maxillary first premolars, (C) mandibular first premolars, and (D) mandibular first molars (mesial cusps).
The mean HSB packing density in the incisal quarter is slightly greater on the palatal side than on the labial side for maxillary anterior teeth and the reverse is the case for the mandibular anterior teeth (Table 1).
For the posterior teeth, the mean HSB packing density is greatest across the occlusal surfaces with the exception of the occluso-lingual segment of the lower first premolars. The general trend is for the palatal cusps (represented by the combined incisal quarter and both occluso-palatal upper segments) of the maxillary posterior teeth to have a slightly greater mean HSB packing density than the buccal cusps (represented by the combined incisal quarter and both occluso-buccal segments) (Table 1). The reverse is the case for the mandibular posterior teeth.
Intra-tooth group comparison of mean Hunter-Schreger Band packing densities: mesiodistal plane
The mean HSB packing density in the mesiodistal plane for the different types of teeth examined is given in Table 2. The data show that the overall pattern of HSB packing density is very similar, but not identical, on the mesial and distal sides. It is least in the cervical quarter and rises through the lower and upper middle quarters to reach a maximum in the incisal quarter. For all tooth groups examined, the mean packing density in the incisal quarter is 2.5–3.5 times greater than that of the cervical quarter.
Table 2.
Mean Hunter-Schreger Band (HSB) packing density (HSBs mm−1) in each tooth segment on the mesial, occlusal and distal surfaces of teeth examined (SDs in parentheses).
 |
Examination of the incisal edges of the maxillary and mandibular central incisors revealed an absence of HSBs in this region. For the posterior teeth, the mean HSB packing density is greatest across the occlusal surfaces. The general trend is for all mesiodistal occlusal segments to have similar mean HSB packing densities, with a trend for slightly increased mean HSB packing densities in the centre of the occlusal surface, rather than at the marginal ridge areas.
Intra-tooth group comparison of mean Hunter-Schreger Band packing densities: statistical analysis
When individual segments within anterior teeth were compared, it was found that segments within maxillary central incisors, mandibular central incisors, maxillary canines, and mandibular canines, respectively, were significantly different to each other (P < 0.05).
When segments within individual posterior tooth groups were compared, it was found that, within each tooth group, the segments on the axial surfaces (i.e. cervical quarter, lower middle quarter, upper middle quarter, and incisal quarter) were significantly different to each other (P < 0.05). In maxillary first premolars, the axial surface segments were also significantly different to the occlusal surface segments (P < 0.05). In the other three posterior tooth groups, there were occasional comparisons that were not significantly different; these combinations commonly involved the incisal quarter, and occasionally the upper middle quarter, on the axial surfaces. One exception was noted with mandibular first premolars, where the mean HSB packing density in the lower middle, upper middle, and incisal quarters was not different to the occluso-lingual surface.
Representative images of Hunter-Schreger Bands observed
Representative images of the appearance of HSBs in each tooth group were recorded under optimum lighting conditions using the Nikon photomicroscope. HSB patterns of general interest or curiosity were noted. Although no attempt was made to quantify the occurrence of these, patterns of interest are reported below.
Regions of enamel devoid of Hunter-Schreger Bands
In the cervical quarters of axial surfaces in many teeth (Fig. 5).
Along the mesiodistal length of the incisal edge of maxillary and mandibular central incisors; in some teeth the transition between this and the adjacent enamel of the proximal surface where HSBs were numerous was quite marked (Fig. 6).
In parts of the occlusal surface in some premolars and molars.
Fig. 5.
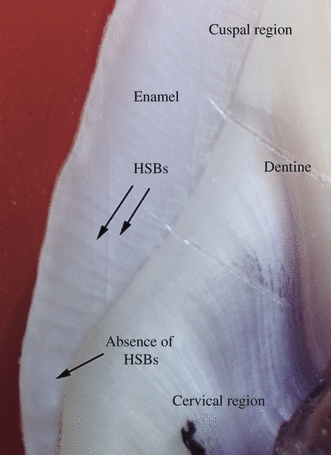
Hunter-Schreger Band (HSB) distribution in the lower middle quarters and cervical quarters of enamel from the palatal surfaces of a maxillary canine. Note the region devoid of HSBs in the cervical quarter.
Fig. 6.
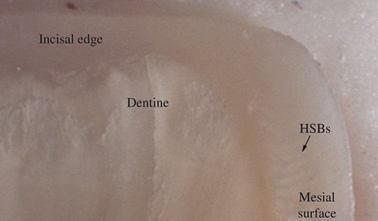
Hunter-Schreger Band (HSB) distribution along the mesial surface and incisal edge of a maxillary central incisor tooth. Note that the incisal edge shown is devoid of HSBs.
The arrangement of Hunter-Schreger Bands over cusp tips, incisal edges, and marginal ridges
HSB patterns over cusp tips were often unclear, although some examples of cup-shaped, inter-digitating and radiating HSB patterns were observed (Fig. 7).
The alignment of HSBs in the marginal ridges conformed to a similar radiating pattern.
Fig. 7.
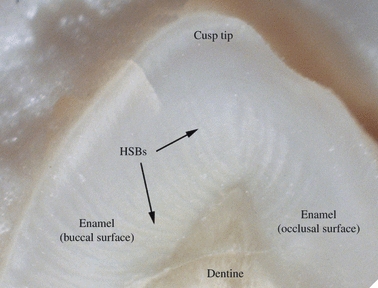
Hunter-Schreger Band (HSB) distribution in a mesiodistal section over the palatal cusp tip of a maxillary premolar.
Variations in the direction/curvature of Hunter-Schreger Bands
Fig. 8.
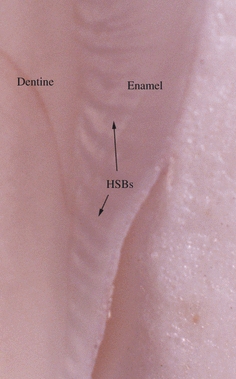
Hunter-Schreger Band (HSB) distribution along the mesiolingual surface of a mandibular first molar. Note the change in shape and orientation of the HSBs along this specimen (from cervical to cuspal) and also that many HSBs do not reach the external enamel surface.
Fig. 9.
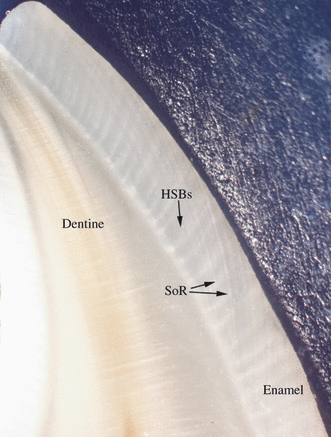
Hunter-Schreger Band (HSB) and Striae of Retzius (SoR) distribution along the labial surface of a mandibular central incisor. Note regions where the SoR appear to interrupt the HSBs.
HSBs in the cervical regions tended to be ‘short and stubby’;
HSBs in the lower middle quarters tended to be straight and directed cervically;
HSBs in the upper middle quarters tended to be narrower and more curved than those in the more cervical regions;
HSBs in the incisal quarters and the occlusal surfaces tended to be quite thin, those on the axial surfaces and upper occlusal surfaces tended to curve cuspally, and those in the mid-occlusal regions tended to be thin and straight.
Variations in the distance from the amelodentinal junction at which the Hunter-Schreger Bands were evident
Most commonly, the HSBs clearly passed from the ADJ to the EES (Figs 1 and 9).
In some regions, the HSBs were not visible in the outer third of the enamel (Fig. 8).
In some teeth, the inter-relationship of HSBs and the Striae of Retzius was quite subtle; in others the interruption of the HSBs by the striae was quite marked (Fig. 9).
Discussion
The present study reveals that HSB distribution throughout the human dentition occurs in a very controlled, almost exquisite, pattern. It is widely accepted that the varying alignment of enamel prisms occurs as a consequence of the patterns of movement of ameloblasts. One theory for the cause of this ameloblast displacement is that they are pushed into new positions by the pressures exerted on them during amelogenesis (Osborn, 1970, 1973). This suggests an over-riding developmental control that, perhaps by determining the sequence of pressures within the developing enamel, causes the necessary movement of the ameloblasts to produce the consequent pattern of HSBs.
Primitive enamel prisms or prism-like structures have been reported in therapsid reptiles of about 180 million years ago (Sahni, 1987) and some other reptilian groups (Torii, 1998) but prismatic enamel is essentially a mammalian characteristic (Poole, 1956; Osborn & Hillman, 1979; Line & Novaes, 2005). For the most part, HSBs in human teeth tend to be straight or curved depending on their position within the crown of the tooth under inspection, although the pattern can be more complicated in the tips of cusps (Osborn, 1968). In other mammals, and particularly among the carnivores, HSB patterns can often be quite dissimilar to the relatively uncomplicated arrangement in humans (Steffen, 1997) and this variation may be linked to the nature of their diet (Koenigswald, 1997; Rensberger, 1997).
Enamel must be sufficiently rigid to resist deformation, while incorporating structural modifications to resist abrasion and fracture. A high mineral content increases its resistance to deformation during mastication but this also increases its brittleness and susceptibility to fracture. The minimum work required to fracture a sample of enamel in its weakest direction, parallel to the long axes of the prisms, is a mere 13 J m−2, whereas the corresponding value for dentine fracture is 270 J m−2 (Rasmussen et al. 1976). The patterns of prism decussation introduce ‘crack-stopping’ properties that significantly increase the overall fracture resistance of enamel (Rensberger, 1997). Each time a prism crosses an HSB it changes direction and an increased packing density of HSBs implies a greater proportion of prism decussations. These decussation patterns are quite complex in animals and often depend on the dietary habits of the animals concerned. Rensberger & Koenigswald (1980) found that, when a large number of enamel prisms within rhinoceros enamel intercept an abrading surface obliquely, the rate of wear is only about 35% of the rate when most of the prisms are parallel to the surface. This was confirmed by Boyde & Fortelius (1986) who concluded that the most important factor in the abrasion resistance of enamel is the direction of the prism axes with respect to the occlusal/chewing surface and enamel prisms that are perpendicular to the chewing surface offer the greatest resistance to abrasion (Boyde & Fortelius, 1986). Studies of various animal groups have indicated that enamel prism direction tends to be asymmetrical with respect to the long axis of the tooth, with the prisms being oriented to ensure minimal abrasion during function (Rensberger & Koenigswald, 1980; Young et al. 1987; Stern et al. 1989; Rensberger, 1997).
Analysis of the data from this study corresponds well with the findings reported from animal studies. Significantly greater mean HSB packing densities are observed on the occlusal surface and most axial surface segments at, or above, the upper middle quarter. It is also noteworthy that the HSB packing densities are not symmetrically distributed around the long axis of the tooth but are greatest in sites exposed to considerable loading forces during articulation and mastication, such as the palatal surfaces of maxillary canines, the labial surfaces of mandibular canines, and the occlusal surfaces of posterior teeth. These surfaces have important functions during articulation and mastication, leaving these exposed to significant loading forces in comparison to other regions.
To date, the properties of HSBs have been poorly investigated in human dentitions and their role in human teeth has been mainly inferred from comparative anatomical studies. Certain aspects of HSB packing densities and distributions clearly have beneficial roles in clinical techniques such as enamel bonding, where the production of a roughened or uneven enamel surface through the application of an acid is desirable (Soetopo & Hardwick, 1978). In addition, some conditions, including abfraction and cracked tooth syndrome, appear to be passively facilitated by HSB distributions, occurring in areas where HSB packing densities are relatively low, such as the cervical regions of the tooth crown (Burke et al. 2000; Lynch & McConnell, 2002).
The lack of HSBs in the cervical enamel regions may reflect the fact that the enamel in this region is not subjected to occlusal loading and that it is also quite thin. The lack of HSBs along the incisal edges of the maxillary and mandibular central incisors may be a consequence of the configuration of the retreating ameloblasts because the shape of the straight incisal edge is quite different to the curved nature of the rest of the crown but it has biomechanical advantages. The lack of HSBs in this area ensures that the rapid abrasion of this HSB-free zone will lead to the exposure of an underlying flat HSB-rich region and the resulting enamel edges will be kept sharp and efficient for incising food. This is similar to a finding by Boyde (1997) who observed that the orientation of the enamel decussations in rhinoceros enamel facilitated localized regions of preferential wear of the occlusal surface creating a serrated surface leading to enhanced mastication and ultimately improved nutrition. Another advantage relates to the loading forces to which the enamel is exposed. Considerable loading of the enamel can occur at the incisal edge. Preferential wear to increase the surface area at the incisal edge would distribute these forces across a wider area, thereby reducing the risk of enamel damage and fracture. A similar pattern was noted in some canines, where diminished HSB packing densities were observed at the cusp tip and load-bearing region.
The reasons for the lack of HSBs on some occlusal surfaces of posterior teeth is interesting. It is unlikely that this would confer a biomechanical advantage to this region where food is crushed. A lack of HSBs may indicate an absence of prisms or that prisms are present but do not decussate. Difficulties can arise during the formation of occlusal fissures as a consequence of crowding of the ameloblasts and reduced access of these cells to nutrition (Boyde, 1989). In such circumstances the enamel may be largely or entirely aprismatic or the conditions are such that decussation is not possible and all of the prisms are similarly orientated.
Conclusion
This study demonstrates that HSB patterns show regional variations in their distribution throughout the enamel of human teeth. HSBs are most concentrated in regions exposed to the greatest functional demand, such as the occlusal surfaces of posterior teeth for chewing and the surfaces of maxillary and mandibular canines for guiding mandibular movement. It is suggested that HSB patterns have developed as a consequence of an evolutionary process. This is in keeping with similar patterns observed in the animal kingdom but it has not previously been reported in the human dentition.
In human teeth, HSB patterns should be considered as a factor in the development and progress of certain clinical conditions that affect enamel, including tooth wear, the resistance of enamel to fracture, cracked tooth syndrome, enamel bonding, and abfraction. Further investigation of the rôle of HSB patterns in the understanding of these clinically significant areas is warranted.
Acknowledgments
The assistance of Professor J. P. Fraher (Professor Emeritus, Department of Anatomy, University College Cork, Ireland) in allowing the use of departmental facilities for the laboratory work detailed in this study is greatly appreciated.
References
- Baud CA, Held AS. Silberfärbung, Rontgenmikrographie und Mineralgehalt der Zahnhartgewebe. Dtsch Zahnärztl Z. 1956;11:309–314. cit. Osborn JW (1965) The nature of the Hunter-Schreger Bands in enamel. Arch Oral Biol10, 929–933. [Google Scholar]
- Berkovitz BKB, Holland GR, Moxham BJ. A Colour Atlas and Text of Oral Anatomy, Histology, and Embryology. 2nd edn. London: Mosby-Wolfe; 1995. pp. 109–124. [Google Scholar]
- Boyde A. The Structure and Development of Mammalian Enamel. London, UK: University of London; 1964. Ph.D. Thesis. [Google Scholar]
- Boyde A. Enamel. In: Oksche A, Vollrath L, editors. Handbook of Microscopic Anatomy, Vol. VI: Teeth. Berlin: Springer-Verlag; 1989. pp. 309–473. [Google Scholar]
- Boyde A. Microstructure of enamel. In: Chadwick D, Cardew G, editors. Dental Enamel (Ciba Foundation Symposium 205) Chichester: Wiley; 1997. pp. 18–31. [DOI] [PubMed] [Google Scholar]
- Boyde A, Fortelius M. Development, structure and function of rhinoceros enamel. Zool J Linn Soc. 1986;87:181–214. [Google Scholar]
- Burke FJT, Johnston N, Wiggs RB, et al. An alternative hypothesis from veterinary science for the pathogenesis of noncarious cervical lesions. Quintessence Int. 2000;31:475–482. [PubMed] [Google Scholar]
- Gustafson G. The structure of human dental enamel. Odontol Tidskr. 1945;53(Supplement):1–150. [Google Scholar]
- Hoffman-Axthelm W. History of Dentistry. Chicago: Quintessence Publishing; 1981. [Google Scholar]
- Hollander F, Bödecker CF, Applebaum E, et al. A study of the bands of Schreger by histological and grenz-ray methods. Dent Cosmos. 1935;77:12–20. [Google Scholar]
- Homma K. Historical studies on the Striae of Hunter-Schreger. Dent Jpn. 1990;27:141–145. [PubMed] [Google Scholar]
- Hunter J. The Natural History of the Human Teeth: Explaining their Structure, Use, Formation, Growth, and Disease including a Practical Treatise on the Diseases of the Teeth: Intended as a Supplement to the Natural History of these Parts. 2nd edn. Birmingham, AL, USA: 1778. Facsimile Reproduction published in 1980 by The Classics of Medicine Library. [Google Scholar]
- Koenigswald Wv. Evolutionary trends in the differentiation of mammalian enamel ultrastructure. In: Koenigswald Wv, Sander PM., editors. Tooth Enamel Microstructure. Rotterdam: Balkema; 1997. pp. 203–235. [Google Scholar]
- Koenigswald Wv, Sander PM. Introduction. In: Koenigswald Wv, Sander PM., editors. Tooth Enamel Microstructure. Rotterdam: Balkema; 1997a. pp. 1–3. [Google Scholar]
- Koenigswald Wv, Sander PM. Glossary of terms used for enamel microstructures. In: Koenigswald Wv, Sander PM., editors. Tooth Enamel Microstructure. Rotterdam: Balkema; 1997b. pp. 267–280. [Google Scholar]
- Line SRP, Novaes PD. The development and evolution of mammalian enamel: structural and functional aspects. Braz J Morphol Sci. 2005;22:67–72. [Google Scholar]
- Lynch CD, McConnell RJ. The cracked tooth syndrome. J Can Dent Assoc. 2002;68:470–475. [PubMed] [Google Scholar]
- Mortell JF, Peyton FA. Observations of Hunter-Schreger Bands. J Dent Res. 1956;35:804–813. doi: 10.1177/00220345560350052201. [DOI] [PubMed] [Google Scholar]
- Osborn JW. The nature of the Hunter-Schreger bands in enamel. Arch Oral Biol. 1965;10:929–935. doi: 10.1016/0003-9969(65)90086-5. [DOI] [PubMed] [Google Scholar]
- Osborn JW. Directions and interrelationship of prisms in cuspal and cervical enamel of human teeth. J Dent Res. 1968;47:395–402. doi: 10.1177/00220345680470030901. [DOI] [PubMed] [Google Scholar]
- Osborn JW. The mechanism of prism formation in teeth: a hypothesis. Calcif Tissue Res. 1970;5:115–132. doi: 10.1007/BF02017542. [DOI] [PubMed] [Google Scholar]
- Osborn JW. Variations in structure and development of enamel. In: Melchior AH, Zarb GA, editors. Oral Sciences Reviews 3: Dental Enamel. Copenhagen: Munksgaard; 1973. pp. 3–83. [PubMed] [Google Scholar]
- Osborn JW. A 3-dimensional model to describe the relation between prism directions, parazones and diazones, and the Hunter-Schreger bands in human tooth enamel. Arch Oral Biol. 1990;35:869–878. doi: 10.1016/0003-9969(90)90065-i. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Hillman J. Enamel structure in some therapsids and mesozoic mammals. Calcif Tissue Int. 1979;29:47–61. doi: 10.1007/BF02408055. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Roberts AM. Optical fringe effects at prism borders in human tooth enamel sections. J Microsc. 1971;93:123–128. doi: 10.1111/j.1365-2818.1971.tb02274.x. [DOI] [PubMed] [Google Scholar]
- Poole DFG. The structure of the teeth of some mammal-like reptiles. Q J Microsc Sci. 1956;97:303–312. [Google Scholar]
- Rasmussen ST, Patchin RE, Scott DB, et al. Fracture properties of human enamel and dentin. J Dent Res. 1976;55:154–164. doi: 10.1177/00220345760550010901. [DOI] [PubMed] [Google Scholar]
- Rensberger JM. Mechanical adaptation in enamel. In: Koenigswald Wv, Sander PM., editors. Tooth Enamel Microstructure. Rotterdam: Balkema; 1997. pp. 237–257. [Google Scholar]
- Rensberger JM, Koenigswald Wv. Functional and phylogenetic interpretation of enamel microstructure in rhinoceroses. Paleobiology. 1980;6:477–495. [Google Scholar]
- Sahni A. Evolutionary aspects of reptilian and mammalian enamel structure. Scanning Microsc. 1987;1:1903–1912. [PubMed] [Google Scholar]
- Schreger D. Beitrag zur Geschichte der Zähne. Beitr Zergliederungskunst. 1800;1:1–7. [Google Scholar]
- Shimada Y, Tagami J. Effects of regional enamel and prism orientation on resin bonding. Oper Dent. 2003;28:20–27. [PubMed] [Google Scholar]
- Soetopo DRB, Hardwick JL. Mechanism of adhesion of polymers to acid-etched enamel. J Oral Rehab. 1978;5:69–80. doi: 10.1111/j.1365-2842.1978.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Steffen C. Differentiations in Hunter-Schreger bands of carnivores. In: Koenigswald Wv, Sander PM., editors. Tooth Enamel Microstructure. Rotterdam: Balkema; 1997. pp. 123–136. [Google Scholar]
- Stern D, Crompton AW, Skobe Z. Enamel ultrastructure and masticatory function in molars of the American opossum, Didelphis virginiana. Zool J Linn Soc. 1989;95:311–334. [Google Scholar]
- Torii S. Origin of enamel prisms and Hunter-Schreger bands in reptilian enamel. Connect Tissue Res. 1998;38:45–51. doi: 10.3109/03008209809017019. [DOI] [PubMed] [Google Scholar]
- Young WG, McGowan M, Daley TJ. Tooth enamel structure in the koala, Phascolarctos cinereus– some functional interpretations. Scanning Microsc. 1987;1:1925–1934. [PubMed] [Google Scholar]


