Abstract
Intensive artificial selection has led to the production of the modern broiler chicken, which over the last few decades has undergone a dramatic increase in growth rate and noticeable changes in body conformation. Unfortunately, this has been associated with musculoskeletal abnormalities which have altered the walking ability of these birds, raising obvious welfare concerns, as well as causing economic losses. Here we present a comparative study of ancestral and derived muscle anatomy in chickens to begin to tease apart how evolutionary alterations of muscle form in chickens have influenced their locomotor function and perhaps contributed to lameness. We measured the muscle architectural properties of the right pelvic limb in 50 birds, including the Giant Junglefowl, a commercial strain broiler and four pureline commercial broiler breeder lines (from which the broiler populations are derived) to identify which features of the broiler’s architectural design have diverged the most from the ancestral condition. We report a decline in pelvic limb muscle mass in the commercial line birds that may compromise their locomotor abilities because they carry a larger body mass. This greater demand on the pelvic limb muscles has mostly led to changes in support at the hip joint, revealing significantly larger abductors and additionally much larger medial rotators in the broiler population. Differences were seen within the commercial line bird populations, which are likely attributed to different selection pressures and may reflect differences in the walking ability of these birds. In addition, Junglefowl seem to have both greater force-generating capabilities and longer, presumably faster contracting muscles, indicative of superior musculoskeletal/locomotor function. We have provided baseline data for generating hypotheses to investigate in greater depth the specific biomechanical constraints that compromise the modern broiler’s walking ability and propose that these factors should be considered in the selection for musculoskeletal health in the chickens of the future. Our new anatomical data for a wide range of domestic and wild-type chickens is useful in a comparative context and for deeper functional analysis including computer modelling/simulation of limb mechanics.
Keywords: artificial selection, broiler chicken, junglefowl, lameness, locomotion, muscle architecture, musculoskeletal system
Introduction
Livestock breeders have deliberately exploited artificial selection for millennia, producing animals with desired characteristics whose phenotypic attributes vary dramatically within a species. Artificial selection of broiler chickens (chickens raised specifically for meat production from ancestral Gallus gallus) has moved from simple mass selection for live weight to include multiple selection criteria for growth-related production traits to increase economic gains and meet the demands of the consumer. The result is an extreme organism, the modern broiler chicken (or simply ‘broiler’), which over 60 years has undergone a 300% increase in growth rate (Knowles et al. 2008) and marked conformation changes, including a significantly larger pectoral muscle mass (Barton, 1994; Lilburn, 1994; Webster, 1995; Nicholson, 1998; Corr et al. 2003a; Schmidt et al. 2009). Unfortunately, strong associations have been made linking skeletal abnormalities to these anatomical changes (Riddel, 1985; Nestor et al. 1987; Lilburn, 1994; Webster, 1995; Julian, 1998; Vestergaard & Sanotra, 1999; Kestin et al. 2001), suggesting their bodies are not evolving in harmony with these traits and perhaps predisposing them to lameness.
Musculoskeletal abnormalities affect approximately 28% of the broiler population (Knowles et al. 2008) and are therefore of vast welfare and economic importance (Thorp, 1994; Bennett et al. 1999; McGeown et al. 1999; Sandoe et al. 1999; Waldenstedt, 2006). Possible environmental/management causes have been well investigated (Riddel, 1985; Nestor et al. 1987; Lilburn, 1994; Thorp, 1994; Webster, 1995; Julian, 1998; Sorenson et al. 1999; Su et al. 1999; Vestergaard & Sanotra, 1999; Kestin et al. 2001; Scott, 2002; Dawkins et al. 2004; Mench, 2004; Brickett et al. 2007; Buijs et al. 2009). However, other studies have shown that the differing physical conformation of the broilers is linked to gait alteration and is an important factor influencing leg health (Corr et al. 2003a; Skinner Noble & Teeter, 2009). To determine whether artificial selection has produced broilers with biomechanical constraints that compromise their walking ability, the functional anatomy of the broiler musculoskeletal system must be further investigated. A simple biomechanical perspective would suggest that a better relationship between production traits (e.g. growth rate; edible mass) and ‘healthy’ locomotor-related traits could be evolved through targeted artificial selection. This requires a better understanding of how the broiler’s muscles transmit the forces that are necessary for support and movement through the environment.
To date, muscle architecture in chickens and more specifically broilers is almost completely unstudied. Only a few studies have detailed basic galliform anatomy (Hudson, 1937; Hudson et al. 1959; George & Berger, 1966), with fewer still providing quantitative anatomical data (in quail, Clark & Alexander, 1975; turkey, Roberts et al. 1997; Roberts, 2001; guinea fowl, Henry et al. 2005; Rubenson et al. 2006). This study is therefore a first step to investigate how evolutionary changes in the pelvic limb of chickens may have influenced their locomotor ability and perhaps contributed to lameness. Because muscle architecture bridges the gap between shape/anatomy (at the organ level) and behaviour/performance (at the whole organism level), we aim to present a quantitative, comparative study of ancestral (approximate wild type; Giant Junglefowl) and derived (commercial line/broiler) muscle anatomy in chickens.
Architectural properties used to calculate the effective physiological cross-sectional area (PCSA; Gans & Bock, 1965) take into account the effect of pennate muscle fascicles on maximizing force per unit area in muscles. PCSA is thus greater in pennate muscles and is directly proportional to the maximum force that can be generated by the muscle (Burkholder et al. 1994; Lieber & Friden, 2000). Muscle contraction velocity and range of motion are also proportional to fibre (or fascicle) length. Hence, quantification of the architectural properties of muscles can expound muscle design and performance by directly relating anatomical form to biomechanical function. However, other physiological properties such as fibre type characteristics or intrinsic properties, including activation and relaxation times, also play major roles (Close, 1972; Bennett et al. 1989; Josephson, 1999; Herrel et al. 2007; James et al. 2007); these will be the focus of a future study.
To further investigate the effects of selection pressure (and other evolutionary patterns that may have influenced muscle architecture) on the modern broiler, we make an additional comparison to the pureline commercial broiler breeder lines (pureline A, B, C and D). These represent the top level of modern breeding programmes – i.e. birds from which the broiler populations are derived selected for optimal breeder and broiler performance (Fig. 1). While there is no reason why these birds should have different pelvic limb anatomy, differences in general body shape exist, and we investigated these for functional significance. Finally, we carry out a longitudinal study by comparing the broilers with Giant Junglefowl at two points: at the same absolute age (6 weeks) and when the Giant Junglefowl are fully grown (15 weeks) and have bodyweights more closely approaching those of broilers. This allows us to distinguish between differences arising as a result of the higher postnatal growth rate in the broilers and an extended period of growth in the Junglefowl, as well as looking at the direct result of artificial selection. We propose that the Giant Junglefowl will have pelvic limb muscles that are longer, presumably faster contracting and with a wider range of motion, and with higher force-generating capabilities compared to commercial line birds (broiler and pureline populations), in which stability and economical force generation maybe more important to sustain efficient locomotion.
Fig. 1.
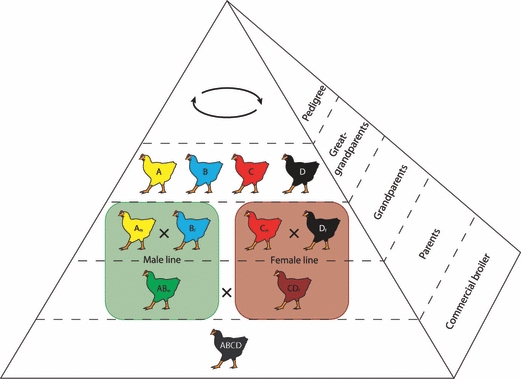
A typical modern broiler chicken breeding programme, represented as a pyramid where each level represents a generation. The great-grandparent line/purelines on the top of the production line are where desired traits are selected across four lines. Within the pedigree segment are the specific male and female lines, with the males typically selected for heritable growth and production traits and the female lines selected for early growth and conformation (Anthony, 1998). The commercial broiler (fifth generation) is derived from the cross of a male and female parent line.
Methods
In this study, fresh male bird cadavers of the four purelines (n = 5 per group), the commercial strain broiler (n = 10) and the Giant Junglefowl (adult and juvenile; n = 10 per group) were used (Table 1). Only male birds were used because the prevalence of skeletal deformity is greater in male domestic birds, including chickens, turkeys and ostriches (Haye & Simons, 1978; Randall & Mills, 1981; Duff et al. 1987; Bezuidenhout & Burger, 1993). Thus studies of male birds are more likely to reveal fundamental constraints on musculoskeletal health. The commercial strain broiler is a hybrid developed in the UK in the 1970s, and is one of the most widely distributed products in markets around the world. The Giant Junglefowl is a typical progenitor obtained from a closed flock at the University of Arkansas (Gyles et al. 1967). It is not directly ancestral to the broiler lineage but is a reasonable and easily accessible proxy for the ancestral condition of Gallus gallus. The 6- and 15-week populations are referred to as juvenile and adult Junglefowl, respectively (see also Allen et al. 2009).
Table 1.
Subject data: body mass was measured directly from the cadavers and limb muscle mass was determined by adding the masses of all pelvic limb muscles removed from the leg.
| Juvenile Junglefowl | Adult Junglefowl | Pureline A | Pureline B | Pureline C | Pureline D | Broiler | |
|---|---|---|---|---|---|---|---|
| n (sample size) | 10 | 10 | 5 | 5 | 5 | 5 | 10 |
| Age (weeks) | 6 | 15 | 6 | 6 | 6 | 6 | 6 |
| Body mass (kg) | 0.63 ± 0.02* | 1.94 ± 0.1* | 3.20 ± 0.05* | 2.82 ± 0.02* | 2.52 ± 0.05* | 2.37 ± 0.08* | 2.79 ± 0.06* |
| Limb muscle mass (% bodyweight) | 5.69 ± 0.1* | 7.73 ± 0.5* | 7.04 ± 0.5 | 6.58 ± 0.2 | 6.16 ± 0.6 | 7.31 ± 1.1 | 6.27 ± 0.1 |
Measured values reported are means ± SEM.
Significant differences at the 0.05 level.
The right pelvic limb from each individual was dissected, with each muscle and respective tendon being identified and systematically removed. Four architectural measurements were then taken; muscle mass (Mm), fascicle length (Lf), muscle belly length and pennation angle (θ). Muscle mass was measured on an electronic balance (± 0.1 g), fascicle length was measured from at least five random sites within the muscle belly using digital callipers (± 0.1 mm), muscle belly length was measured as the length from the origin of the most proximal muscle fibres to the insertion of the most distal fibres, and the pennation angle was measured at least five times using a goniometer (± 1°). Repeated measurements were essential to account for any differences that may be seen across an individual muscle and to ensure mean values used for further calculations are representative of the overall architecture of the muscle. Physiological cross-sectional area (PCSA) was then calculated (Eq. 1; ρ = muscle density: Sacks & Roy, 1982; Powell et al. 1984):
| (1) |
The density value assumed was 1.06 g cm−3, the standard value for mammalian muscle (Mendez & Keys, 1960). Due to the rapid muscle growth seen in the commercial line birds (both pureline and broiler populations), a preliminary test was carried out following Archimedes’ principle to ensure the density of chicken muscle was a similar value to validate the use of this number in the current study (n = 4; 1.05 ± 0.08 g cm−3 (mean ± SD)).
Many scaling (body-size dependent) effects have been reported in vertebrates (Alexander, 1985; Biewener, 1989, 1991, 2000; Christiansen, 1999; Diaz, 2002), therefore we normalized our data to negate the effect of body size to make valid comparisons across bird populations. If birds scale isometrically following the principles of geometric similarity, all linear dimensions should scale in proportion to one another (Biewener, 2003). Therefore, based on the principle that area is proportional to body mass2/3 and length is proportional to body mass1/3 (Alexander et al. 1981) PCSA and fascicle length measurements were normalized accordingly (Eqs 2 and 3):
| (2) |
| (3) |
Muscle mass is represented as a percentage of body mass for functional groups (approximated from anatomy) because this calculation allowed us to compare where the main mass of muscle lies when considering the movement of the limb and gives an indication of mechanical power capacity for each joint, as power is proportional to muscle mass (Alexander, 1974).
Here we infer basic, qualitative aspects of muscle function (e.g. extensor, abductor, medial rotator) from muscle lines of action (as is standard practice in functional anatomy) and from functional (including quantitative biomechanical) analyses of homologous muscles in other galliform taxa (e.g. Jacobsen & Hollyday, 1982; Gatesy, 1994, 1999b; Hutchinson & Gatesy, 2000; Ellerby & Marsh, 2006). However, our inferences must be viewed as hypotheses that deserve rigorous testing with biomechanical analysis via experiments and/or mathematical modelling. Yet this does not devalue the role of architecture in inspiring hypothesis formulation and testing, particularly with large comparative datasets such as ours.
The computer package spss (Statistical Package for the Social Sciences, Chicago, IL, USA) was used for the statistical analysis to look for differences between the PCSA of individual muscles, differences between masses of functional muscle groups, and also any differences between the modern broiler, the purelines and the Giant Junglefowl. Where assumptions of normal distribution and equal variances could be met, a one-way analysis of variance (anova) was used to test the differences among several means for significance without increasing the Type I error rate. P-values (< 0.05 deemed significant) were taken into consideration when analysing the data and drawing conclusions. For all non-parametric data, a Kruskal–Wallis one-way anova was used to compare the medians between groups and populations. A subsequent Mann–Whitney U-test, incorporating the Bonferroni correction (adjusting for multiple comparisons), was applied on all significant results to determine which groups or populations differ. For non-parametric data, medians and interquartile ranges are presented.
Results
All muscles excluding the most distal muscles of the tarsometatarsus and foot (deemed too small to measure reliably), were identified in the right pelvic limb of all seven bird groups (two Junglefowl ages, four purelines and the broiler), totalling four measurements per muscle for 40 muscles in all 50 birds. Descriptions of the individual muscles, including their origins, insertions, action and abbreviations used in figures and in the text are presented in Table 2. Origins and insertions did not vary between these groups and generally agreed with other published data on Galliformes (Hudson, 1937; Hudson et al. 1959; George & Berger, 1966). Some minor variation was observed that was inconsistent and subtle, and so is not detailed here.
Table 2.
Origins, insertions and functional groups for the major muscles of the chicken (Gallus gallus) pelvic limb.
| Muscle (abbreviation) | Origin | Insertion | Functional group |
|---|---|---|---|
| M. iliotibialis cranialis (IC) | Dorsal iliac crest (cranial end of ilium) | Medial side of patella tendon | Hip flexor, knee extensor |
| M. iliotibialis lateralis postacetabularis (PIL) | Dorsal and dorsolateral region of iliac crest | Distal end of femur, on patella tendon | Hip abductor, hip extensor, knee extensor |
| M. iliotibialis lateralis preacetabularis (AIL) | Dorsal and dorsolateral region of iliac crest | Cranial aspect of distal femur by aponeurosis over FMTM | Hip abductor, hip flexor, knee extensor |
| M. iliofibularis (ILFB) | Dorsolateral region of postacetabular ilium | Lateral surface of fibula | Hip extensor, hip abductor, knee flexor |
| M. flexor cruris lateralis pelvica (FCLP) | Caudal end of ilium and adjacent caudal vertebrae | Femur and tibiotarsus via pars accessoria | Hip extensor, hip abductor, knee flexor |
| M. flexor cruris lateralis accessoria (FCLA) | Extension of FCLP | Distal end of femur, intercondylar region and proximal tibiotarsus | Hip extensor, hip abductor |
| M. flexor cruris medialis (FCM) | Caudolateral surface of edge of ischium | Proximal tibiotarsus, medial aspect | Hip extensor, hip abductor, knee flexor |
| M. caudofemoralis pars caudalis (CFC) | Ventrolateral surface of pygostyle | Proximal femur shaft, caudal aspect | Hip extensor |
| M. caudofemoralis pars pelvica (CFP) | Lateral ridge of ilium and ventrolateral surface of ischium | Proximal femur shaft, caudal aspect | Hip extensor |
| M. ischiofemoralis (ISF) | Lateral surface of ischium | Lateral surface of trochanteric crest | Hip extensor, lateral rotator |
| M. iliofemoralis externus (IFE) | Lateral ridge (processus supratrochantericus) of supra-acetabular ilium | Lateral surface of trochanteric crest | Hip abductor |
| M. iliofemoralis internus (IFI) | Ventral surface of ilium, deep to origin of ITM | Caudomedial surface of the proximal end of the femur | Hip flexor, hip adductor |
| M. iliotrochantericus caudalis (ITC) | Lateral surface of preacetabular ilium fossa | Lateral surface of trochanteric crest | Hip flexor, medial rotator |
| M. iliotrochantericus cranialis (ITCR) | Ventral edge of preacetabular ilium | Lateral surface of trochanteric crest, distal to ITC | Hip flexor, medial rotator |
| M. iliotrochantericus medius (ITM) | Ventral surface of ilium, caudal to ITCR | Lateral surface of trochanteric crest, caudal aspect | Hip flexor, medial rotator |
| M. obturatorius (O) | Ventral portion of ischium, part of pubis and ilium (postacetabular pelvis) | Lateral surface of trochanteric crest | Hip flexor, lateral rotator, adductor |
| M. puboischiofemoralis pars lateralis (PIFL) | Pubis | Lateral side of shaft of femur | Hip extensor |
| M. puboischiofemoralis pars medialis (PIFM) | Pubis | Medial side of shaft of femur | Hip extensor |
| M. ambiens (AMB) | Pectineal (preacetabular) process of ilium | Lateral head of fibula, underneath FPD II | Hip flexor, hip adductor, knee extensor |
| M. femorotibialis lateralis (FMTL) | Lateral surface of femur | Lateral side of proximal end of tibiotarsus via patella tendon | Knee extensor |
| M. femorotibialis medialis (FMTM) | Lateral and cranial surface of femur | Proximal end of tibiotarsus via patella tendon | Knee extensor |
| M. femorotibialis intermedius (FMTIM) | Medial surface of femur | Medial side of proximal end of tibiotarsus | Knee extensor |
| M. gastrocnemius pars lateralis (GL) | Lateral side of distal end of femur | Tarsometatarsus via common tendon with GM, GIM and FCLA | Knee flexor, ankle extensor |
| M. gastrocnemius pars medialis (GM) | Around patella tendon, and cranial aspect of tibiotarsus | Tarsometatarsus via common tendon with GL, GIM and FCLA | Knee flexor, ankle extensor |
| M. gastrocnemius pars intermedia (GIM) | Medial condyle of femur | Tarsometatarsus via common tendon with GL, GM and FCLA | Knee flexor, ankle extensor |
| M. fibularis longus (FL) | Proximal end of tibiotarsus | Proximolateral corner of tibial cartilage and tendon of flexores perforati digiti III | Ankle extensor, digit flexor |
| M. fibularis brevis (FB) | Craniolateral surface of tibiotarsus and cranial and medial surface of fibula | Lateral aspect of proximal tarsometatarsus | Ankle medial rotator and abductor |
| M. tibialis cranialis caput femorale (TCF) | Lateral condyle of femur | Cranial surface of proximal tarsometatarsus | Knee extensor, ankle flexor |
| M. tibialis cranialis caput tibiale (TCT) | Anterior tibial crest | Cranial surface of proximal tarsometatarsus | Ankle flexor |
| M. plantaris (PLT) | Caudomedial side of proximal end of tibiotarsus | Medial side of tibial cartilage, proximal end | Unknown |
| M. popliteus (POP) | Caudomedial surface of head of fibula | Caudal surface of proximal part of tibiotarsus | Unknown; tibia/fibula rotator |
| M. extensor digitorum longus (EDL) | Cranial surface of tibiotarsus | Phalanges of digits II, III and IV, distal end of distal phalanx | Ankle flexor, digit extensor |
| M. flexor digitorum longus (FDL) | Caudal surface of tibiotarsus | Phalanges of digits II, III and IV, distal end of distal phalanx | Ankle extensor, digit flexor |
| M. flexor hallucis longus (FHL) | Caudal surface of distal end of femur | Distal phalanx of the hallux | Knee flexor, ankle extensor, digit flexor |
| M. flexores perforantes et perforati digiti II (FPPD II) | Lateral condyle of femur | Digit II, distal end of intermediate phalanx | Knee flexor, ankle extensor, digit flexor |
| M. flexores perforantes et perforati digiti III (FPPD III) | Lateral condyle of femur, adjacent to FPPD II | Digit III, proximal end of proximal phalanx | Knee flexor, ankle extensor, digit flexor |
| M. flexores perforati digiti II (FPD II) | Head of fibula via patella tendon, lateral aspect | Digit II, proximal end of proximal phalanx | Ankle extensor, digit flexor |
| M. flexores perforati digiti III (FPD III) | Adjacent to FPD II, caudally | Digit III, distal end of intermediate phalanx | Ankle extensor, digit flexor |
| M. flexores perforati digiti IV (FPD IV) | Lateral condyle of femur | Digit IV, proximal end of intermediate phalanx | Knee flexor, ankle extensor, digit flexor |
Initial observations of the pelvic limb showed a marked proximal to distal reduction in muscle mass across all groups, as in many other tetrapods. The more proximal limb muscles tended to be long-fascicled, parallel-fibred muscles, with the exception of the M. femorotibiales (multi-pennated) and M. ambiens (bipennate), which presumably function as knee extensors. Other pennate muscles found close to the hip joint, such as M. iliotrochantericus caudalis and M. ischiofemoralis, all seem involved with either flexion or extension of the hip and rotation about the longitudinal axis of the femur. The more distal muscles tended to be short-fascicled, pennate muscles, with short muscle bellies and long tendons. The exception was M. popliteus, whose muscle function is likely involved with rotation of the tibia and fibula (Fuss, 1996). Based on muscle mass alone, the largest muscles of the pelvic limb were M. iliotibialis lateralis postacetabularis and M. gastrocnemius pars medialis, which are both hip and knee or ankle extensors, with the latter also contributing to flexion of the knee. Muscle data (muscle mass, fascicle length, pennation angle and PCSA) from the broiler population can be found in the Appendix. Except where noted, reported values are means ± SD.
The specialization of the pelvic limb muscles towards either greater force-generating capacity or fast contraction in all the bird populations (Figs 2–4) revealed that the long-fascicled, parallel-fibred muscles of the proximal limb and the M. tibialis cranialis (tibial head; TCT) of the distal part of the limb have smaller force-generation capabilities than the M. iliotrochantericus caudalis (ITC), M. femorotibialis medialis (FMTM), M. fibularis longus (FL), M. gastrocnemius pars medialis (GM) and M. gastrocnemius pars lateralis (GL), which are capable of producing much greater forces. The latter muscles are primarily the hip, knee and ankle extensors (and rotators), and have a greater force-generating capacity and/or have short pennate muscle fibres. These normalized values did not differ significantly in the Junglefowl population from its juvenile stage through to adulthood, but the PCSA of the GM, GL, FMTM and ITC were significantly larger than those found for the commercial line birds (both broiler and pureline populations).
Fig. 2.
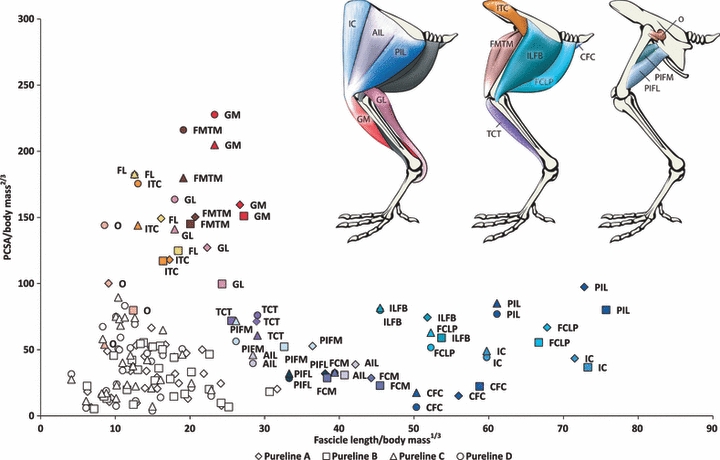
The PCSA against fascicle length for the pelvic limb muscles of the purelines A, B, C and D. Values (means) are normalized for comparison. The colour of the labelled data points for each muscle matches the colour of that particular muscle in the anatomical diagram above. The uncoloured points represent the remaining muscles of the pelvic limb.
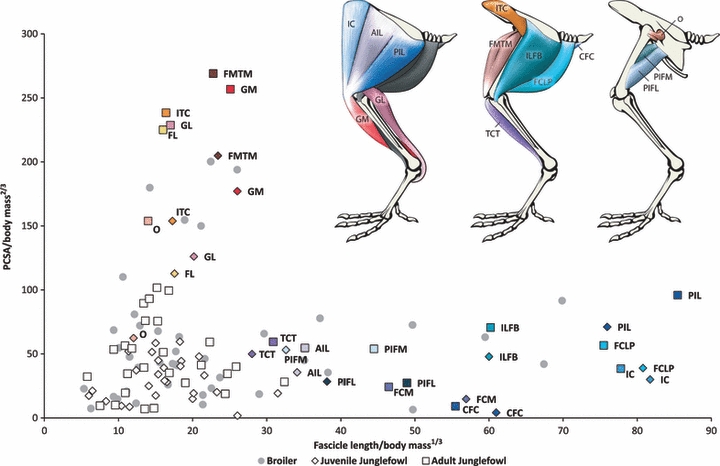
The PCSA against fascicle length for the pelvic limb muscles of the juvenile and adult Junglefowl population. Values (means) are normalized for comparison. The colour of the labelled data points for each muscle matches the colour of that particular muscle in the anatomical diagram above. The uncoloured points represent the remaining muscles of the pelvic limb. The data points for the broiler population are shaded in grey.
Within the pureline populations we found that there are distinct differences between male lines (A and B) and female lines (C and D; see Fig. 1). In these cases the female lines have muscles which are more capable of greater force generation, and the males lines have muscles which are longer-fibred, and presumably faster-contracting or have a wider range of motion (Fig. 2). The broiler population exhibited similar muscle characteristics to all four pureline groups (Fig. 3).
Fig. 3.
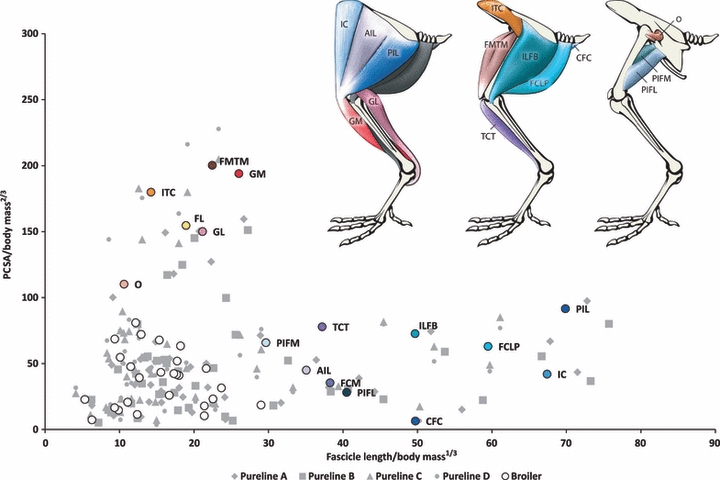
The PCSA against fascicle length for the pelvic limb muscles of the broiler population. Values (means) are normalized for comparison. The colour of the labelled data points for each muscle matches the colour of that particular muscle in the anatomical diagram above. The uncoloured points represent the remaining muscles of the pelvic limb. The data points for the pureline populations are shaded in grey.
The greatest muscle mass in all bird populations was for the hip extensors, knee flexors and abductors (Fig. 5). The digital extensor muscle mass did not vary across groups. Within the Junglefowl populations, the relative muscle mass of functional groups generally increased across ontogeny, apart from the medial rotators, which decreased in mass quite dramatically. The adult Junglefowl generally had the largest muscle mass per functional group overall, but despite the age difference between the adult Junglefowl and the commercial line birds, the difference in pelvic limb muscle mass, although statistically significant, was still small (< 1% of body mass). Compared to the broiler population, the juvenile Junglefowl had smaller hip and ankle extensors, and smaller adductor and abductor muscle mass. These two populations also had substantially larger medial rotators than the other bird populations. Within the pureline populations, pureline A and C had significantly larger abductors and ankle flexors and pureline B likewise followed this trend (although this was not statistically significant). The interquartile range was large within this population, and this variation was continually seen within all populations in the knee extensors, knee flexors and the ankle extensors. This variation was greater in pureline A and B, and smaller in pureline C and D. Despite this variation, pureline C and D had significantly larger knee flexors.
Fig. 5.
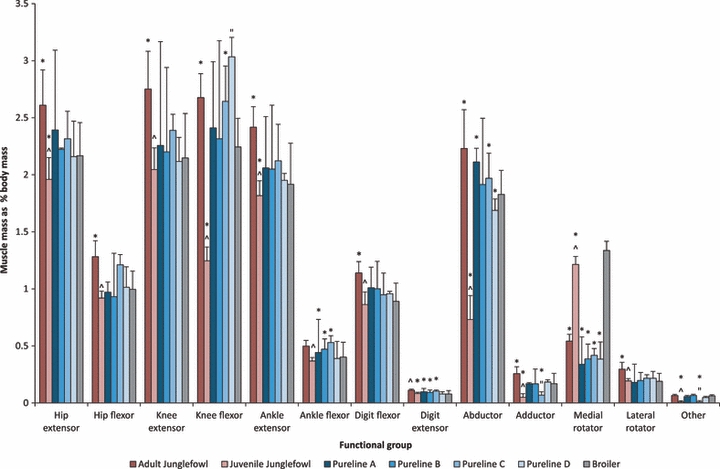
Functional distribution of muscle mass within the pelvic limb for all commercial line birds and Junglefowl populations. *Significant difference at the 0.05 level to the broiler population. ˇ ”Significant difference at the 0.05 level within the Junglefowl populations and pureline populations, respectively. Data are median ± interquartile range.
Discussion
Although it is known that domestication and the resulting selection pressures for growth and yield faced by commercial line birds have altered their basic morphology, the extent to which these birds deviate from their ancestral condition is unknown. More specifically, little is known about how features of their architectural design (and consequently functional anatomy) have been indirectly and unintentionally influenced by selection pressures aimed at producing rapidly growing birds with a large pectoral muscle mass. Whilst we cannot infer directly how these changes may affect posture and/or limb loading, our analysis provides baseline data for generating hypotheses to investigate in greater depth whether any anatomical features potentially predispose broilers to lameness or are otherwise responsible for their unusual gait.
Previous studies of broilers have shown a shift in metabolic resources to develop additional breast muscle that alters the growth of other organs, leading to a decline of pelvic limb muscle mass (Harshaw & Rector, 1940; Miller, 1968; Nestor et al. 1985, 1987; Katanbaf et al. 1988; Lilburn, 1994; Berri et al. 2007; Schmidt et al. 2009). Our data support these previous studies, but also indicate that the decline in pelvic limb muscle mass is relatively small (< 1%) between the Junglefowl populations and the commercial line birds, as reported by Wall & Anthony (1995). We further confirm their rapid growth rates, with broilers gaining 2 kg more body mass than their wild counterparts at the same age, and exceeding the body mass of the adult Junglefowl population (Table 1).
The proximal to distal reduction in muscle mass (limb tapering) reported here has been previously reported for other birds and ‘cursorial’ animals (e.g. Alexander et al. 1981; Hutchinson, 2004; Payne et al. 2005; Smith et al. 2006; Williams et al. 2007a,b;) as well as less cursorial crocodylians (Allen et al. 2010). The presence of this limb tapering in non-cursorial broiler chickens represents phylogenetic inertia and/or a dissociation between cursoriality and limb tapering. Limb tapering is thought to reflect a specialization for power versus force development. Proximal muscles tend to be specialized for generating power to move the centre of mass, whereas more distal muscles with long tendons tend to act as springs (Alexander, 1974), in addition to reducing mechanical work through elastic energy storage in tendons (Alexander, 1991). However, the larger body mass of broiler chickens will require greater forces to overcome inertia (Corr et al. 2003a) and this may be compromised by having to carry this extra weight on limbs with similar power-producing capabilities to their wild counterparts. This is because an added load increases the mass that must be accelerated and supported against gravity, increasing the mechanical work that must be done (McGowan et al. 2006).
Previous studies have shown that selection for a large pectoral muscle mass and increased bodyweight has put greater demands on the pelvic muscles in farmed turkeys (Abourachid, 1993). This hypothesis was also proposed by Corr et al. (2003b) who showed that broilers seem to take wider steps and have increased stance times, linked to their apparent instability. Most birds maintain a sub-horizontal femur during walking to bring their feet under their centre of mass (Storer, 1971; Alexander, 1983; Manion, 1984; Gatesy, 1999a). As a result, movements of the femur are restricted (Gatesy & Biewener, 1991; Gatesy, 1999a) and most of the body’s forward progression is produced by knee flexion/extension (Gatesy, 1999a; Reilly, 2000). Birds also require large force-producing muscles acting about the hip joint during stance to stabilize the femur and centre of mass (Jacobsen & Hollyday, 1982; Hutchinson, 2004). Our data show that across all bird populations, it is mainly the hip, knee and ankle extensors that constitute the greatest proportion of pelvic limb muscle mass. These are all involved with support of the limb during the stance phase and have either a high force-generating capacity (indicated by a large PCSA) or long parallel-fibred muscles for faster contractions or a wider range of motion. The latter muscles likely have a greater role in movement of the limb during locomotion.
Balance in birds is also achieved by long-axis rotation of the femur controlled by pelvic musculature including M. iliotrochantericus caudalis (ITC), preventing abduction of the limb and maintaining the foot under the midline of the body (Hutchinson & Gatesy, 2000). Within the Junglefowl populations, medial rotator mass decreased with age, whereas abductor muscle mass increased significantly. Hence stabilization of the femur might be achieved primarily by the medial rotators through more juvenile stages, relying progressively more on the abductors when fully mature, when mediolateral stability may become more important to maintain. Large abductors may also be needed to swing the leg laterally during the swing phase and clear the wide body wall. The broilers are effectively juveniles so their large medial rotators also fit this pattern. However, there could be other possible explanations related potentially to a wider pelvis or increase waddling, but this is purely speculative without further biomechanical analyses.
Within the pureline populations, the medial rotator muscle mass remained small, but they had extremely large abductors similar to the adult Junglefowl. These abductors are also the main extensors and flexors of the limb, and these seem to develop at a faster rate than the medial rotator muscle mass at the hip. Gait analysis has shown that these birds experience increased mediolateral forces (Corr et al. 2003b) and that their femora are in a more abducted and medially rotated position when flexed (Abourachid, 1993). This could result in relative increases of the hip and knee joint moments, due to the change in mediolateral long-axis rotation of the bone (Carrano & Biewener, 1999; Gatesy, 1999a). Large abductors may therefore be more favourable in these more juvenile populations, or simply a consequence of a functional demand for larger hip and knee extensors/flexors.
Interestingly, we also found a large abductor muscle mass in the broiler population, but this was combined with a significantly larger medial rotator mass. This suggests that the commercial broiler has perhaps evolved additional three-dimensional support at the hip, which may be associated with this population experiencing larger mediolateral forces (and mediolateral roll) than the purelines from which it is derived. Our data reveal that purelines A, B and C have the largest abductors of the commercial line birds, although variation within each population was high. Pureline D had the smallest abductor muscle mass. Therefore the previously reported increase in mediolateral forces and postural change (Corr et al. 2007) may be greatest in the commercial broiler and pureline A–C bird populations, and seen to a lesser extent in pureline D. Experimental analyses are required to fully test these anatomically based speculations.
The pureline populations face similar growth-related selection pressures to the broiler population, therefore it was expected that the architectural changes would be similar. However, our data indicate that the demands on the pelvic limb muscles of the individual commercial line bird populations may be variable. The interquartile ranges for the muscle mass of the functional groups in pureline A and B were also substantially higher for the knee extensors, flexors and ankle extensors. This variation was not seen across all bird populations. We propose that limb support and indeed joint support is highly variable within these bird populations and could be a predisposing factor in their susceptibility to lameness. Bone also has an inherently limited rate of development, so always lags behind muscle development (Bain & Watkins, 1993; Rath et al. 2000). This is exaggerated when muscle development is forced and is also likely to be a contributing factor. Skeletal abnormalities, which contribute to lameness, are certainly not exclusive to commercial line chickens. They have been found in other farm-reared birds including ostriches (Squire & More, 1998; Huchzermeyer, 2002) and turkeys (Duff et al. 1987), which suggests these species share common biomechanical and anatomical design constraints associated with obligate bipedalism and the various commercial selection pressures they are subjected to as part of the breeding programmes.
We have provided new quantitative anatomical data, including a total of 8000 measurements, for a wide range of domestic and wild-type chickens that are useful in a comparative context and for computer modelling/simulation of limb mechanics. Our analysis has highlighted commonalities within these bird populations as well as revealing where the modern broiler has deviated from its ancestral condition. We conclude that it is mainly the hip, knee and ankle extensors (‘antigravity’ muscles) that have either a greater force-generating capacity or have longer parallel-fibred muscles for fast contraction, which are all involved with support of the limb during the stance phase. The Junglefowl have both greater force-generating capabilities and longer, presumably faster-contracting muscles, indicative of improved locomotor function, supporting our initial predictions. However, this does not necessarily correlate with joints that have a large range of motion. Those muscles that can produce large velocities may not do this if placed in the skeleton with a very large moment arm. Moment arm measurements will need to be collected to more fully understand the functional roles of the pelvic limb muscles, in conjunction with experimental gait analyses and biomechanical models. Additionally, more data on muscle physiology are required to characterize any differences between these populations (e.g. maximal contraction velocity, maximal stress or activation/relaxation times).
Finally, we have emphasized that more detailed biomechanical analysis using experiments and modelling is required to fully determine how pelvic limb muscles control locomotion in chickens and how this capacity has evolved. Artificial selection of chickens appears to have led most strikingly to specializations related to support of the hip joint, although these were variable within the commercial line birds and may reflect differences in the walking ability of these birds. It is our hope that these data will not only form the foundation of a deeper understanding of pelvic limb function, dysfunction and lameness in commercial chickens, but also inspire methods to select for improved musculoskeletal health in the chickens of the future.
Acknowledgments
We thank Roy Mutimer, Kate Barger and the staff at Cobb Vantress Inc. for their assistance and generous support of this work. This project was funded by a BBSRC CASE Industrial PhD Studentship Award in collaboration with Cobb Vantress Inc. We appreciate dissection assistance from students at the Royal Veterinary College, including Alice Rawlinson, Victoria Jux, Rachel Simmons and Sharon Warner. Special thanks are due to Julia Molnar, who designed the illustration for Fig. 1 and the anatomical drawings for Figs 2–4.
Appendix
Broiler muscle data: Values are means ± SD. Bold values represent those muscles which have the greater force-generating capacity or the longer-fibred muscles with wide functional ranges.
| Muscle (abbreviation) | Muscle mass (g) | Muscle belly length (mm) | Fascicle length (mm) | Pennation angle (degrees) | PCSA (mm2) |
|---|---|---|---|---|---|
| M. iliotibialis cranialis (IC) | 8.24 ± 1.03 | 107.3 ± 6.4 | 94.9 ± 11.2 | 0 | 42.0 ± 9.8 |
| M. iliotibialis lateralis postacetabularis (PIL) | 19.44 ± 2.76 | 110.4 ± 18.3 | 92.5 ± 20.9 | 0 | 91.6 ± 21.7 |
| M. iliotibialis lateralis preacetabularis (AIL) | 4.16 ± 0.70 | 70.7 ± 20.7 | 55.2 ± 19.3 | 0 | 45.0 ± 14.9 |
| M. iliofibularis (ILFB) | 10.60 ± 0.40 | 76.5 ± 6.5 | 69.9 ± 4.4 | 0 | 72.6 ± 9.7 |
| M. flexor cruris lateralis pelvica (FCLP) | 11.01 ± 1.97 | 93.7 ± 6.0 | 83.7 ± 7.6 | 0 | 63.1 ± 9.3 |
| M. flexor cruris lateralis accessoria (FCLA) | 2.91 ± 0.47 | 40.7 ± 8.7 | 29.8 ± 6.0 | 0 | 46.3 ± 9.6 |
| M. flexor cruris medialis (FCM) | 3.35 ± 0.39 | 61.3 ± 23.0 | 56.9 ± 4.4 | 0 | 28.2 ± 4.4 |
| M. caudofemoralis pars caudalis (CFC) | 0.94 ± 0.15 | 82.5 ± 6.9 | 69.9 ± 8.6 | 0 | 6.5 ± 13.1 |
| M. caudofemoralis pars pelvica (CFP) | 2.16 ± 0.43 | 58.3 ± 3.0 | 25.5 ± 3.3 | 0 | 41.0 ± 10.3 |
| M. ischiofemoralis (ISF) | 1.94 ± 0.32 | 42.2 ± 8.1 | 13.2 ± 3.2 | 19 ± 7 | 68.6 ± 22.8 |
| M. iliofemoralis externus (IFE) | 0.41 ± 0.11 | 21.1 ± 4.5 | 14.5 ± 3.5 | 0 | 14.7 ± 5.2 |
| M. iliofemoralis internus (IFI) | 0.42 ± 0.12 | 21.9 ± 2.4 | 17.4 ± 2.6 | 0 | 11.5 ± 1.9 |
| M. iliotrochantericus caudalis (ITC) | 8.50 ± 1.21 | 44.3 ± 2.4 | 20.9 ± 1.2 | 26 ± 5 | 189.6 ± 61.6 |
| M. iliotrochantericus cranialis (ITCR) | 1.76 ± 0.31 | 32.7 ± 8.0 | 14.2 ± 2.2 | 25 ± 7 | 54.8 ± 17.7 |
| M. iliotrochantericus medius (ITM) | 0.43 ± 0.04 | 17.2 ± 2.6 | 13.1 ± 3.7 | 0 | 16.6 ± 5.1 |
| M. obturatorius (O) | 3.53 ± 0.62 | 32.1 ± 6.1 | 16.4 ± 6.9 | 21 ± 3 | 110.1 ± 52.6 |
| M. puboischiofemoralis pars lateralis (PIFL) | 3.79 ± 0.98 | 61.2 ± 8.0 | 52.5 ± 5.3 | 0 | 35.5 ± 12.8 |
| M. puboischiofemoralis pars medialis (PIFM) | 5.79 ± 0.90 | 56.0 ± 8.3 | 43.1 ± 6.4 | 0 | 65.8 ± 17.0 |
| M. ambiens (AMB) | 0.79 ± 0.15 | 45.7 ± 5.4 | 21.0 ± 12.4 | 18 ± 7 | 20.8 ± 8.4 |
| M. femorotibialis lateralis (FMTL) | 2.51 ± 0.72 | 53.0 ± 8.7 | 17.7 ± 7.3 | 21 ± 3 | 72.1 ± 36.8 |
| M. femorotibialis medialis (FMTM) | 13.45 ± 1.50 | 69.4 ± 6.1 | 31.5 ± 6.4 | 18 ± 6 | 200.3 ± 65.4 |
| M. femorotibialis intermedius (FMTIM) | 3.07 ± 0.65 | 53.1 ± 4.7 | 17.6 ± 5.4 | 22 ± 4 | 80.9 ± 32.6 |
| M. gastrocnemius pars lateralis (GL) | 9.78 ± 1.55 | 77.8 ± 13.4 | 29.8 ± 4.4 | 24 ± 5 | 150.0 ± 45.4 |
| M. gastrocnemius pars medialis (GM) | 16.31 ± 2.47 | 113.0 ± 8.4 | 37.9 ± 5.5 | 18 ± 3 | 193.9 ± 61.0 |
| M. gastrocnemius pars intermedia (GIM) | 1.56 ± 0.46 | 40.8 ± 4.9 | 48.8 ± 4.4 | 21 ± 4 | 18.6 ± 6.5 |
| M. fibularis longus (FL) | 8.18 ± 1.52 | 86.6 ± 4.8 | 26.6 ± 12.6 | 24 ± 4 | 164.2 ± 62.0 |
| M. fibularis brevis (FB) | 0.42 ± 0.09 | 48.3 ± 10.8 | 6.75 ± 2.9 | 19 ± 4 | 22.8 ± 14.4 |
| M. tibialis cranialis caput femorale (TCF) | 6.27 ± 0.74 | 77.2 ± 7.8 | 41.8 ± 12.9 | 21 ± 3 | 63.4 ± 21.2 |
| M. tibialis cranialis caput tibiale (TCT) | 2.77 ± 0.26 | 77.0 ± 9.8 | 36.6 ± 19.5 | 19 ± 7 | 45.4 ± 17.1 |
| M. plantaris (PLT) | 1.51 ± 0.26 | 43.1 ± 5.3 | 31.7 ± 5.3 | 0 | 23.2 ± 5.5 |
| M. popliteus (POP) | 0.16 ± 0.04 | 13.5 ± 1.6 | 8.8 ± 1.9 | 0 | 7.3 ± 3.9 |
| M. extensor digitorum longus (EDL) | 2.26 ± 0.32 | 85.2 ± 5.1 | 24.3 ± 4.7 | 20 ± 2 | 42.4 ± 9.3 |
| M. flexor digitorum longus (FDL) | 3.45 ± 0.36 | 82.2 ± 6.2 | 27.3 ± 13.0 | 20 ± 3 | 59.56 ± 21.79 |
| M. flexor hallucis longus (FHL) | 1.81 ± 0.83 | 59.3 ± 2.6 | 21.0 ± 6.7 | 24 ± 4 | 33.4 ± 12.3 |
| M. flexores perforantes et perforati digiti II (FPPD II) | 1.55 ± 0.80 | 54.7 ± 11.3 | 17.8 ± 2.6 | 20 ± 4 | 33.9 ± 10.3 |
| M. flexores perforantes et perforati digiti III (FPPD III) | 2.67 ± 0.92 | 66.0 ± 7.2 | 25.0 ± 8.1 | 27 ± 7 | 51.9 ± 23.3 |
| M. flexores perforati digiti II (FPD II) | 1.21 ± 0.40 | 45.6 ± 10.3 | 21.4 ± 7.7 | 21 ± 3 | 26.0 ± 10.5 |
| M. flexores perforati digiti III (FPD III) | 2.09 ± 0.48 | 60.0 ± 9.7 | 19.69 ± 7.8 | 27 ± 12 | 47.7 ± 26.4 |
| M. flexores perforati digiti IV (FPD IV) | 2.29 ± 0.64 | 54.1 ± 7.8 | 26.9 ± 1.9 | 19 ± 6 | 41.7 ± 16.1 |
References
- Abourachid A. Mechanics of standing in birds: functional explanation of lameness problems in giant turkeys. Br Poult Sci. 1993;34:887–898. doi: 10.1080/00071669308417649. [DOI] [PubMed] [Google Scholar]
- Alexander RM. The mechanics of a dog jumping, Canis familiaris. J Zool. 1974;173:549–573. [Google Scholar]
- Alexander RM. Allometry of the leg bones of moas (Dinornithes) and other birds. J Zool. 1983;200:215–231. [Google Scholar]
- Alexander RM. The maximum forces exerted by animals. J Exp Biol. 1985;115:231–238. doi: 10.1242/jeb.115.1.231. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Energy-saving mechanisms in walking and running. J Exp Biol. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Jayes AS, Maloiy GMO, et al. Allometry of the leg muscles of mammals. J Zool. 1981;194:539–552. [Google Scholar]
- Allen V, Paxton H, Hutchinson JR. Variation in center of mass estimates for extant sauropsids, and its importance for reconstructing inertial properties of extinct archosaurs. Anat Rec. 2009;292:1442–1461. doi: 10.1002/ar.20973. [DOI] [PubMed] [Google Scholar]
- Allen V, Elsey RM, Jones N, et al. Functional specialization and ontogenetic scaling of limb anatomy in Alligator mississippiensis. J Anat. 2010;216:423–445. doi: 10.1111/j.1469-7580.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony NB. A review of genetic practices in poultry: efforts to improve meat quality. J Muscle Food. 1998;9:25–33. [Google Scholar]
- Bain SD, Watkins BA. Local modulation of skeletal growth and bone modelling in poultry. J Nutr. 1993;123:317–322. doi: 10.1093/jn/123.suppl_2.317. [DOI] [PubMed] [Google Scholar]
- Barton NF. Proceedings of 9th European Poultry Conference. Glasgow: 1994. Breeding meat type poultry for the future targets for selection, limits to performance and market requirements for chicken; pp. 33–38. [Google Scholar]
- Bennett AF, Garland G, Jr, Else PL. Individual correlation of morphology muscle mechanics and locomotion in a salamander. Am J Physiol. 1989;256:R1200–R1208. doi: 10.1152/ajpregu.1989.256.6.R1200. [DOI] [PubMed] [Google Scholar]
- Bennett RM, Christiansen K, Clifton-Hadley RS. Direct costs of endemic diseases of farm animals in Great Britain. Vet Rec. 1999;145:376–377. doi: 10.1136/vr.145.13.376. [DOI] [PubMed] [Google Scholar]
- Berri C, Bihan-Duval EL, Debut M, et al. Consequence of muscle hypertrophy on characteristics of pectoralis major muscle and breast meat quality of broiler chickens. J Anim Sci. 2007;85:2005–2011. doi: 10.2527/jas.2006-398. [DOI] [PubMed] [Google Scholar]
- Bezuidenhout A, Burger WP. The incidence of tibiotarsal rotation in the ostrich (Struthio camelus) J S Afr Vet Med Assoc. 1993;64:159–161. [PubMed] [Google Scholar]
- Biewener AA. Mammalian terrestrial locomotion and size: mechanical design principles define limits. Bioscience. 1989;39:776–783. [Google Scholar]
- Biewener AA. Musculoskeletal design in relation to body size. J Biomech. 1991;24:19–29. doi: 10.1016/0021-9290(91)90374-v. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Scaling in Biology. Oxford: Oxford University Press; 2000. Scaling of terrestrial support: differing solutions to mechanical constraints of size; pp. 51–66. [Google Scholar]
- Biewener AA. Animal Locomotion. Oxford: Oxford University Press; 2003. [Google Scholar]
- Brickett KE, Dahiya JP, Classen HL, et al. Influence of dietary nutrient density, feed form, and lighting on growth and meat yield of broiler chickens. Poult Sci. 2007;86:2172–2181. doi: 10.1093/ps/86.10.2172. [DOI] [PubMed] [Google Scholar]
- Buijs S, Keeling L, Rettenbacher S, et al. Stocking density effects on broiler welfare: identifying sensitive ranges for different indicators. Poult Sci. 2009;88:1536–1543. doi: 10.3382/ps.2009-00007. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, et al. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Carrano MT, Biewener AA. Experimental alteration of limb posture in the chicken (Gallus gallus) and its bearings on the use of birds as analogs for dinosaur locomotion. J Morphol. 1999;240:237–249. doi: 10.1002/(SICI)1097-4687(199906)240:3<237::AID-JMOR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Christiansen P. Scaling of the limb bones to body mass in terrestrial mammals. J Morphol. 1999;239:167–190. doi: 10.1002/(SICI)1097-4687(199902)239:2<167::AID-JMOR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Clark J, Alexander RM. Mechanics of running by quail (Coturnix) J Zool. 1975;176:87–113. [Google Scholar]
- Close RL. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52:129–196. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Corr SA, Gentle MJ, McCorquodale CC, et al. The effect of morphology on the musculoskeletal system of the modern broiler. Anim Welf. 2003a;12:145–157. [Google Scholar]
- Corr SA, Gentle MJ, McCorquodale CC, et al. The effect of morphology on walking ability in the modern broiler: a gait analysis study. Anim Welf. 2003b;12:159–171. [Google Scholar]
- Corr SA, McCorquodale C, McDonald J, et al. A force plate study of avian gait. J Biomech. 2007;40:2037–2043. doi: 10.1016/j.jbiomech.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Dawkins MS, Donnelly CA, Jones TA. Chicken welfare is influenced more by housing conditions than by stocking density. Nature. 2004;427:342–344. doi: 10.1038/nature02226. [DOI] [PubMed] [Google Scholar]
- Diaz JI. Differential scaling of locomotor performance in small and large terrestrial mammals. J Exp Biol. 2002;205:2897–2908. doi: 10.1242/jeb.205.18.2897. [DOI] [PubMed] [Google Scholar]
- Duff SRI, Hocking PM, Field RK. The gross morphology of skeletal disease in adult male breeding turkeys. Avian Pathol. 1987;16:635–651. doi: 10.1080/03079458708436412. [DOI] [PubMed] [Google Scholar]
- Ellerby DJ, Marsh RL. The energetic costs of trunk and distal-limb loading during walking and running in guinea fowl Numida meleagris II. Muscle energy use as indicated by blood flow. J Exp Biol. 2006;209:2064–2075. doi: 10.1242/jeb.02227. [DOI] [PubMed] [Google Scholar]
- Fuss FK. Tibiofibular junction of the South African Ostrich (Struthio camelus australis) J Morphol. 1996;227:213–226. doi: 10.1002/(SICI)1097-4687(199602)227:2<213::AID-JMOR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gans C, Bock WJ. The functional significance of muscle architecture – a theoretical analysis. Ergeb Anat Entwicklungsgesch. 1965;38:115–142. [PubMed] [Google Scholar]
- Gatesy SM. Neuromuscular diversity in archosaur deep dorsal thigh muscles. Brain Behav Evol. 1994;43:1–14. doi: 10.1159/000113619. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. Guineafowl hind limb function I: cineradiographic analysis and speed effects. J Morphol. 1999a;240:127–142. doi: 10.1002/(SICI)1097-4687(199905)240:2<115::AID-JMOR3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. Guineafowl hind limb function II: electromyographic analysis and motor pattern evolution. J Morphol. 1999b;240:127–142. doi: 10.1002/(SICI)1097-4687(199905)240:2<127::AID-JMOR4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Biewener AA. Bipedal locomotion: effects of speed, size and limb posture in birds and humans. J Zool. 1991;224:127–147. [Google Scholar]
- George JC, Berger AJ. Avian Myology. New York: Academic Press; 1966. [Google Scholar]
- Gyles NR, Miley JL, Brown CJ. The responses of resistant and susceptible strains of chickens and their F1 and F2 crosses to subcutaneous inoculations with Rous Sarcoma virus. Poult Sci. 1967;46:465–472. doi: 10.3382/ps.0460789. [DOI] [PubMed] [Google Scholar]
- Harshaw HM, Rector RR. The composition of turkeys as affected by age and sex. Poult Sci. 1940;19:404–411. [Google Scholar]
- Haye U, Simons PCM. Twisted leg in broilers. Br Poult Sci. 1978;19:549–557. doi: 10.1080/00071667808416512. [DOI] [PubMed] [Google Scholar]
- Henry HT, Ellerby DJ, Marsh RL. Performance of guinea fowl Numida meleagris during jumping requires storage and release of elastic energy. J Exp Biol. 2005;208:3293–3302. doi: 10.1242/jeb.01764. [DOI] [PubMed] [Google Scholar]
- Herrel A, Schaerlaeken V, Meyers JJ, et al. The evolution of cranial design and performance in squamates: consequences of skull-bone reduction on feeding behaviour. Integr Comp Biol. 2007;47:107–117. doi: 10.1093/icb/icm014. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer FW. Diseases of farmed crocodiles and ostriches. Rev Sci Tech. 2002;21:265–276. doi: 10.20506/rst.21.2.1334. [DOI] [PubMed] [Google Scholar]
- Hudson GE. Studies on the muscles of the pelvic appendage in birds. Am Midl Nat. 1937;18:1–108. [Google Scholar]
- Hudson GE, Lanzillotti PJ, Edwards GD. Muscles of the pelvic limb in galliform birds. Am Midl Nat. 1959;61:1–67. [Google Scholar]
- Hutchinson JR. Biomechanical modeling and sensitivity analysis of bipedal running ability I. Extant taxa. J Morphol. 2004;262:421–440. doi: 10.1002/jmor.10241. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR, Gatesy SM. Adductors, abductors and the evolution of archosaur locomotion. Paleobiology. 2000;26:734–751. [Google Scholar]
- Jacobsen RD, Hollyday M. A behavioural and electromyographic study of walking in the chick. J Neuro-physiol. 1982;48:238–256. doi: 10.1152/jn.1982.48.1.238. [DOI] [PubMed] [Google Scholar]
- James RS, Navas Carlos A, Herrel A. How important are skeletal muscle mechanics in setting limits on jumping performance? J Exp Biol. 2007;210:923–933. doi: 10.1242/jeb.02731. [DOI] [PubMed] [Google Scholar]
- Josephson RK. Dissecting muscle power output. J Exp Biol. 1999;202:3369–3375. doi: 10.1242/jeb.202.23.3369. [DOI] [PubMed] [Google Scholar]
- Julian RJ. Rapid growth problems: ascites and skeletal deformities in broilers. Poult Sci. 1998;77:1773–1780. doi: 10.1093/ps/77.12.1773. [DOI] [PubMed] [Google Scholar]
- Katanbaf MN, Dunnington EA, Siegel PB. Allomorphic relationships from hatching to 56 days in parental lines and F1 crosses of chickens selected 27 generations for high and low body weight. Growth Dev Aging. 1988;52:11–22. [PubMed] [Google Scholar]
- Kestin SC, Gordon S, Su G, et al. Relationships in broiler chickens between lameness, liveweight, growth rate and age. Vet Rec. 2001;148:195–197. doi: 10.1136/vr.148.7.195. [DOI] [PubMed] [Google Scholar]
- Knowles TG, Kestin SC, Haslam SM, et al. Leg disorders in broiler chickens: prevalence, risk factors and prevention. PLoS ONE. 2008;3:e1545. doi: 10.1371/journal.pone.0001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lilburn MS. Skeletal growth of commercial poultry species. Poult Sci. 1994;73:897–903. doi: 10.3382/ps.0730897. [DOI] [PubMed] [Google Scholar]
- Manion BL. University of Illinois; 1984. The effects of size and growth on hindlimb locomotion in the chicken. PhD thesis. [Google Scholar]
- McGeown D, Danbury TC, Waterman-Pearson AE, et al. Effects of carprofen on lameness in broiler chickens. Vet Rec. 1999;144:668–671. doi: 10.1136/vr.144.24.668. [DOI] [PubMed] [Google Scholar]
- McGowan CP, Duarte HA, Main JB, et al. The effects of load carrying on metabolic cost and hindlimb muscle dynamics in guinea fowl (Numida meleagris) J Appl Physiol. 2006;101:1060–1069. doi: 10.1152/japplphysiol.01538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mench JA. Measuring and Auditing Broiler Welfare. Wallingford, UK: CABI; 2004. Lameness. [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Miller BF. Comparative yield of different size turkey carcasses. Poult Sci. 1968;47:1570–1574. [Google Scholar]
- Nestor KE, Bacon WL, Saif YM, et al. The influence of genetic increases in shank width on body weight, walking ability and reproduction of turkeys. Poult Sci. 1985;64:2248–2255. doi: 10.3382/ps.0642248. [DOI] [PubMed] [Google Scholar]
- Nestor KE, Bacon WL, Moorhead PD, et al. Comparison of bone and muscle growth in turkey lines selected for increased body weight and increased shank width. Poult Sci. 1987;66:1421–1428. doi: 10.3382/ps.0661421. [DOI] [PubMed] [Google Scholar]
- Nicholson D. Research, is it the broiler industry’s partner into the new millennium? Worlds Poult Sci J. 1998;54:271–278. [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, et al. Functional specialisation of the equine pelvic limb. J Anat. 2005;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, et al. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Randall CJ, Mills CPJ. Observations on leg deformity in broilers with particular reference to the intertarsal joint. Avian Pathol. 1981;10:407–431. doi: 10.1080/03079458108418492. [DOI] [PubMed] [Google Scholar]
- Rath NC, Huff GR, Huff W, et al. Factors regulating bone maturity and strength in poultry. Poult Sci. 2000;79:1024–1032. doi: 10.1093/ps/79.7.1024. [DOI] [PubMed] [Google Scholar]
- Reilly SM. Locomotion in the quail (Coturnix japonica): the kinematics of walking and increasing speed. J Morphol. 2000;243:173–185. doi: 10.1002/(SICI)1097-4687(200002)243:2<173::AID-JMOR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Riddel C. Skeletal deformities in poultry. Adv Vet Sci Comp Med. 1985;25:278–310. [PubMed] [Google Scholar]
- Roberts TJ. Muscle force and stress during running in dogs and wild turkeys. Bull Mus Comp Zool. 2001;156:283–295. [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, et al. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Rubenson J, Henry HT, Dimoulas PM, et al. The cost of running uphill: linking organismal and muscle energy use in guinea fowl (Numida meleagris) J Exp Biol. 2006;209:2395–2408. doi: 10.1242/jeb.02310. [DOI] [PubMed] [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hindlimb muscles of cats: functional significance. J Morphol. 1982;173:185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Sandoe P, Nielsen BL, Christensen LG, et al. Staying good while playing God – the ethics of breeding farm animals. Anim Welf. 1999;8:313–328. [PubMed] [Google Scholar]
- Schmidt CJ, Persia ME, Feierstein E, et al. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult Sci. 2009;88:2610–2619. doi: 10.3382/ps.2009-00055. [DOI] [PubMed] [Google Scholar]
- Scott TA. Evaluation of lighting programs, diet density, and short-term use of mash as compared to crumbled starter to reduce incidence of sudden death syndrome in broiler chicks to 35 days of age. Can J Anim Sci. 2002;82:375–383. [Google Scholar]
- Skinner Noble DO, Teeter RG. An examination of anatomic, physiologic, and metabolic factors associated with well-being of broilers differing in field gait score. Poult Sci. 2009;88:2–9. doi: 10.3382/ps.2006-00450. [DOI] [PubMed] [Google Scholar]
- Smith NC, Wilson AM, Jespers KJ, et al. Muscle architecture and functional anatomy of the pelvic limb of the Ostrich (Struthio camelus) J Anat. 2006;209:765–779. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson P, Su G, Kestin SC. The effect of photoperiod: scotoperiod on leg weakness in broiler chickens. Poult Sci. 1999;78:336–342. doi: 10.1093/ps/78.3.336. [DOI] [PubMed] [Google Scholar]
- Squire BT, More SJ. Factors on farms in eastern Australia associated with the development of tibiotarsal rotation in ostrich chicks. Aust Vet J. 1998;76:110–117. doi: 10.1111/j.1751-0813.1998.tb14541.x. [DOI] [PubMed] [Google Scholar]
- Storer W. Avian Biology. New York: Academic Press; 1971. Adaptive radiation of birds. [Google Scholar]
- Su G, Sorenson P, Kestin SC. Meal feeding is more effective than early feed restriction at reducing the prevalence of leg weakness in broiler chickens. Poult Sci. 1999;78:949–955. doi: 10.1093/ps/78.7.949. [DOI] [PubMed] [Google Scholar]
- Thorp BH. Skeletal disorders in the fowl – a review. Avian Pathol. 1994;23:203–236. doi: 10.1080/03079459408418991. [DOI] [PubMed] [Google Scholar]
- Vestergaard KS, Sanotra GS. Relationships between leg disorders and changes in the behaviour of broiler chickens. Vet Rec. 1999;144:205–209. doi: 10.1136/vr.144.8.205. [DOI] [PubMed] [Google Scholar]
- Waldenstedt L. Nutritional factors of importance for optimal leg health in broilers: a review. Anim Feed Sci Technol. 2006;126:291–307. [Google Scholar]
- Wall CW, Anthony NB. Inheritance of carcass variables when giant junglefowl and broilers achieve a common physiological body weight. Poult Sci. 1995;74:231–236. doi: 10.3382/ps.0740231. [DOI] [PubMed] [Google Scholar]
- Webster J. Animal Welfare: A Cool Eye towards Eden. Cambridge, MA: Blackwell Science; 1995. [Google Scholar]
- Williams SB, Wilson AM, Payne RC. Functional specialisation of the thoracic limb of the hare (Lepus europeus) J Anat. 2007a;210:419–505. doi: 10.1111/j.1469-7580.2007.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Payne RC, Wilson AM. Functional specialisation of the pelvic limb of the hare (Lepus europeus) J Anat. 2007b;210:472–490. doi: 10.1111/j.1469-7580.2007.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


