Abstract
Context: Some studies suggest altered pituitary functioning and TSH production with aging.
Objective: Our objective was to test the hypothesis that less TSH production occurs despite comparable hypothyroxinemia with advancing age.
Design: We retrospectively studied adult outpatients of all ages with confirmed hypothyroidism and documented their TSH and free T4 concentrations.
Participants: Two populations of 112 patients were subdivided into four age groups: 1) patients newly diagnosed with primary hypothyroidism and 2) thyroid cancer patients undergoing l-T4 withdrawal in preparation for diagnostic or therapeutic radioiodine.
Main Outcome Measure: The relationship between paired free T4 and TSH concentrations and patient age was studied.
Results: With spontaneous hypothyroidism, the mean TSH concentration decreased nonsignificantly in each ascending age group with comparable free T4 (FT4) concentrations (<35 yr, 69 mIU/liter; 35–49 yr, 49 mIU/liter; 50–64 yr, 43 mIU/liter; >64 yr, 29 mIU/liter). With iatrogenic hypothyroidism, the mean TSH concentration decreased significantly in each ascending age group (<35 yr, 156 mIU/liter; 35–49 yr, 115 mIU/liter; 50–64 yr, 74 mIU/liter; >64 yr, 46 mIU/liter; P < 0.001) despite similar FT4 concentrations. The relationship between the log-transformed TSH and FT4 was significantly and inversely affected by age in multivariate analyses in both spontaneous hypothyroidism (P = 0.0005) and in iatrogenic hypothyroidism (P < 0.0001).
Conclusions: Age modifies the pituitary set point or response to comparably reduced free T4 concentrations, resulting in lesser serum TSH elevation in older individuals. This phenomenon occurs with both spontaneous and iatrogenic hypothyroidism. This may be an adaptive response in normal aging or a pathological alteration of pituitary function with age.
Age reduces the TSH response to hypothyroxinemia in both spontaneous and iatrogenic hypothyroidism.
The biochemical endpoint used to diagnosis and manage hypothyroidism is the serum TSH concentration. A patient’s physiological response to hypothyroxinemia is manifest by the degree of elevation of their serum TSH concentration.
Previous studies examining cases of spontaneous hypothyroidism have shown various effects of age on the thyrotrope response to reduced T4 concentrations. A 1994 study showed no impact of age and found that patients older than 70 yr had similar serum TSH concentrations to patients younger than 55 yr for similar degrees of hypothyroxinemia (1). A second study showed differential responses only at extremes of age. In this study, patients older than 70 yr had lesser TSH responses to a low total T4 level compared with patients younger than age 30 yr (2). More recently, Carlé et al. (3) reported an inverse relationship between TSH concentrations and age across the age spectrum. These authors studied cases of Hashimoto’s hypothyroidism diagnosed in the Danish community. They found that the median TSH values were significantly higher in patients under age 20 yr compared with those 80 yr and older. They also found in multivariate analyses that both total T4 and age were significantly inversely associated with serum TSH concentrations.
Many thyroid cancer specialists have observed, anecdotally, that younger patients mount a more vigorous pituitary response to thyroid hormone withdrawal protocols, manifest by higher TSH concentrations after a specific period of abstinence from l-T4. This impression is borne out by at least two studies. Schlumberger et al. (4) showed in 1984 that after at least 5 wk off l-T4, the TSH concentration achieved was inversely correlated with the patient’s age. In addition, the mean TSH value was significantly higher in those younger than 45 yr compared with those 45 yr and older. A study of children with thyroid cancer undergoing withdrawal from l-T4 without concurrent use of T3, showed that 12 d off thyroid hormone was sufficient to produce a serum TSH concentration of at least 25 mIU/liter (5). Other studies, either with or without temporary use of T3, have demonstrated in older individuals encompassing the adult age group that longer periods of discontinuation are generally necessary to achieve the requisite TSH concentration (6,7,8,9,10,11,12,13,14,15).
The goal of our study, therefore, was first to confirm that the TSH response to a given degree of hypothyroxinemia was affected by patient age. We then wished to determine whether the inverse relationship between serum TSH and age existed across the entire age spectrum, rather than only at extremes of age. We furthermore wished to extend the investigation to whether this phenomenon was a feature of both spontaneous and iatrogenic hypothyroidism. Finally, we speculated that the same age-TSH relationship would also hold true for subclinical hypothyroidism.
Patients and Methods
Two groups of 112 patients each were obtained through chart review after approval of the project by the Institutional Review Board. The review included medical records available from 1999 through 2009 at either Georgetown University Medical Center or Washington Hospital Center. A search was conducted for all patients with diagnoses of hypothyroidism and thyroid cancer. The paper and electronic records of these patients were reviewed to determine whether there were sufficient data to satisfy the study requirements. Any patients without the required data were excluded. All patients were outpatients, and they were excluded if the chart review suggested the possibility of any concurrent illness or confounding medications. There were no other exclusion criteria. Patients were divided into four age groups as follows: under 35 yr, 35–49 yr, 50–64 yr, and over 64 yr. These age groups were selected to divide the represented age range approximately into quarters yet ensure there were sufficient patients contained within the youngest and oldest groups.
Group with spontaneous hypothyroidism
For the group with spontaneous hypothyroidism, data were abstracted only for patients in whom the elevated TSH concentration had been confirmed with at least one confirmatory TSH reading, and l-T4 therapy was subsequently started. However, all data were from the period before l-T4 initiation. Patients with subclinical hypothyroidism were included if there were at least two confirmatory elevated TSH readings and if l-T4 therapy was maintained. For the patients older than 50 and 80 yr, upper limits of 7 and 10 mIU/liter were used for defining an abnormal TSH based on the data of Surks and Hollowell (16). Patients were also excluded if they were taking any medications known to affect TSH or thyroid hormone concentrations or relationships. Examples of medications leading to exclusion were steroids, dopamine analogs, somatostatin analogs, phenytoin, carbamazepine, sertraline, rifampin, amiodarone, and lithium. The following data were collected from the records of patients with primary hypothyroidism at Georgetown University: patient age, gender, height, weight, body mass index, menopausal status, first elevated TSH with a concomitant free T4 (FT4) concentration, FT4 concentration, serum creatinine concentration obtained within 2 wk of TSH determination, estimated glomerular filtration rate (GFR), year of laboratory evaluation, month of laboratory evaluation, type of hypothyroidism (Hashimoto’s hypothyroidism, surgically induced hypothyroidism, radioiodine therapy for hyperthyroidism, or external beam radiotherapy), and documentation of thyroid peroxidase antibodies. Data about thyroglobulin antibodies were not collected.
Group with iatrogenic hypothyroidism
For the group of thyroid cancer patients undergoing a protocol of withdrawal from l-T4, and thus having iatrogenic hypothyroidism, the only exclusion criteria were missing data, confounding medications, and concurrent illness. Hospitalization for radioiodine administration only was not grounds for exclusion. Patients with all stages of thyroid cancer were included, as were patients undergoing either their first or any subsequent withdrawal protocol. To our knowledge, no patient had functioning metastases that were secreting thyroid hormone. Only patients with concomitant FT4 concentrations that were detectable but less than 0.4 ng/dl were analyzed. The following data were collected from the records of patients with thyroid cancer at Georgetown University and Washington Hospital Center: patient age, gender, height, weight, body mass index, menopausal status, baseline TSH concentration, TSH concentration during withdrawal, FT4 concentration during withdrawal, serum creatinine concentration obtained within 1 wk of TSH determination, estimated GFR, year of protocol, month of protocol, number of days off l-T4 therapy, number of days off T3 therapy, thyroglobulin concentration, thyroglobulin antibody titer, presence of distant metastases, presence of thyroid remnant, type of differentiated thyroid cancer, years elapsed since thyroidectomy, years elapsed since last radioiodine therapy, and reason for withdrawal from l-T4 therapy (radioiodine therapy or diagnostic scanning or both). If data were collected from patients whose thyroidectomy was within the last 12 months, the years elapsed since thyroidectomy was designated as 0. If data were associated with current administration of radioiodine therapy, the years elapsed since radioiodine administration was described as 0.
Laboratory assessments and GFR calculation
Laboratory testing was performed by the clinical laboratory designated by the patient’s insurance company. TSH and FT4 were analyzed by Quest Diagnostics (Madison, NJ), LabCorp (Burlington, NC), Georgetown University Laboratory (Washington, DC), or Washington Hospital Center Laboratory (Washington, DC). TSH assays used by these clinical laboratories employed a third-generation ultrasensitive immunochemiluminometric assay with a sensitivity of 0.01 mIU/liter (laboratory reference ranges approximately 0.4–4.5 mIU/liter). FT4 levels were measured by the clinical laboratories using chemiluminescent immunoassays also. Over the period of this study, the reference range for FT4 was approximately 0.8–1.80 ng/dl. GFR was estimated using the abbreviated Modification of Diet in Renal Disease study equation (GFR = 186 × serum creatinine−1.154 × age−0.203 × sex × race) and reported in milliliters per minute per 1.73 m2 (17,18). Sex adjustments were 1 for males and 0.742 for females; adjustments for race were 1.21 for Black individuals and 1 for non-Black individuals.
Statistical analysis
Biostatistical support was provided by the Georgetown University Medical Center’s General Clinical Research Center. For pairs of continuous variables, scatter plots were constructed to visually display the respective relations. If found to be linear with both variables normally distributed, then the Pearson correlation was computed to statistically describe the significance, strength, and nature of the relation. If the assumption of linearity assumption was violated, then the Spearman rank correlation was used instead.
An independent two-samples t test was used to examine the existence of a statistically significant difference between the means of two groups if the variable being tested was normally distributed in each of the two independent groups. When the normality assumption was violated in either or both groups, the Wilcoxon-Mann-Whitney U test was used.
A one-way ANOVA was used to examine the difference in means of normally distributed variables between groups of three or more. When the normality assumption was violated, the Kruskal-Wallis test was used. The values of continuous variables whose distribution did not follow that of a normal distribution were ranked. A multifactorial ANOVA was then used to examine statistically significant differences among the ranks of the study groups. The analysis of covariance was used to test for statistically significant differences in the slopes of multiple regression lines. A multiple linear regression was used to model the relation between the dependent variable and a varying combination of predictor variables. SAS 9.1.3 was used for all analyses.
Results
The characteristics of the groups with spontaneous and iatrogenic hypothyroidism are shown in Tables 1 and 2, respectively. The spontaneously hypothyroid patients had a mean age of 46 yr and were 73% female. The cause of the hypothyroidism was Hashimoto’s hypothyroidism in 62% of patients. Elevated serum thyroid peroxidase antibodies were documented in 37%; in the remainder, the diagnosis was made on clinical grounds. The mean TSH concentration was 51 mIU/liter. The mean FT4 was 0.76 ng/dl, with 54% of cases being due to subclinical hypothyroidism. The group of patients with iatrogenic hypothyroidism had a mean age of 47 yr and were 77% female. They had been off l-T4 therapy for a mean of 39 d and off exogenous T3 for a mean of 13 d. Seventy-nine percent were receiving their first radioiodine therapy shortly after their thyroidectomy. Seventy-four percent had papillary thyroid cancer, 16% had follicular thyroid cancer, and only 13% had distant metastases. The mean TSH concentration was 105 mIU/liter with a corresponding mean serum FT4 of 0.23 ng/dl. In both the hypothyroid groups, serum creatinine progressively increased with age, whereas GFR progressively decreased with age (see Tables 1 and 2).
Table 1.
Characteristics of patients with spontaneous hypothyroidism (number of patients = 112)
| Characteristic | Mean (sd) | Percentage |
|---|---|---|
| Age (yr) | 46 (15) | |
| Gender | ||
| Female | 73 | |
| Male | 27 | |
| Height (m) | 1.68 (0.10) | |
| Weight (kg) | 73 (16) | |
| BMI | 26 (5) | |
| Menopausal status | ||
| Premenopausal | 43 | |
| Postmenopausal | 30 | |
| Not applicable | 27 | |
| TSH (mIU/liter) | 51 (73) | |
| FT4 (ng/dl) | 0.76 (0.35) | |
| Serum creatinine (mg/dl) | ||
| All ages | 0.99 (0.27) | |
| <35 yr | 0.91 (0.22) | |
| 35–49 yr | 0.94 (0.22) | |
| 50–64 yr | 1.05 (0.28) | |
| >64 yr | 1.21 (0.36) | |
| GFR (ml/min · 1.73 m2) | ||
| All ages | 83 (30) | |
| <35 yr | 99 (36) | |
| 35–49 yr | 87 (27) | |
| 50–64 yr | 71 (18) | |
| >64 yr | 58 (15) | |
| Type of hypothyroidism | ||
| Overt | 46 | |
| Subclinical | 54 | |
| Cause of hypothyroidism | ||
| Hashimoto’s hypothyroidism | 62 | |
| Surgery | 21 | |
| Radioiodine therapy | 11 | |
| External beam radiotherapy | 6 | |
| Presence of elevated thyroid peroxidase antibodies | ||
| Yes | 37 | |
| No | 35 | |
| Not determined | 28 |
Table 2.
Characteristics of patients with iatrogenic hypothyroidism (number of patients = 112)
| Characteristic | Mean (sd) | Percentage |
|---|---|---|
| Age (yr) | 47 (15) | |
| Gender | ||
| Female | 77 | |
| Male | 23 | |
| Height (m) | 1.72 (0.09) | |
| Weight (kg) | 79 (19) | |
| BMI | 28 (7) | |
| Menopausal status | ||
| Premenopausal | 46 | |
| Postmenopausal | 31 | |
| Not applicable | 23 | |
| TSH (mIU/liter) | 105 (75) | |
| FT4 (ng/dl) | 0.23 (0.09) | |
| Serum creatinine (mg/dl) | ||
| All ages | 1.22 (0.30) | |
| <35 yr | 1.09 (0.17) | |
| 35–49 yr | 1.13 (0.23) | |
| 50–64 yr | 1.24 (0.29) | |
| >64 yr | 1.67 (0.27) | |
| GFR (ml/min · 1.73 m2) | ||
| All ages | 64 (21) | |
| <35 yr | 75 (19) | |
| 35–49 yr | 68 (19) | |
| 50–64 yr | 59 (19) | |
| >64 yr | 39 (10) | |
| Baseline serum TSH (mIU/liter) | ||
| All ages | 2.0 (1.5) | |
| <35 yr | 1.7 (1.3) | |
| 35–49 yr | 1.7 (1.1) | |
| 50–64 yr | 2.1 (1.2) | |
| >64 yr | 3.1 (2.5) | |
| Days off l-T4 | 39 (5) | |
| Days off T3 | 13 (1) | |
| Thyroglobulin (ng/ml) | 85 (444), median 0 | |
| Presence of thyroglobulin antibodies | ||
| No | 83 | |
| Yes | 17 | |
| Distant metastases | ||
| No | 87 | |
| Yes | 13 | |
| Presence of thyroid remnant | ||
| Yes | 86 | |
| No | 14 | |
| Type of differentiated thyroid cancer | ||
| Papillary | 74 | |
| Follicular | 16 | |
| Other variant | 10 | |
| Years elapsed since thyroidectomy | 0.5 (1.2) | |
| Years elapsed since last radioiodine therapy | 0.3 (0.7) | |
| Initial radioiodine therapy | 79 | |
| Subsequent radioiodine therapy or diagnostic scanning | 21 |
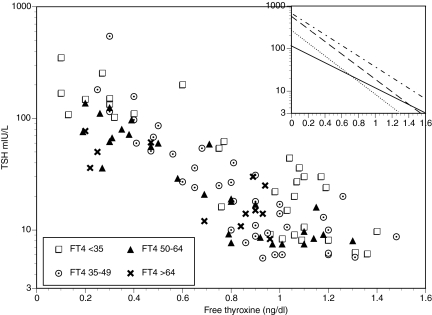
For the patients with spontaneous hypothyroidism, there was a statistically significant inverse correlation between FT4 and log10 TSH (Spearman correlation coefficient = −0.795, P value <0.0001). The slope of the relationship was −1.131 ± 0.074 with an intercept of 2.29 (TSH 194) (see Fig. 1). The relationship between FT4 and log10 TSH was significantly affected by age (P value = 0.0005). None of the following variables including gender, height, weight, BMI, menopausal status, year of laboratory evaluation, month of laboratory evaluation, type of hypothyroidism, or presence of thyroid peroxidase antibodies had any significant influence on the relationship between FT4 and log10 TSH. However, cause of hypothyroidism was a significant predictor in the model (p value = 0.042). Serum creatinine and estimated GFR were associated with TSH concentrations (P < 0.001), but were not significant predictors when age was included in the model.
Figure 1.
Relationship between FT4 and TSH in the spontaneously hypothyroid group according to age. For all patients together, the Spearman correlation coefficient = −0.795, P value <0.0001. The inset shows the slopes within the four age groups: dots and dashes, under 35 yr; dashes, 35–49 yr; dots, 50–64 yr; solid line, over 64 yr.
Patients were divided into 4 age groups as follows: <35 yr (n = 32), 35–49 yr (n = 38), 50–64 yr (n = 30), and >64 yr (n = 12). For each group there was a statistically significant inverse correlation between FT4 and log10 TSH (Spearman correlations: coefficient = −0.799, P value <0.0001; coefficient = −0.850, P value <0.0001; coefficient = −0.867, P value <0.0001; and coefficient = −0.606, P value = 0.036, respectively). The slope of the relationship was not statistically different for each age group. Separate intercepts on the y-axis could be visualized for each age group, but these were not statistically different (see inset in Fig. 1). When employing the same four age groups, neither the mean TSH nor the mean FT4 were statistically different for each group (see Table 3).
Table 3.
Differences in mean TSH and mean FT4 values with patients divided into four age groups
| Age groups (yr) | Number | TSH | FT4
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mean (mIU/liter) | Log-transformed mean TSH | sd (mIU/liter) | P value (for differences between age groups) | Mean (ng/dl) | sd (ng/dl) | P value (for differences between age groups using Kruskal-Wallis test) | ||
| Spontaneous hypothyroidism | ||||||||
| <35 | 32 | 69 | 1.83 | 82 | 0.45a | 0.82 | 0.41 | 0.16a |
| 35–49 | 38 | 49 | 1.69 | 92 | 0.81 | 0.31 | ||
| 50–64 | 30 | 43 | 1.63 | 39 | 0.67 | 0.35 | ||
| >64 | 12 | 29 | 1.46 | 22 | 0.68 | 0.30 | ||
| Iatrogenic hypothyroidism | ||||||||
| <35 | 28 | 156 | 2.19 | 98 | <0.0001b | 0.23 | 0.09 | 0.94a |
| 35–49 | 40 | 115 | 2.06 | 70 | 0.24 | 0.11 | ||
| 50–64 | 30 | 74 | 1.86 | 31 | 0.23 | 0.07 | ||
| >64 | 14 | 46 | 1.66 | 20 | 0.22 | 0.09 | ||
Kruskal-Wallis test.
ANOVA.
For the patients with iatrogenic hypothyroidism, there was a very narrow range of FT4 values, and there was no significant inverse correlation between FT4 and log10 TSH. Results for the group with iatrogenic hypothyroidism showed a statistically significant association between log10 TSH levels and FT4 after adjusting for age (P value <0.0001). Only age was a significant predictor in the model (P value <0.0001). All other variables including gender, height, weight, BMI, baseline TSH concentration before withdrawal from l-T4, menopausal status, year of laboratory evaluation, month of laboratory evaluation, days off l-T4, days off T3, thyroglobulin concentration, presence of thyroglobulin antibodies, distant metastases, presence of thyroid remnant, type of differentiated thyroid cancer, years since thyroidectomy, and years since last radioiodine therapy were not significant predictors in the model. Serum creatinine and estimated GFR, however, were again associated with TSH concentrations (P = 0.005 and P = 0.003, respectively) but were not significant predictors when age was included in the model.
When patients with iatrogenic hypothyroidism were again divided into four age groups [<35 yr (n = 28), 35–49 yr (n = 40), 50–64 yr (n = 30), and >64 yr (n = 14)], there was a statistically significant difference in the mean TSH concentrations among the four age groups (ANOVA based on ranks P value <0.0001) (see Table 3). After multiplicity adjustments, statistically significant differences in mean TSH concentrations were found between age groups under 35 and 35–49 yr (P value = 0.0476), age groups under 35 and 50–64 yr (P value <0.0001), and age groups under 35 and over 64 yr (P value <0.0001). There were also significant differences between age groups 35–49 and 50–64 yr (P value = 0.0028), and age groups 35–49 and over 64 yr (P value <0.001). Finally, there were also significant differences between age groups 50–64 and over 64 yr (P value = 0.019). On the other hand, there were no statistically significant differences in mean FT4 levels between the four age groups (Kruskal-Wallis test P value = 0.939).
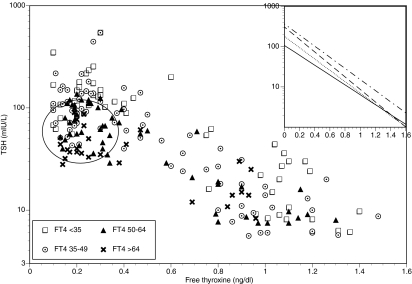
Finally, when the FT4 values and TSH values from the patients with both spontaneous and iatrogenic hypothyroidism were combined, there was a significant inverse relationship between FT4 and log10 TSH (Spearman correlation coefficient = −0.681, P value <0.0001). The slope of this relationship was −1.063 ± 0.043, and the intercept was 2.1 (TSH 125) (see Fig. 2). When these combined data were again divided into the same four age groups used previously [<35 yr (n = 60), 35–49 yr (n = 78), 50–64 yr (n = 60), and >64 yr (n = 26)], there were again significant relationships between FT4 and log10 TSH (P value <0.0001 in each group). The slopes of the relationships were not statistically different between age groups; neither were the intercepts on the y-axis statistically different (see inset in Fig. 2).
Figure 2.
Relationship between FT4 and TSH for all hypothyroid patients (spontaneous and iatrogenic causes combined) according to age. For all patients together, the Spearman correlation coefficient = −0.681, P value <0.0001. Most of the values for the group with iatrogenic hypothyroidism fall within the outlined circle. The inset shows the slopes within the four age groups: dots and dashes, under 35 yr; dashes, 35–49 yr; dots, 50–64 yr; solid line, over 64 yr.
Discussion
In the present study, we confirm that the magnitude of the pituitary response to hypothyroidism, as judged by the serum TSH concentration, is affected by patient age. In contrast to several previous studies, we found this relationship to be present across the entire age spectrum, including in the middle-aged years. We found this relationship to hold true regardless of whether the etiology of the primary hypothyroidism was spontaneous or iatrogenic. Moreover, the cause of the spontaneous hypothyroidism (Hashimoto’s hypothyroidism vs. other causes) did not appear to obscure the finding of a relationship. As a novel finding, we also showed that the relationship extended to cases of confirmed subclinical hypothyroidism.
We did not find that the mean TSH values seen in cases of spontaneous hypothyroidism were different for different age groups, as has previously been found by others. We speculate that this could be explained by the fact that these patients represent a diverse group in whom other factors such as duration of disease, severity of hypothyroidism, and cause of hypothyroidism may all have influenced the magnitude of the TSH response achieved. Patients with overt hypothyroidism would be expected to have had unrecognized disease for a much longer period than patients with subclinical disease, with patients thus coming to attention at different points in the course of their disease. The inclusion of patients with hypothyroidism caused by surgery, radioiodine therapy, and radiation treatment may have also contributed to differing presentation of hypothyroidism. However, when examining the patients with iatrogenic hypothyroidism, we demonstrated significantly different mean TSH values within the different age groups. We believe this occurred, in contrast to the finding in the group with spontaneous hypothyroidism, because of the controlled conditions of the hypothyroidism, in which all patients underwent the same period of l-T4 deprivation. This uniformity may have allowed the effect of age to become manifest. It is well documented that there may be a delay in the ability of the pituitary to signal a low T4 concentration with a high TSH value after previous suppression by high T4 levels (19,20). This effect did not seem to be evident in our group with iatrogenic hypothyroidism, because there was no relationship between baseline TSH concentrations and hypothyroid TSH levels. However, 79% of our patients were undergoing their initial radioiodine therapy after thyroidectomy, and their most recent TSH value was normal.
TSH is known to be cleared through both hepatic metabolism and renal excretion (21,22). It is interesting that older patients had lower TSH concentrations, despite the fact that based on animal data, both these avenues of clearance would be expected to be reduced in older individuals (23). It is known that increased serum creatinine levels can be a consequence of severe hypothyroidism (24,25,26). This has also been documented in the setting of the iatrogenic hypothyroidism used in the management of thyroid cancer patients, where serum creatinine elevations have been reported in approximately 90% of patients (24,27). Such hypothyroidism-induced declines in renal function have been associated with metformin toxicity (28), lithium toxicity (29), and rhabdomyolysis (30) in case reports. In our study, we were not able to document baseline creatinine levels in our patients, but the serum creatinine concentrations measured when the patients were hypothyroid appeared to be higher than the reported age-related reference ranges (31,32). Similarly, GFR values were lower than those typically seen in euthyroid patients of similar ages (33,34). In our patients, in addition to evidence of decreased renal function with increasing degrees of hypothyroidism, there was also a decline in renal function with age. Because our older patients could potentially have augmentation of their TSH concentrations on the basis of their diminished renal function, this suggests that the attenuation in their pituitary response to hypothyroxinemia may be even more profound than is apparent from our data.
The serum FT4 concentrations seen in the hypothyroid state in our study were not different between age groups, regardless of the cause of the hypothyroidism. It has also previously been shown that FT4 levels do not change with age in euthyroid individuals. For example, FT4 concentrations did not differ between young and old in 22,116 outpatients without thyroid disease in the United States (35) or between young and old in 387 subjects without thyroid disease studied in Europe (36). In addition, FT4 levels also were not different in younger control groups and a group comprised entirely of healthy centenarians (37). These data would suggest that TSH alterations in response to hypothyroidism are not secondary to subtle alterations in FT4 levels themselves. The daily l-T4 dose required to maintain euthyroidism is also known to decrease with age (38,39,40,41,42). It is possible that this reduced requirement with age may be partially due to altered body composition and changes in l-T4 metabolism or clearance with age. Alternatively, there could be an altered pituitary response to circulating thyroid hormones.
It is unclear from these data whether this phenomenon of age-related decrease in TSH concentrations is due to altered responsiveness of the pituitary to reduced FT4 concentrations, a modified pituitary set point, a reduced capacity for production of TSH, or a combination of these factors. Each of these changes could either represent a normal adaptation to age or a pathological change with its onset in older age groups. For example, there could be less vigorous secretion of TSH in older individuals due to a protective down-regulation of the pituitary-thyroid axis. Alternatively, there could be impaired production of TSH due to pathological deterioration of pituitary function or reserve with age. It has been shown that TSH pulse amplitude is decreased at night in the elderly subjects compared with younger subjects, suggestive of alteration of the pituitary-thyroid axis with age (43). TSH responses to TRH have been reported to be normal in the elderly in some studies (43) but diminished in other studies (36).
TSH reference intervals are impacted by age, with a shift to higher TSH concentrations in older age groups (16,35). These latter findings, if they imply that higher TSH concentrations are normal in older age groups, might also suggest that a lesser TSH response to hypothyroxinemia in older individuals may be protective. This would certainly be in concert with the findings that higher TSH concentrations are associated with longevity in older individuals (44). An alternative contradictory or opposite conclusion that could be drawn from our data are that the serum TSH may not completely reflect the degree of hypothyroxinemia experienced by older patients.
The major shortcomings of this study are due to its retrospective design. In addition to the statistical limitations inherent in any retrospective study, the design of the study predicated that assays from four different clinical laboratories were run at different points in time and were then pooled. This is obviously not an ideal situation and could be avoided if funding was secured to conduct a prospective study using uniform assays for both TSH and FT4 measurement. A prospective study would also have permitted tighter processing of patients and more sophisticated manipulations to examine whether TSH levels tended to plateau, or reach a plateau earlier, in different age groups. This would have increased the robustness of the findings. As a counterpoint to these weaknesses, however, the strengths of the study are that subjects had confirmed hypothyroidism of two different causes (spontaneous and iatrogenic) and were of ages spanning the age spectrum. In addition, we included patients with subclinical hypothyroidism and were able to collect fairly extensive clinical data on our subjects.
The clinical consequences of these findings are clear with respect to the l-T4 withdrawal protocols used to prepare thyroid cancer patients for diagnostic scanning or radioiodine therapy. If this method of preparation is selected, the patient’s age should be considered, because the period of withdrawal, and attendant symptomatic hypothyroidism, can likely be considerably shortened in younger patients, with the desired TSH concentration of 30 mIU/liter (11) still being achieved. With respect to interpreting thyroid function tests in cases of spontaneous hypothyroidism, it is apparent that in older individuals, a particular TSH value may be associated with a greater degree of hypothyroxinemia. Thus, the possibility of significant hypothyroxinemia in older individuals should be entertained despite a modest degree of TSH elevation. However, the implication of this observation for the treatment of hypothyroidism in older patients has yet to be determined.
Acknowledgments
The assistance of medical students Dipti Patel and David Hur is gratefully acknowledged.
Footnotes
This work was supported by Grant M01-RR020359 from the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health. This analysis constitutes the fellowship and resident research projects of R.O. and S.M., respectively.
Present addresses for R.O.: Pacific Medical Center, Seattle, Washington.
Present addresses for S.M.: Upper Chesapeake Medical Center, Bel Air, Maryland.
Disclosure Summary: R.O., S.M., H.N.-M., and J.J. have nothing to disclose. The Washington Hospital Center receives funding to support clinical thyroid cancer protocols from Pfizer and Exelixis. K.D.B. has written articles for Medscape and UpToDate and is on the Board of the American Thyroid Association, is Deputy Editor of the Journal of Clinical Endocrinology and Metabolism, and is on the U.S. Food and Drug Administration Endocrine Advisory Committee.
First Published Online May 19, 2010
Abbreviations: FT4, Free T4; GFR, glomerular filtration rate.
References
- Doucet J, Trivalle C, Chassagne P, Perol MB, Vuillermet P, Manchon ND, Menard JF, Bercoff E 1994 Does age play a role in clinical presentation of hypothyroidism? J Am Geriatr Soc 42:984–986 [DOI] [PubMed] [Google Scholar]
- Wiener R, Utiger RD, Lew R, Emerson CH 1991 Age, sex, and serum thyrotropin concentrations in primary hypothyroidism. Acta Endocrinol (Copenh) 124:364–369 [DOI] [PubMed] [Google Scholar]
- Carlé A, Laurberg P, Pedersen IB, Perrild H, Ovesen L, Rasmussen LB, Jorgensen T, Knudsen N 2007 Age modifies the pituitary TSH response to thyroid failure. Thyroid 17:139–144 [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Charbord P, Fragu P, Lumbroso J, Parmentier C, Tubiana M 1980 Circulating thyroglobulin and thyroid hormones in patients with metastases of differentiated thyroid carcinoma: relationship to serum thyrotropin levels. J Clin Endocrinol Metab 51:513–519 [DOI] [PubMed] [Google Scholar]
- Kuijt WJ, Huang SA 2005 Children with differentiated thyroid cancer achieve adequate hyperthyrotropinemia within 14 days of levothyroxine withdrawal. J Clin Endocrinol Metab 90:6123–6125 [DOI] [PubMed] [Google Scholar]
- Liel Y 2002 Preparation for radioactive iodine administration in differentiated thyroid cancer patients. Clin Endocrinol (Oxf) 57:523–527 [DOI] [PubMed] [Google Scholar]
- Grigsby PW, Siegel BA, Bekker S, Clutter WE, Moley JF 2004 Preparation of patients with thyroid cancer for 131I scintigraphy or therapy by 1–3 weeks of thyroxine discontinuation. J Nucl Med 45:567–570 [PubMed] [Google Scholar]
- Golger A, Fridman TR, Eski S, Witterick IJ, Freeman JL, Walfish PG 2003 Three-week thyroxine withdrawal thyroglobulin stimulation screening test to detect low-risk residual/recurrent well-differentiated thyroid carcinoma. J Endocrinol Invest 26:1023–1031 [DOI] [PubMed] [Google Scholar]
- Sánchez R, Espinosa-de-los-Monteros AL, Mendoza V, Brea E, Hernández I, Sosa E, Mercado M 2002 Adequate thyroid-stimulating hormone levels after levothyroxine discontinuation in the follow-up of patients with well-differentiated thyroid carcinoma. Arch Med Res 33:478–481 [DOI] [PubMed] [Google Scholar]
- Maxon 3rd HR, Smith HS 1990 Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am 19:685–718 [PubMed] [Google Scholar]
- Edmonds CJ, Hayes S, Kermode JC, Thompson BD 1977 Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol 50:799–807 [DOI] [PubMed] [Google Scholar]
- Hilts SV, Hellman D, Anderson J, Woolfenden J, Van Antwerp J, Patton D 1979 Serial TSH determination after T3 withdrawal or thyroidectomy in the therapy of thyroid carcinoma. J Nucl Med 20:928–932 [PubMed] [Google Scholar]
- Goldman JM, Line BR, Aamodt RL, Robbins J 1980 Influence of triiodothyronine withdrawal time on 131I uptake postthyroidectomy for thyroid cancer. J Clin Endocrinol Metab 50:734–739 [DOI] [PubMed] [Google Scholar]
- Davids T, Witterick IJ, Eski S, Walfish PG, Freeman JL 2006 Three-week thyroxine withdrawal: a thyroid-specific quality of life study. Laryngoscope 116:250–253 [DOI] [PubMed] [Google Scholar]
- Serhal DI, Nasrallah MP, Arafah BM 2004 Rapid rise in serum thyrotropin concentrations after thyroidectomy or withdrawal of suppressive thyroxine therapy in preparation for radioactive iodine administration to patients with differentiated thyroid cancer. J Clin Endocrinol Metab 89:3285–3289 [DOI] [PubMed] [Google Scholar]
- Surks MI, Hollowell JG 2007 Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- Levey A, Greene T, Kusek J 2000 A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11:A0828 (Abstract) [Google Scholar]
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F 2006 Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254 [DOI] [PubMed] [Google Scholar]
- Chung YJ, Lee BW, Kim JY, Jung JH, Min YK, Lee MS, Lee MK, Kim KW, Chung JH 2006 Continued suppression of serum TSH level may be attributed to TSH receptor antibody activity as well as the severity of thyrotoxicosis and the time to recovery of thyroid hormone in treated euthyroid Graves’ patients. Thyroid 16:1251–1257 [DOI] [PubMed] [Google Scholar]
- Ross DS, Daniels GH, Gouveia D 1990 The use and limitations of a chemiluminescent thyrotropin assay as a single thyroid function test in an out-patient endocrine clinic. J Clin Endocrinol Metab 71:764–769 [DOI] [PubMed] [Google Scholar]
- Cuttelod S, Lemarchand-Béraud T, Magnenat P, Perret C, Poli S, Vannotti A 1974 Effect of age and role of kidneys and liver on thyrotropin turnover in man. Metabolism 23:101–113 [DOI] [PubMed] [Google Scholar]
- Constant RB, Weintraub BD 1986 Differences in the metabolic clearance of pituitary and serum thyrotropin (TSH) derived from euthyroid and hypothyroid rats: effects of chemical deglycosylation of pituitary TSH. Endocrinology 119:2720–2727 [DOI] [PubMed] [Google Scholar]
- Connors JM, DeVito WJ, Hedge GA 1984 The effects of the duration of severe hypothyroidism and aging on the metabolic clearance rate of thyrotropin (TSH) and the pituitary TSH response to TSH-releasing hormone. Endocrinology 114:1930–1937 [DOI] [PubMed] [Google Scholar]
- Tuttle RM, Leboeuf R, Robbins RJ, Qualey R, Pentlow K, Larson SM, Chan CY 2006 Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med 47:1587–1591 [PubMed] [Google Scholar]
- Karanikas G, Schütz M, Szabo M, Becherer A, Wiesner K, Dudczak R, Kletter K 2004 Isotopic renal function studies in severe hypothyroidism and after thyroid hormone replacement therapy. Am J Nephrol 24:41–45 [DOI] [PubMed] [Google Scholar]
- Montenegro J, González O, Saracho R, Aguirre R, González O, Martínez I 1996 Changes in renal function in primary hypothyroidism. Am J Kidney Dis 27:195–198 [DOI] [PubMed] [Google Scholar]
- Kreisman SH, Hennessey JV 1999 Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch Intern Med 159:79–82 [DOI] [PubMed] [Google Scholar]
- Bernet VJ 2004 Reversible renal insufficiency attributable to thyroid hormone withdrawal in a patient with type 2 diabetes mellitus. Endocr Pract 10:339–344 [DOI] [PubMed] [Google Scholar]
- Phillips BD, Gopalakrishnan G, Gohh R, Hennessey JV 2008 Lithium toxicity precipitated by profound hypothyroidism. Thyroid 18:651–654 [DOI] [PubMed] [Google Scholar]
- Espiritu RP, Stan MN 2008 Rhabdomyolysis after withdrawal of thyroid hormone in a patient with papillary thyroid cancer. Endocr Pract 14:1023–1026 [DOI] [PubMed] [Google Scholar]
- Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F 2008 Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta 396:49–55 [DOI] [PubMed] [Google Scholar]
- Tiao JY, Semmens JB, Masarei JR, Lawrence-Brown MM 2002 The effect of age on serum creatinine levels in an aging population: relevance to vascular surgery. Cardiovasc Surg 10:445–451 [DOI] [PubMed] [Google Scholar]
- Lindeman RD, Tobin J, Shock NW 1985 Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33:278–285 [DOI] [PubMed] [Google Scholar]
- Lindeman RD 1990 Overview: renal physiology and pathophysiology of aging. Am J Kidney Dis 16:275–282 [DOI] [PubMed] [Google Scholar]
- Surks MI, Boucai L 2010 Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 95:496–502 [DOI] [PubMed] [Google Scholar]
- Sell MA, Schott M, Tharandt L, Cissewski K, Scherbaum WA, Willenberg HS 2008 Functional central hypothyroidism in the elderly. Aging Clin Exp Res 20:207–210 [DOI] [PubMed] [Google Scholar]
- Mariotti S, Barbesino G, Caturegli P, Bartalena L, Sansoni P, Fagnoni F, Monti D, Fagiolo U, Franceschi C, Pinchera A 1993 Complex alteration of thyroid function in healthy centenarians. J Clin Endocrinol Metab 77:1130–1134 [DOI] [PubMed] [Google Scholar]
- Jonklaas J 2010 Sex and age differences in levothyroxine dosage requirement. Endocr Pract 16:71–79 [DOI] [PubMed] [Google Scholar]
- Sawin CT, Herman T, Molitch ME, London MH, Kramer SM 1983 Aging and the thyroid. Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med 75:206–209 [DOI] [PubMed] [Google Scholar]
- Cunningham JJ, Barzel US 1984 Lean body mass is a predictor of the daily requirement for thyroid hormone in older men and women. J Am Geriatr Soc 32:204–207 [DOI] [PubMed] [Google Scholar]
- Rosenbaum RL, Barzel US 1982 Levothyroxine replacement dose for primary hypothyroidism decreases with age. Ann Intern Med 96:53–55 [DOI] [PubMed] [Google Scholar]
- Young RE, Jones SJ, Bewsher PD, Hedley AJ 1984 Age and the daily dose of thyroxine replacement therapy for hypothyroidism. Age Ageing 13:293–303 [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Klibanski A, Rowe JW, Elahi D 1991 Age-related alterations in pulsatile secretion of TSH: role of dopaminergic regulation. Am J Physiol 260:E486–E491 [DOI] [PubMed] [Google Scholar]
- Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG 2004 Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]