Abstract
Context: The very clinical trial, the Women’s Health Initiative, which definitely established the antifracture efficacy of estrogen therapy, led to the demise of estrogen treatment as a viable, long-term option for prevention of bone loss in postmenopausal women due to the well-publicized adverse effects of estrogen plus progestin therapy on a number of nonskeletal endpoints. Given the diminishing clinical use of estrogen, it is logical to question whether estrogen regulation of bone remains a relevant issue at a clinical or basic research level.
Evidence Acquisition: Findings of this update are based on a PubMed search and the author’s knowledge of the field.
Evidence Synthesis: Basic and clinical studies on the mechanisms of estrogen effects on bone will continue to provide potential novel drug targets for the prevention and treatment of osteoporosis. At a clinical level, it is clear that even the low levels of estrogen present in postmenopausal women have a significant impact on bone turnover, leading to a more aggressive approach to prevent bone loss in patients with breast cancer on aromatase inhibitors. Conversely, increasing these low estrogen levels with small doses of estrogen may have beneficial skeletal effects in postmenopausal women without adverse effects on reproductive tissues. Finally, the search continues for new selective estrogen receptor modulators with beneficial effects on bone and other tissues.
Conclusions: Even in the post-WHI era, basic and clinical investigation on estrogen and bone will continue to yield important insights that not only expand our knowledge at a basic level but also impact the health of our aging population.
Even in the post-Women’s Health Initiative era, basic and clinical investigation on estrogen and bone will continue to yield important insights that impact the health of our aging population.
In a seminal paper published 70 yr ago (1), Fuller Albright made the observations that “a survey of 42 cases [of “idiopathic” osteoporosis] 65 yr or under showed that 40 were women after the menopause; there were only two males; there were no cases in women before the menopause. This form of osteoporosis was found in several women of the premenopause age, who had undergone a surgical menopause. In brief, it is our belief that idiopathic osteoporosis is postmenopausal osteoporosis.” Using careful calcium balance studies, Albright went on to demonstrate the effects of estrogen treatment in postmenopausal women on total body calcium retention, and essentially laid the framework for decades of subsequent work attempting to define, at the cellular, tissue, and organismal level, the effects of estrogen on bone metabolism. Albright’s findings, followed by exhaustive observational data (2) on the protective effects of estrogen on bone, led to estrogen therapy (combined with a progestin in women with an intact uterus) as the mainstay for the prevention and treatment of postmenopausal osteoporosis. Ironically, the very clinical trial, the Women’s Health Initiative (WHI), that definitively established the antifracture efficacy of estrogen therapy led to the demise of estrogen treatment as a viable, long-term option for prevention of bone loss in postmenopausal women due to the well-publicized adverse effects of estrogen plus progestin therapy on the risk of breast cancer, cardiovascular disease, and dementia (3). And yet, despite the diminishing clinical use of estrogen (4), there remains considerable interest in better defining the mechanisms of estrogen action on bone; increasing recognition of the critical role of estrogen on bone metabolism in men; the clinical importance of even the small amounts of estrogen present in postmenopausal women on bone loss and fracture risk, as evidenced by the growing experience on the skeletal effects of aromatase inhibitors in women with breast cancer; and an ongoing search for alternate approaches to deliver estrogenic activity to bone without adverse effects on other tissues by using either very low doses of estrogen, selective estrogen receptor modulators (SERMs), or combinations of the two. The evidence for the role played by estrogen in bone metabolism in men has recently been reviewed in this journal (5); this brief update considers each of the other areas noted above and attempts to define, even in the post-WHI era, why it is important to continue, at both a basic and clinical level, the enduring line of investigation on the skeletal effects of estrogen started by Albright 70 yr ago.
Mechanisms of Estrogen Action on Bone
Although the effects of estrogen on bone are generally considered in the context of estrogen deficiency after the menopause, it is important to recognize that the major physiological effects of estrogen are likely manifest during skeletal growth and pregnancy, and the importance of estrogen deficiency is most relevant during lactation. Considerable evidence now implicates a major role for estrogen in the acquisition of bone mass during growth in both sexes (6) and during pregnancy; the high circulating levels of estrogen likely have important effects not only on bone but also, as Albright surmised in his original treatise (1), on extraskeletal calcium homeostasis, such as intestinal and renal calcium handling (6). Moreover, rodent and human studies have now established that the suppression of estrogen levels during lactation, combined with increases in PTHrP levels, are responsible for mobilization of calcium from skeletal stores into breast milk (7,8). In terms of evolution, therefore, the provision of adequate calcium in breast milk for the needs of the neonatal skeleton is probably the major reason why estrogen deficiency leads to increased bone resorption and skeletal calcium mobilization. As such, although bone loss after lactation is largely reversible (8), whereas postmenopausal bone loss is not, loss of bone after the menopause is likely an evolutionarily unintended consequence of the fundamental reproductive effects of estrogen on calcium homeostasis.
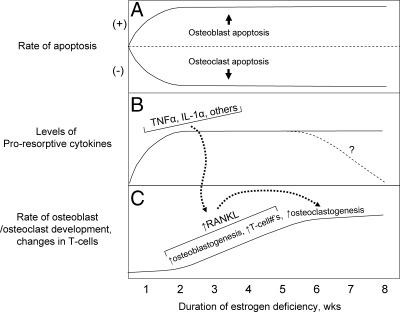
Based on a compilation of findings from rodent and human studies, Fig. 1 provides a working model of changes after acute estrogen deficiency that ultimately result in mobilization of calcium from the skeleton and bone loss, with the caveat that the detailed molecular mechanisms of estrogen action on bone are not covered in this overview. There is now convincing evidence that estrogen, acting via estrogen receptor (ER)α, stimulates osteoclast apoptosis (9,10) and, conversely, suppresses osteoblast and osteocyte apoptosis (11,12,13) (Fig. 1A). Thus, estrogen deficiency is associated with an increase in the lifespan of osteoclasts and a concomitant decrease in osteoblast lifespan. Physiologically, this is exactly what the rodent or human mother needs in the early days postpartum—a stimulation of bone resorption (resulting in increased calcium release from bone) and at least a relative deficit in bone formation (leading to less calcium returning to the skeleton). These effects of estrogen deficiency are accentuated by the proresorptive actions of PTHrP (7), resulting in fairly extensive mobilization of calcium, principally from trabecular bone (7,14), for the needs of the newborn through breast milk.
Figure 1.
Working model of changes and potential time course of these changes in osteoclast/osteoblast apoptosis (A), in proresorptive cytokines (B), and in osteoblastogenesis, increases in T cells, and osteoclastogenesis (C) after the induction of estrogen deficiency. Please see text for further discussion. Reproduced from Khosla (53) with permission.
Estrogen deficiency is also associated with increases in bone marrow levels of a number of proresorptive cytokines, including TNF-α, IL-1α, and others (6) (Fig. 1B). Although much of the data on estrogen regulation of these cytokines has come from studies in rodents, the most definitive evidence supporting a role for TNF-α and IL-1β in mediating increases in bone resorption after estrogen deficiency in vivo in humans comes from the work of Charatcharoenwitthaya et al. (15). In this study, transdermal estradiol was administered to 42 early postmenopausal women for 60 d to suppress bone resorption. Estrogen treatment was then discontinued, and the subjects were randomly assigned to intervention groups receiving 3 wk of injections with saline, the IL-1 receptor 1 blocker, anakinra, or the soluble p75 TNF receptor, etanercept (which binds and thereby inhibits TNF action). These investigators found that either IL-1 or TNF-α blockade reduced the estrogen deficiency-induced increase in bone resorption by approximately 50%, although TNF-α blockade appeared to be more effective (due to potential toxicity, both blockers could not be administered simultaneously). These findings thus demonstrated that in humans, as in rodents, at least part of the effects of estrogen deficiency on increasing bone resorption are mediated via TNF-α and IL-1.
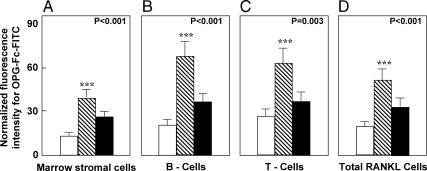
As shown in Fig. 1C, these proresorptive cytokines expand the pool of osteoclast precursor cells and also increase expression of the key molecule regulating osteoclast development, activity, and lifespan—receptor activator of nuclear factor κB ligand (RANKL)—by osteoblastic and other cells in the bone microenvironment (16). The direct demonstration that estrogen deficiency leads to increases in RANKL production came from studies by Eghbali-Fatourechi et al. (17). These investigators isolated bone marrow mononuclear cells expressing RANKL on their surfaces by two-color flow cytometry using fluorescein isothiocyanate (FITC)-conjugated osteoprotegerin (OPG)-Fc as a probe. The cells were characterized as preosteoblastic marrow stromal cells, B lymphocytes, or T lymphocytes by using antibodies against alkaline phosphatase, CD20, and CD3, respectively, in 12 premenopausal women (group A), 12 early postmenopausal women (group B), and 12 age-matched, estrogen-treated postmenopausal women (group C). As shown in Fig. 2, fluorescence intensity of OPG-Fc-FITC, an index of the surface concentration of RANKL per cell, was increased in group B over groups A and C by 2- to 3-fold for marrow stromal cells, B cells, T cells, and total RANKL-expressing cells. Moreover, in the merged groups, RANKL expression per cell correlated directly with the bone resorption markers, serum C-telopeptide of type I collagen and urine N-telopeptide of type I collagen (NTX), in all three cell types and inversely with serum estradiol levels for total RANKL-expressing cells. These data thus demonstrated that up-regulation of RANKL on bone marrow cells is an important determinant of increased bone resorption induced by estrogen deficiency. However, whether the effects of estrogen on RANKL production are direct or indirect (e.g. mediated via alterations in other proresorptive cytokines) is presently unclear.
Figure 2.
Changes in OPG-Fc-FITC fluorescence as an index of mean RANKL surface concentration per cell in marrow stromal cells (A), B cells (B), T cells (C), and all cells (D) are shown for premenopausal women (white bars), estrogen-deficient postmenopausal women (stippled bars), and estrogen-treated postmenopausal women (black bars). P values by ANOVA are as indicated. ***, P < 0.001 vs. the premenopausal women. Reproduced from Eghbali-Fatourechi et al. (17) with permission.
Finally, as shown in Fig. 1C, estrogen deficiency up-regulates osteoblastogenesis (18) and expands the number of T cells (19); both osteoblastic and T cells produce RANKL (16), which leads to enhanced osteoclast development. At a conceptual level, the changes depicted in Fig. 1 likely apply to both rodents and humans, but the timeframe for these changes may be species-specific. For example, the period of lactation before weaning is relatively short (∼3 wk) in rodents compared with the often prolonged phase of breast feeding (up to several years) in humans. Thus, the rapidity and relative importance of the changes depicted in Fig. 1 may be quite different in rodents vs. humans.
From a clinical perspective, the importance of understanding the mechanisms depicted in Fig. 1 and other, as yet unknown, pathways regulated by estrogen in bone is the possibility of developing novel therapeutic targets to prevent bone loss and treat osteoporosis. Thus, compounds that enhance osteoclast apoptosis [e.g. estrogen (20), SERMs (9), and bisphosphonates (21)], inhibit osteoblast/osteocyte apoptosis [e.g. estrogen (20), SERMs (22), PTH (23), and modulators of Wnt signaling, such as an antibody to the Wnt antagonist, sclerostin (24)], block proresorptive cytokines (e.g. TNF-α or IL-1 blockers), or block RANKL [e.g. the humanized monoclonal antibody to RANKL, denusomab (25)], all represent possible treatments for osteoporosis that, at a mechanistic level, reverse one or more of the changes after estrogen deficiency summarized in Fig. 1. Based on the progress already made to date, it is likely that further basic studies aimed at more clearly defining the cellular and molecular mechanisms of the pleiotropic effects of estrogen on bone will identify even more potential therapeutically useful pathways.
Importance of Low Residual Estrogen Levels in Postmenopausal Women
Before the widespread use of aromatase inhibitors in women with breast cancer, the importance of the low residual estrogen levels in postmenopausal women on bone metabolism was first demonstrated by Heshmati et al. (26), who assessed the skeletal effects of blockade of estrogen synthesis in 42 normal postmenopausal women randomly assigned to receive either placebo or the aromatase inhibitor, letrozole, for 6 months. Letrozole treatment did not affect bone formation markers but, as compared with the placebo group, increased bone resorption markers [urine 24-h pyridinoline by 13.3% (P < 0.05) and 24-h urine deoxypyridinoline by 14.2% (P < 0.05)]. These data indicated that even the low serum estrogen levels present in late postmenopausal women exerted a restraining effect on bone resorption and raised significant concerns regarding the potential skeletal side effects of aromatase inhibitors.
There are currently two types of aromatase inhibitors available: nonsteroidal agents (anastrozole and letrozole) are reversible aromatase inhibitors, whereas the steroidal agent, exemestane, is an irreversible inactivator of the aromatase enzyme (27). The skeletal effects of each of these agents are considered briefly below.
Anastrozole
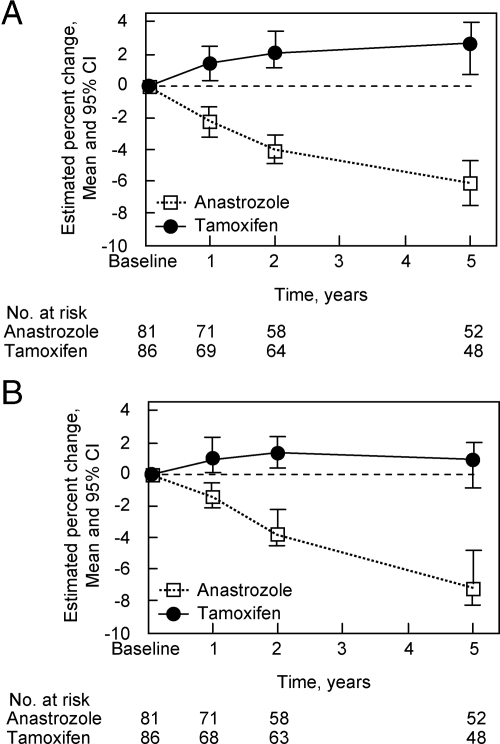
The most robust data on the effects of anastrozole on bone metabolism come from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) study (28) in which 9366 postmenopausal women with a mean age of 64 yr who had completed primary therapy for breast cancer were randomized to receive anastrozole, tamoxifen, or both. After a median follow-up of 33 months, anastrozole improved disease-free survival, time-to-disease recurrence, and incidence of contralateral primary breast cancer compared with either tamoxifen alone or both tamoxifen and anastrozole (28). However, over 5 yr of follow-up, anastrozole was associated with an increased risk of any fracture compared with tamoxifen [odds ratio, 1.49; 95% confidence interval (CI), 1.25–1.77; P < 0.0001] as well as increased spine fracture risk (odds ratio, 1.68; 95% CI, 1.04–2.71; P < 0.03) (28). A total of 197 women from the monotherapy arms of the ATAC trial were recruited into a bone substudy, and as shown in Fig. 3, although tamoxifen was associated with small increases in spine and hip bone mineral density (BMD), anastrozole treatment resulted in significant decreases in BMD at these sites (−6.1% at the spine and −7.2% at the total hip) (29). Consistent with the earlier findings of Heshmati et al. (26), anastrozole was also associated with an increase in urine NTX (+15%; 95% CI, 3–25%).
Figure 3.
Mean percentage change in BMD after 1, 2, and 5 yr in the bone substudy from the ATAC trial (29). Bars represent 95% CI. A, Lumbar spine change over time; B, total hip change over time. Reproduced from Eastell et al. (29) with permission.
Letrozole
In the MA-17 study (30), 5187 patients with a mean age of 62 yr who had completed 5 yr of adjuvant tamoxifen therapy were randomized to receive either letrozole or placebo for an additional 5 yr. After a median follow-up of 2.4 yr, the estimated 4 yr disease-free survival was increased from 87% in the placebo group to 93% in the letrozole group (P = 0.001). Although there was no significant difference in the incidence of clinical fractures in the letrozole group compared with the placebo group (3.6 vs. 2.9%; P = 0.24), in a bone subprotocol, patients on letrozole (n = 122) had a significant decrease in total hip BMD over 24 months (−3.6%) compared with control subjects (n = 104; −0.71%; P = 0.044), with a similar pattern for spine BMD (letrozole, −5.35%; control, −0.7%; P = 0.008) (31).
In the fracture outcome arm of the Breast International Group (BIG) 1-98 study, 4895 patients with breast cancer randomized to either letrozole or tamoxifen for 5 yr were evaluated (32). Fracture incidence was significantly higher in the letrozole group compared with the tamoxifen group (9.3 vs. 6.5%), with a hazard ratio of 1.38 (95% CI, 1.13–1.69). Statistically significant risk factors for fracture included age, smoking history, osteoporosis at baseline, previous fracture, and previous hormone replacement therapy.
Exemestane
Because of its steroidal structure and its structural relationship with androstenedione, exemestane may have somewhat different effects on bone metabolism than anastrozole or letrozole (27). Thus, in a 2-yr, double-blinded, randomized trial that included 147 postmenopausal women with breast cancer (mean age, 60 yr), changes in spine BMD with exemestane (−2.17%) were not significantly different from women given placebo (−1.84%; P = 0.56). However, decreases in femoral neck BMD in the exemestane group (−2.72%) were significantly greater than those in the placebo group (−1.48%; P = 0.024) (33). The Intergroup Exemestane Study (IES) was a double-blind, randomized trial comparing 5 yr of tamoxifen (n = 2380) with 2–3 yr of tamoxifen, followed by 2–3 yr of exemestane (n = 2362) (34). After a median follow-up of 31 months, the exemestane group experienced significant reductions in first cancer-related events, disease-free survival, and contralateral breast cancer. There was a nonsignificant increase in the incidence of clinical fractures in the exemestane group (3.1%) compared with the tamoxifen group (2.3%; P = 0.08). In a bone substudy (n = 206), patients who switched from tamoxifen to exemestane had 2.7 and 3.2% decreases in spine BMD at 6 and 12 months, respectively, compared with a change of 0.2% at 6 and 12 months in the tamoxifen group (P = 0.0001 vs. the exemestane group) (35).
There is, thus, considerable evidence that even the low residual levels of estrogen present in postmenopausal women are important in reducing bone resorption and that women treated with any of the three currently used aromatase inhibitors are at increased risk of bone loss. A caveat to the available data, however, is that virtually all of the skeletal outcomes have been compared with women treated with tamoxifen, which is a SERM that has positive effects on bone (36); thus, although it is plausible that all aromatase inhibitors increase bone loss and fracture risk compared with no treatment, the magnitude of the increased risk of bone loss and fracture risk relative to a true control group may be overestimated due to the comparisons with tamoxifen. Nonetheless, expert groups such as the American Society of Clinical Oncology (37) and the UK Expert Group (38) have appropriately provided algorithms on osteoporosis prevention and treatment for women initiating treatment with aromatase inhibitors. These involve close follow-up of BMD and a generally more aggressive approach to initiating pharmacological therapy than in postmenopausal women not on aromatase inhibitors.
Alternate Approaches to Estrogen Replacement Therapy
Very low dose estrogen therapy
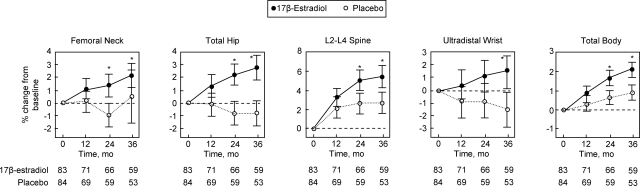
The converse of the observation that the low estrogen levels present in postmenopausal women continue to have a significant impact on bone resorption has been the demonstration that even small increases in these low levels have beneficial effects on bone. Thus, given the adverse nonskeletal effects of conventional doses of estrogen therapy [0.625 mg/d of conjugated equine estrogen (CEE), equivalent to 1.0 mg/d of 17β-estradiol], Prestwood et al. (39) tested whether one quarter of the conventional dose (0.25 mg/d of 17β-estradiol) prevented bone loss in healthy older postmenopausal women without significant adverse effects. Healthy, community-dwelling postmenopausal women who were older than 65 yr at enrollment were randomized to receive 0.25 mg/d of 17β-estradiol (n = 83) or placebo (n = 84); all women who had not had a hysterectomy received 100 mg/d of oral micronized progesterone for 2-wk periods every 6 months. Over 3 yr of follow-up, there were significant increases in BMD in the estrogen group compared with the placebo group at all sites assessed (Fig. 4). The bone resorption marker, serum NTX, was 24% lower in the estrogen vs. the placebo group (P = 0.008). Serum estradiol levels were (median, 95% CI) 9.1 (10.1–30.9) in the placebo group vs. 26.1 (23.4–28.8) in the estrogen group, the latter being well below the median value of 95 pg/ml found with a conventional transdermal 17β-estradiol patch delivering 0.1 mg estradiol/d (40). The adverse effect profile was similar in the estrogen and placebo groups: specifically, there were no statistically significant differences in breast tenderness, changes in endometrial thickness, or in abnormal mammograms.
Figure 4.
Effects of low dose 17β-estradiol and placebo on BMD. Values are mean percentage change with 95% CI. Asterisk represents difference compared with the placebo group: femoral neck, P = 0.03 for 24 months and P < 0.001 for 36 months; total hip, P < 0.001 for 24 and 36 months; L2–L4 spine, P = 0.02 for 24 months and P < 0.001 for 36 months; ultradistal radius, P < 0.001 for 36 months; and total body, P = 0.02 for 24 months and P < 0.001 for 36 months. Reproduced from Prestwood et al. (39) with permission.
Subsequently, Ettinger et al. (41) used an even lower dose of estrogen (unopposed transdermal estradiol, 0.014 mg/d) compared with placebo in 417 postmenopausal women aged 60–80 yr. With this lower dose, median plasma estradiol levels increased in the estrogen group from 4.8 pg/ml at baseline to 8.5 pg/ml at 1 yr and 8.6 pg/ml at 2 yr, and were unchanged in the placebo group. Over 2 yr, lumbar spine BMD increased 2.6% in the estrogen group and 0.6% in the placebo group (P < 0.001 for the between-group difference). Similarly, total hip BMD increased 0.4% in the estrogen group and decreased 0.8% in the placebo group (P < 0.001). Endometrial hyperplasia developed in one of the 208 women in the estrogen group and none of the 209 women in the placebo group.
The evidence, then, from these studies indicates that very low dose estrogen therapy, which keeps serum estradiol levels well below the premenopausal range, is effective in improving BMD. However, the optimal low dose of estrogen still remains to be defined. In addition, whereas low-dose estrogen therapy appears to be a promising approach for some postmenopausal women, it remains to be seen whether large-scale studies examining fracture and safety endpoints (breast cancer, cardiovascular events, and others) will ever be initiated using this approach, given the growing list of alternative agents to prevent and treat osteoporosis (42).
SERMs
The results of the WHI (3) provided a strong impetus for the development of SERMs, with the goal of identifying compounds with beneficial skeletal effects, but without the adverse breast, endometrial, cardiovascular, and other effects associated with estrogen therapy. SERMs are nonsteroidal compounds with tissue-specific actions that are believed to be due to the fact that these drugs induce a different conformation of the ER than estradiol (43). Depending on the tissue, these compounds lead to recruitment of different coactivators and corepressors than estradiol (43). However, conformational changes alone may not explain all actions of SERMs on target cells. For example, recent studies in mice with targeted deletion of the ERα amino-terminal A/B domain suggest that stimulation of ERα by SERMs with minimal activation of the amino-terminal activation domain activation function-1 might preserve beneficial vascular effects but minimize effects on reproductive tissues (44). As much as we have learned about the biology of the ER and the molecular pharmacology of SERMS, however, it has been very difficult to reach conclusions regarding the clinical activity of a given SERM without conducting the appropriate clinical trials.
Raloxifene is the only SERM currently approved by the U.S. Food and Drug Administration for the treatment of postmenopausal osteoporosis. Previous studies have shown that raloxifene reduced vertebral fracture risk by approximately 30–50% relative to placebo in postmenopausal women with osteoporosis, although it did not protect against nonvertebral or hip fractures (45). Raloxifene has also been shown to decrease the risk of developing breast cancer (46) and lead to no significant change in cardiovascular events (47). However, raloxifene does increase the risk of venous thromboembolic disease and fatal stroke and also leads to an increase in hot flushes (47).
Development of two other SERMs, idoxifene and levormeloxifene, was abandoned due to adverse uterine effects not seen with raloxifene, particularly increased endometrial thickness (48). Although a more recent SERM, arzoxifene, showed initial promise for beneficial effects on BMD with no differences from placebo for effects on endometrial hyperplasia, carcinoma, uterine polyps, vaginal bleeding, and surprisingly, climacteric symptoms (49), it appears that development of this compound has been abandoned, likely due to results from the Generation Trial (48). In that study, arzoxifene did not significantly reduce nonvertebral fractures, cardiovascular events, or cognitive decline.
Cummings et al. (50) recently reported on results of the Postmenopausal Evaluation and Risk-Reduction with Lasofoxifene (PEARL) trial. This large, randomized study assigned 8556 women between the ages of 59 and 80 yr with a BMD T score of −2.5 or less at the femoral neck or spine to receive once-daily lasofoxifene (either 0.25 or 0.5 mg) or placebo for 5 yr. The overall results of the study were fairly positive: lasofoxifene treatment was associated with reduced risks of nonvertebral and vertebral fractures, ER-positive breast cancer, coronary heart disease, and stroke. However, lasofoxifene was also associated with an increased risk of venous thromboembolic events and hot flushes. Of some concern, the 0.25-mg-dose group had 7.0 deaths per 1000 person-years, compared with 5.1 deaths per 1000 person-years (P = 0.05); this increase was not observed in the 0.5-mg group, but the impact of these findings on ultimate review and approval of this drug by the FDA remains uncertain at this point.
Another SERM, basedoxifene, is currently in clinical trials (48) and is scheduled for review by the FDA sometime in 2010. This SERM has also been tested in a somewhat novel approach in combination with CEEs, with the rationale that such a combination would improve BMD and hot flushes, but without some of the other adverse effects on the endometrium and breast associated with estrogen therapy alone. Thus, Lindsay et al. (51) enrolled 3397 postmenopausal women and randomized them to placebo, raloxifene, or three doses of basedoxifene in combination with either 0.625 or 0.45 mg of CEE. Over 2 yr, BMD increased significantly more with all basedoxifene/CEE doses compared with placebo at the lumbar spine and total hip, and for most basedoxifene/CEE doses compared with raloxifene at the lumbar spine. Importantly, the basedoxifene (20 mg)/CEE (0.625 or 0.45 mg) combination significantly reduced the number of hot flushes (by 52–86% vs. by 17% for placebo) and improved measures of vaginal atrophy, lipid parameters, and homocysteine levels, with a similar incidence of breast pain and other adverse events compared with placebo (52). Thus, the combination of a SERM with estrogen may represent a fruitful direction in hormone therapy of postmenopausal women, but clearly larger scale studies with fracture and other safety endpoints are needed to fully evaluate this approach.
Summary and Conclusions
Despite the devastating impact of the WHI on estrogen replacement therapy as a viable option for the prevention and treatment of osteoporosis in women, the important line of investigation on the mechanisms of estrogen effects on bone started by Fuller Albright in the 1940s continues to yield important insights and potential novel drug targets for osteoporosis treatment. In addition, the increasing recognition that even the low circulating estrogen levels present in postmenopausal women have a significant impact on bone turnover has had a major clinical impact on our management of patients with breast cancer being treated with aromatase inhibitors. Conversely, it appears that even small increases in these very low estrogen levels may have beneficial skeletal effects without some of the adverse nonskeletal sequelae associated with conventional dose estrogen therapy. Finally, the search continues for newer generations of SERMs that have beneficial effects on bone, cardiovascular events, breast cancer risk, and other tissues. Combinations of a SERM with carefully chosen doses of estrogen may also provide a novel approach to hormone therapy. Thus, even in the post-WHI era, basic and clinical investigation on estrogen and bone will continue to yield important insights that not only expand our knowledge at a basic level but also impact the health of our aging population.
Footnotes
This work was supported by National Institutes of Health Grants AG004875, AG028936, and AR027065.
Disclosure Summary: S.K. has served on an advisory board for Pfizer.
Abbreviations: CEE, Conjugated equine estrogen; CI, confidence interval; ER, estrogen receptor; FITC, fluorescein isothiocyanate; NTX, N-telopeptide of type I collagen; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor κB ligand; SERM, selective estrogen receptor modulator.
References
- Albright F 1940 Post-menopausal osteoporosis. Trans Assoc Am Physicians 55:298–305 [Google Scholar]
- Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR 1995 Estrogen replacement therapy and fractures in older women. Ann Intern Med 122:9–16 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Parente L, Uyehara C, Larsen W, Whitcomb B, Farley J 2008 Long-term impact of the women’s health initiative on HRT. Arch Gynecol Obstet 277:219–224 [DOI] [PubMed] [Google Scholar]
- Khosla S 2010 Update in male osteoporosis. J Clin Endocrinol Metab 95:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- VanHouten JN, Wysolmerski JJ 2003 Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 144:5521–5529 [DOI] [PubMed] [Google Scholar]
- Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M 1996 Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA 276:549–554 [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S 2007 Estrogen prevents bone loss via estrogen receptor α and induction of fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC 2010 The estrogen receptor-α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24:323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC 2005 Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti- versus pro-apoptotic effects of estrogens. J Biol Chem 280:4632–4638 [DOI] [PubMed] [Google Scholar]
- Tomkinson A, Reeve J, Shaw RW, Noble BS 1997 The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab 82:3128–3135 [DOI] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S 2000 Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC 1998 A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 67:693–701 [DOI] [PubMed] [Google Scholar]
- Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL 2007 Effect of blockade of TNF-a and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res 22:724–729 [DOI] [PubMed] [Google Scholar]
- Kearns AE, Khosla S, Kostenuik PJ 2008 Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29:155–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL 2003 Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 111:1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC 1998 Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. J Clin Invest 101:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzmann MN, Pacifici R 2006 Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116:1186–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 [DOI] [PubMed] [Google Scholar]
- Russell RG, Watts NB, Ebetino FH, Rogers MJ 2008 Mechanisms of action of bisphosphonates: similarities and differences and their potential influences on clinical efficacy. Osteoporos Int 19:733–759 [DOI] [PubMed] [Google Scholar]
- Mann V, Huber C, Kogianni G, Collins F, Noble B 2007 The antioxidant effect of estrogen and selective estrogen receptor modulators in the inhibition of osteocyte apoptosis in vitro. Bone 40:674–684 [DOI] [PubMed] [Google Scholar]
- Jilka RL 2007 Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Westendorf JJ, Oursler MJ 2008 Building bone to reverse osteoporosis and repair fractures. J Clin Invest 118:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C 2009 Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765 [DOI] [PubMed] [Google Scholar]
- Heshmati HM, Khosla S, Robins SP, O'Fallon WM, Melton 3rd LJ, Riggs BL 2002 Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J Bone Miner Res 17:172–178 [DOI] [PubMed] [Google Scholar]
- Ghazi M, Roux C 2009 Hormonal deprivation therapy-induced osteoporosis in postmenopausal women with breast cancer. Best Pract Res Clin Rheumatol 23:805–811 [DOI] [PubMed] [Google Scholar]
- Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, Group AT 2005 Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60–62 [DOI] [PubMed] [Google Scholar]
- Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G 2008 Effect of anastrozole on bone mineral density: 5-year results from the Anastrozole, Tamoxifen, Alone or in Combination Trial 18233230. J Clin Oncol 26:1051–1057 [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL 2003 A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802 [DOI] [PubMed] [Google Scholar]
- Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, Shenkier TN, Tozer RG, Palmer MJ, Shepherd LE, Liu S, Tu D, Goss PE 2006 Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA. 17. J Clin Oncol 24:3629–3635 [DOI] [PubMed] [Google Scholar]
- Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Láng I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A 2007 Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25:486–492 [DOI] [PubMed] [Google Scholar]
- Lønning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Paolini J, Polli A, Massimini G 2005 Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol 23:5126–5137 [DOI] [PubMed] [Google Scholar]
- Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C 2004 A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092 [DOI] [PubMed] [Google Scholar]
- Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM, Coombes RC 2007 Skeletal effects of exemestane on bone-mineral density, bone, biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol 8:119–127 [DOI] [PubMed] [Google Scholar]
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL 1992 Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 326:852–856 [DOI] [PubMed] [Google Scholar]
- Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, Cauley JA, Blumenstein BA, Albain KS, Lipton A, Brown S 2003 American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21:4042–4057 [DOI] [PubMed] [Google Scholar]
- Reid DM 2009 Prevention of osteoporosis after breast cancer. Maturitas 64:4–8 [DOI] [PubMed] [Google Scholar]
- Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M 2003 Ultralow-dose micronized 17β-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA 290:1042–1048 [DOI] [PubMed] [Google Scholar]
- Lufkin EG, Wahner HW, O'Fallon WM, Hodgson SF, Kotowicz MA, Lane AW, Judd HL, Caplan RH, Riggs BL 1992 Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med 117:1–9 [DOI] [PubMed] [Google Scholar]
- Ettinger B, Ensrud KE, Wallace R, Johnson KC, Cummings SR, Yankov V, Vittinghoff E, Grady D 2004 Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 104:443–451 [DOI] [PubMed] [Google Scholar]
- Khosla S 2009 Increasing options for the treatment of osteoporosis. N Engl J Med 361:818–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell DP 2003 Mining the complexities of the estrogen signaling pathways for novel therapeutics. Endocrinology 144:4237–4240 [DOI] [PubMed] [Google Scholar]
- Billon-Galés A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF 2009 The transactivating function 1 of estrogen receptor α is dispensable for the vasculoprotective actions of 17β-estradiol. Proc Natl Acad Sci USA 106:2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E, Crans GG, Diez-Perez A, Pinette KV, Delmas PD 2006 Anti-vertebral fracture efficacy of raloxifene: a meta-analysis. Osteoporos Int 17:313–316 [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon Jr ER, Wade 3rd JL, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N 2006 Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727–2741 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK, Raloxifene Use for The Heart (RUTH) Trial Investigators 2006 Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355:125–137 [DOI] [PubMed] [Google Scholar]
- de Paula FJ, Rosen CJ 2010 Developing drugs to treat osteoporosis: lessons learned? Expert Opin Pharmacother 11:867–869 [DOI] [PubMed] [Google Scholar]
- Bolognese M, Krege JH, Utian WH, Feldman R, Broy S, Meats DL, Alam J, Lakshmanan M, Omizo M 2009 Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab 94:2284–2289 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R, Investigators PS 2010 Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 362:686–696 [DOI] [PubMed] [Google Scholar]
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G 2009 Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 92:1045–1052 [DOI] [PubMed] [Google Scholar]
- Lobo RA, Pinkerton JV, Gass ML, Dorin MH, Ronkin S, Pickar JH, Constantine G 2009 Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal systems and effects on metabolic parameters and overall safety profile. Fertil Steril 92:1025–1038 [DOI] [PubMed] [Google Scholar]
- Khosla S 2007 Estrogen and the death of osteoclasts: a fascinating story. BoneKEy-Osteovision 4:267–272 [Google Scholar]