Abstract
Context: Antagonism of protein kinase Cβ (PKCβ) restores endothelial function in experimental models of diabetes and prevents vascular dysfunction in response to hyperglycemia in healthy humans.
Objective: We tested the hypothesis that PKCβ antagonism would improve vascular function in subjects with type 2 diabetes compared with healthy control subjects.
Design: The effect of PKCβ was evaluated in a randomized, placebo-controlled, double-blinded crossover trial.
Setting: The study was performed in the outpatient setting of a university medical center.
Participants: Thirteen subjects with type 2 diabetes without evidence of cardiovascular disease and 15 healthy control subjects were recruited via newspaper advertisement.
Intervention: Subjects underwent a randomized, double-blind, crossover, placebo-controlled trial of the selective PKCβ antagonist ruboxistaurin mesylate. Subjects received each treatment for 14 d.
Main Outcome Measure: Endothelium-dependent and endothelium-independent vasodilation of forearm resistance vessels was measured with mercury-in-silastic, strain-gauge plethysmography during intraarterial administration of methacholine chloride and verapamil, respectively. Markers of inflammation, fibrinolysis, endothelial damage, and oxidative stress were measured after each treatment.
Results: Endothelium-dependent vasodilation of forearm resistance vessels was attenuated in diabetic subjects when compared with healthy subjects (P = 0.001). Endothelium-independent vasodilation did not differ between groups (P value not significant). Ruboxistaurin did not significantly change endothelium-dependent or endothelium-independent vasodilation or blood-based markers of inflammation, fibrinolysis, endothelial damage, and oxidative stress in either diabetic or healthy subjects.
Conclusion: Endothelial dysfunction of forearm resistance vessels was not improved by 2 wk of selective PKCβ inhibition in patients with diabetes. These results suggest that PKCβ does not contribute significantly to vascular dysfunction in otherwise healthy patients with type 2 diabetes.
Protein kinase Cβ inhibition with ruboxistaurin does not improve endothelial function in persons with type 2 diabetes mellitus.
Vascular disease is the primary cause of morbidity and mortality in patients with diabetes mellitus (1). We have previously demonstrated impaired endothelium-dependent, nitric oxide-mediated vasodilation in forearm resistance vessels of subjects with type 2 diabetes (2). The major pathophysiological manifestations of diabetes, including hyperglycemia, free fatty acid excess, and insulin resistance, are associated with impaired endothelial function, suggesting a multiplicity of insults that contribute to vascular dysfunction (1). Recent work has implicated each of these metabolic derangements in vascular dysfunction through protein kinase C (PKC) activation (1).
The β-isoforms of PKC are preferentially activated by hyperglycemia and excess free fatty acid concentrations in the vasculature (3,4) and may be a particularly important cause of endothelial dysfunction in diabetes. We demonstrated previously that selective antagonism of PKCβ with ruboxistaurin mesylate prevents vascular dysfunction induced by hyperglycemia in healthy humans (5). Accordingly, the purpose of this investigation was to test the hypothesis that selective inhibition of PKCβ with ruboxistaurin would improve forearm resistance vessel endothelial function and blood-based markers of endothelial activation in patients with type 2 diabetes mellitus.
Subjects and Methods
Subjects
Type 2 diabetic and healthy volunteers were recruited via newspaper advertisement and provided written, informed consent. All subjects underwent screening, consisting of a medical history, physical examination, and laboratory studies, including a complete blood cell count, serum chemistries, and a lipid profile. Subjects with uncontrolled hypertension (blood pressure >140/90 mm Hg despite medication), uncontrolled diabetes (hemoglobin A1c >11%), glomerular filtration rate less than 60 ml/min, history of tobacco use, low-density lipoprotein (LDL) or total cholesterol higher than 75th percentile for age and gender, and cardiovascular or other disease were excluded, as were subjects currently taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statins. The protocol was approved by the Human Research Committee of Brigham and Women’s Hospital.
Study design
This was a randomized, double-blind, placebo-controlled, crossover design trial. Subjects were randomized to ruboxistaurin (Eli Lilly and Co., Indianapolis, IN), 32 mg orally once daily or matching placebo for 14 d before and on the morning of each vascular function study. This dose was chosen based on previous work (5) and pharmacokinetic analysis (6). After a minimum 2-wk washout period, subjects then crossed over and received the other medication. Female participants underwent vascular testing during the same menstrual phase. Cyclooxygenase inhibitors, alcohol, and caffeine were prohibited for 12 h before the study morning. All subjects were studied in the morning in the postabsorptive state, fasting after the previous midnight.
Vascular reactivity studies
An indwelling catheter was inserted into the brachial artery at the antecubital fossa. After a minimum of 30 min after catheter insertion, baseline mean arterial pressure (MAP) and forearm blood flow were measured. Endothelium-dependent vasodilation was assessed by measuring the forearm blood flow (FBF) response to incremental intraarterial doses of methacholine chloride (0.3, 1.0, 3.0, and 10.0 μg/min). FBF measurements were made during the last 2 min of a 6-min infusion. Endothelium-independent vasodilation was assessed with incremental intraarterial doses of the calcium channel blocker verapamil at doses of 10, 30, 100, and 300 μg/min. FBF was measured by venous-occlusion, mercury-in-silastic, strain-gauge plethysmography (D. E. Hokanson, Issaquah, WA) using established methods (5). The vascular research laboratory was quiet, dimly lit, and temperature controlled at 23 C.
Biomarkers of endothelial activation, fibrinolysis, and oxidative stress
Laboratory analyses included soluble vascular cell adhesion molecule-1 (sVCAM-1) (R&D Systems, Minneapolis, MN; kit no. DVC00, lot no. 258373), nitrotyrosine (NT) (Hycult Biotechnology, Uden The Netherlands), plasminogen activator inhibitor-1 (PAI-1) (R&D Systems; kit no. DSE100, lot no. 258454), high-sensitivity C-reactive protein (hsCRP) (Wako Diagnostics, Richmond, VA), and von Willebrand factor (vWF) (Hyphen BioMed, Neuville sur Oise, France).
Statistical analyses
Descriptive measures are reported as mean ± sd. Experimental measures are reported as mean ± se. Biomarkers are reported as median (interquartile range). Basal FBF was compared by paired two-tailed t tests. Two-way, repeated-measures ANOVA was performed to compare the dose-response curves during each treatment, using the absolute increase in blood flow from the basal flow rate. Laboratory measures were compared by Mann-Whitney U testing for two-way comparisons. Statistical significance was accepted at the 95% confidence level (P < 0.05).
Results
This study included 13 patients with type 2 diabetes mellitus and 15 healthy subjects. Diabetic subjects were older (53 ± 9 vs. 44 ± 12 yr, P = 0.009), had higher levels of fasting glucose (176 ± 63 vs. 94 ± 9 mg/dl, P < 0.001), higher glycated hemoglobin (8.4 ± 1.4 vs. 5.3 ± 0.4%, P < 0.001), and a higher body mass index (30.6 ± 6.4 vs. 25.6 ± 3.5 kg/m2, P = 0.03) than healthy subjects. Type 2 diabetes was present for an average of 7.8 ± 5.4 yr. One subject had mild proteinuria, none had retinopathy, and one had neuropathy. Diabetic and healthy subjects were well matched by sex, MAP, total and LDL cholesterol, and renal function.
Effect of ruboxistaurin on FBF
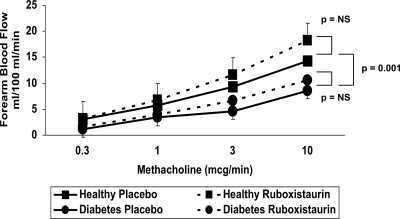
During placebo treatment, basal FBF did not differ between healthy and diabetic subjects (1.8 ± 0.2 vs. 2.2 ± 0.4 ml/100 ml · min, respectively; P > 0.2). Incremental doses of methacholine increased FBF in all subjects, and the methacholine response was attenuated in the diabetic subjects compared with control subjects (P = 0.001; Fig. 1). Adjustment for the baseline difference in age did not significantly attenuate the effect of diabetes on the response to intraarterial methacholine (P = 0.003 for the difference between healthy and diabetic subjects). There was no significant difference in the FBF response to verapamil between healthy and diabetic subjects (P = 0.17).
Figure 1.
Endothelium-dependent vasodilation in healthy and diabetic subjects. A, The increase in FBF induced by incremental methacholine from baseline during placebo and ruboxistaurin treatment is shown. Endothelium-dependent vasodilation in subjects with diabetes (•) was attenuated when compared with healthy control subjects (▪) (P = 0.001). Ruboxistaurin did not affect endothelium-dependent vasodilation in either healthy or diabetic subjects. NS, Not significant.
Ruboxistaurin treatment did not significantly change MAP, heart rate, lipid levels, fasting glucose, or hemoglobin A1c compared with placebo treatment in either healthy or diabetic subjects (Table 1). Treatment with ruboxistaurin did not significantly change basal FBF in healthy subjects (1.8 ± 0.2 vs. 2.2 ± 0.3 ml/100 ml · min for placebo and ruboxistaurin, respectively; P > 0.2) or diabetic subjects (2.2 ± 0.4 vs. 2.2 ± 0.3 ml/100 ml · min for placebo and ruboxistaurin, respectively; P > 0.2). Compared with placebo treatment, ruboxistaurin administration did not change the FBF response to methacholine in either healthy (P > 0.2) or diabetic subjects (P > 0.2; Fig. 1). Similarly, ruboxistaurin did not affect the FBF response to verapamil in either healthy controls (P > 0.2) or diabetic subjects (P > 0.2).
Table 1.
Effect of ruboxistaurin on laboratory measures and endothelial biomarkers
| Healthy
|
Diabetes
|
|||
|---|---|---|---|---|
| Placebo | Ruboxistaurin | Placebo | Ruboxistaurin | |
| Laboratory measures | ||||
| MAP (mm Hg) | 85.3 ± 15.5 | 90.0 ± 17.3 | 98.1 ± 11.2a | 98.7 ± 11.7 |
| Total cholesterol (mg/dl) | 176 ± 27 | 179 ± 27 | 178 ± 33 | 190 ± 39 |
| LDL cholesterol (mg/dl) | 105 ± 23 | 105 ± 23 | 100 ± 39 | 114 ± 39 |
| HDL cholesterol (mg/dl) | 51 ± 16 | 51 ± 16 | 44 ± 13 | 54 ± 39 |
| Triglycerides (mg/dl) | 107 ± 71 | 107 ± 98 | 176 ± 89b | 198 ± 89b |
| Glucose (mg/dl) | 92.6 ± 10.9 | 94.7 ± 10.4 | 185.2 ± 70.2b | 174.6 ± 61.2b |
| ALT (U/liter) | 20 ± 7.5 | 23.6 ± 7.6 | 26.1 ± 16.3 | 26.8 ± 17.8 |
| Endothelial biomarkers | ||||
| hsCRP (mg/liter) | 0.67 (0.46–1.8) | 1.05 (0.52–2.44) | 2.2 (1.25–10.24)a | 1.54 (0.81–3.67) |
| PAI-1 (ng/ml) | 2.03 (1.15–4.10) | 3.41 (1.97–5.79) | 5.62 (3.63–8.09) | 3.23 (1.53–6.92) |
| sVCAM (ng/ml) | 398.4 (292.2–432.9) | 430.3 (360.0–538.3) | 396.0 (314.6–536.6) | 427.4 (309.2–562.8) |
| vWF | 80.4 (56.8–98.1) | 60.9 (20.9–87.3) | 85.3 (49.5–111.8) | 108.0 (51.3–115.1) |
| NT (nm) | 12.2 (1.4–54.0) | 3.2 (0.2–16.2) | 2.1 (0.2–17.8) | 3.5 (0.2–17.8) |
ALT, Alanine aminotransferase; HDL, high-density lipoprotein.
P < 0.05 for comparison with control placebo.
P = 0.001 for comparison with same treatment period in control subjects.
Biomarkers of endothelial activation, fibrinolysis, and oxidative stress
Blood-based markers of vascular function including inflammation (hsCRP and sVCAM), fibrinolysis (PAI-1), endothelial damage (vWF), and oxidative stress (NT) were measured. During placebo treatment, subjects with type 2 diabetes had a higher hsCRP [2.2 (1.25–10.24) mg/liter] compared with healthy subjects [0.67 (0.46-.80), P = 0.039], but no differences were noted in the other markers. Treatment with ruboxistaurin did not significantly change any of these biomarkers in healthy or diabetic subjects (Table 1).
Discussion
In this study, inhibition of PKCβ by ruboxistaurin did not improve endothelium-dependent vasodilation in forearm resistance vessels in patients with type 2 diabetes mellitus. Additional indicators of vascular function (markers of inflammation, oxidative stress, inhibitors of fibrinolysis, and endothelial damage) were also unaffected by inhibition of PKCβ. Therefore, in patients with type 2 diabetes, PKCβ inhibition did not improve several parameters of endothelial function.
PKCβ in diabetes
PKC is a superfamily of cytoplasmic kinases consisting of approximately 10 isoforms (7). Of the family of PKC enzymes, PKCβ activation may be particularly important in diabetes and vascular disease. PKCβ is preferentially expressed in vascular endothelial cells exposed to hyperglycemia or free fatty acids (1) and is activated in animal models of diabetes (4). Inhibition of PKCβ has been shown to improve diabetic vascular dysfunction in experimental models of diabetes. PKCβ decreased insulin-mediated activation of endothelial nitric oxide synthase (8), and conversely, inhibition of PKCβ with ruboxistaurin normalized flow-mediated vasodilation and restored endothelium-dependent vasodilation in mesenteric and coronary arteries in diabetic rat models (9,10,11). We have demonstrated that acute hyperglycemia created by hyperglycemic clamp impairs endothelial function in healthy humans in vivo and that this reduction in the bioavailability of nitric oxide may be prevented with inhibition of PKCβ (5). In other human studies, ruboxistaurin increased skin microvascular blood flow in subjects with diabetic neuropathy compared with placebo (12) and reduced urinary albumin in diabetic subjects with albuminuria (13). These findings suggested that PKCβ activation may represent a viable intracellular vascular therapeutic target in type 2 diabetes.
Despite attenuated forearm microvascular endothelial function in the diabetic subjects, ruboxistaurin did not restore endothelium-dependent vasodilation in our cohort. Our findings are buttressed by a study in humans in conduit arteries and the outcome of recent large clinical trials. Mehta and colleagues (14) evaluated PKCβ inhibition on brachial artery flow-mediated vasodilation in subjects with type 2 diabetes, showing that ruboxistaurin improved conduit artery flow-mediated vasodilation compared with placebo, the difference in vascular function between the groups was related to worsening in the placebo group. In two ophthalmic trials (n = 937) and a trial in diabetic peripheral neuropathy (n = 205), ruboxistaurin failed to meet its primary endpoint (15,16,17). Thus, our findings are consistent with a lack of significant effect on the microvascular dysfunction in diabetes.
The conflicting observations in experimental models and in patients with diabetes is difficult to explain. Although the acute activation of PKCβ by hyperglycemia and excess free fatty acids attenuates endothelial function, it is not certain that PKCβ activation remains relevant in the chronic setting. In our previous study of PKCβ inhibition, subjects underwent a 6-h hyperglycemic clamp. In our current study and in the larger clinical trials, diabetes was present for years. Longer duration of diabetes is associated with greater vascular stiffness (18) and reductions in the bioavailability of nitric oxide (19). Furthermore, other isoforms of PKC, including α and δ, may participate importantly in vascular dysfunction (20). These isoforms would not be affected by ruboxistaurin. Indeed, multiple mechanisms may account for vascular dysfunction, such that PKCβ inhibition alone may not overcome the other perturbations of endothelial function.
Biomarkers of vascular function
To gain further insight into the potential effect of PKCβ inhibition on vascular function, we examined several biomarkers of endothelial activation, fibrinolysis, and oxidant stress. Subjects with type 2 diabetes had higher levels of hsCRP than healthy subjects, but ruboxistaurin did not affect any marker in either group. Mehta and colleagues (14) similarly reported no change in oxidative stress.
Limitations
We chose to study otherwise healthy persons to avoid the potentially confounding effects of dyslipidemia, hypertension, and atherosclerosis on endothelial function. We cannot exclude the possibility that diabetic patients with additional atherosclerotic risk factors or overt evidence of cardiovascular disease may respond differently to ruboxistaurin. Moreover, a larger study cohort may have been able to demonstrate a small effect of ruboxistaurin on vascular function.
Conclusions
PKCβ inhibition with ruboxistaurin did not improve endothelium-dependent vasodilator function in persons with type 2 diabetes mellitus. These results refute the notion that PKCβ contributes to vascular dysfunction in patients with type 2 diabetes.
Footnotes
This work has been supported by grants from the NIH (P01 HL48743 and K23 HL-04169), the American Diabetes Association (ADA 1-06-CD-01), and Eli Lilly. M.A.C. is the Simon C. Fireman Scholar in Cardiovascular Medicine at Brigham and Women’s Hospital.
Disclosure Summary: J.A.B. received grant support from the National Institutes of Health (NIH) and American Diabetes Association for this study. A.B.G., A.G., A.P., and S.K. have nothing to declare. M.A.C. received grant support from the NIH and Eli Lilly for this study.
First Published Online May 5, 2010
Abbreviations: FBF, Forearm blood flow; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; MAP, mean arterial pressure; NT, nitrotyrosine; PAI-1, plasminogen activator inhibitor-1; PKC, protein kinase C; sVCAM-1, soluble vascular cell adhesion molecule-1; vWF, von Willebrand factor.
References
- Beckman JA, Creager MA, Libby P 2002 Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287:2570–2581 [DOI] [PubMed] [Google Scholar]
- Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA 1996 Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 27:567–574 [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL 1992 Preferential elevation of protein kinase C isoform βII and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA 89:11059–11063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H 2000 High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945 [DOI] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA 2002 Inhibition of protein kinase Cβ prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res 90:107–111 [DOI] [PubMed] [Google Scholar]
- Demolle D, DeSuray JM, Onkelinx C 1999 Pharmacokinetics and safety of multiple oral doses of LY333531, a PKCβ inhibitor, in healthy subjects. Clin Pharmacol Ther 65:189 [Google Scholar]
- Mellor H, Parker PJ 1998 The extended protein kinase C superfamily. Biochem J 332:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL 2006 Activation of vascular protein kinase C-β inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 55:691–698 [DOI] [PubMed] [Google Scholar]
- Bohlen HG 2004 Protein kinase βII in Zucker obese rats compromises oxygen and flow-mediated regulation of nitric oxide formation. Am J Physiol Heart Circ Physiol 286:H492–H497 [DOI] [PubMed] [Google Scholar]
- Cotter MA, Jack AM, Cameron NE 2002 Effects of the protein kinase Cβ inhibitor LY333531 on neural and vascular function in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 103:311–321 [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang XL, Lamping KG, Lee HC 2006 Inhibition of protein kinase Cβ protects against diabetes-induced impairment in arachidonic acid dilation of small coronary arteries. J Pharmacol Exp Ther 319:199–207 [DOI] [PubMed] [Google Scholar]
- Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr 3rd EJ, Wolka AM, Vinik AI 2007 A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-β inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care 30:896–902 [DOI] [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW 2005 The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 28:2686–2690 [DOI] [PubMed] [Google Scholar]
- Mehta NN, Sheetz M, Price K, Comiskey L, Amrutia S, Iqbal N, Mohler ER, Reilly MP 2009 Selective PKCβ inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc Drugs Ther 23:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PKC-DMES Study Group 2007 Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol 125:318–324 [DOI] [PubMed] [Google Scholar]
- Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Vignati L, Zhi XE 2006 Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology 113:2221–2230 [DOI] [PubMed] [Google Scholar]
- Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr 3rd EJ 2005 Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase Cβ-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther 27:1164–1180 [DOI] [PubMed] [Google Scholar]
- Mahfouz Badran H, Elnoamany M 2006 Impact of type 2 diabetes mellitus on aortic elastic properties in normotensive diabetes: Doppler tissue imaging study. J Am Soc Echocardiogr 19:1471–1481 [DOI] [PubMed] [Google Scholar]
- Guangda X, Yuhua W 2003 Apolipoprotein e4 allele and endothelium-dependent arterial dilation in type 2 diabetes mellitus without angiopathy. Diabetologia 46:514–519 [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Yang T, Jialal I 2002 α-Tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-α. Diabetes 51:3049–3054 [DOI] [PubMed] [Google Scholar]