Abstract
Context: The mechanisms by which Roux-en-Y gastric bypass surgery (GBP) results in sustained weight loss and remission of type 2 diabetes are not fully understood.
Objective: We hypothesized that the anorexic hormone oxyntomodulin (OXM) might contribute to the marked weight reduction and the rapid improvement in glucose metabolism observed in morbidly obese diabetic patients after GBP.
Methods: Twenty obese women with type 2 diabetes were studied before and 1 month after GBP (n = 10) or after a diet-induced equivalent weight loss (n = 10). Patients from both groups were matched for age, body weight, body mass index, and diabetes duration and control. OXM concentrations were measured during a 50-g oral glucose challenge before and after weight loss.
Results: At baseline, OXM levels (fasting and stimulated values) were indistinguishable between the GBP and the diet group. However, OXM levels rose remarkably in response to an oral glucose load more than 2-fold (peak, 5.25 ± 1.31 to13.8 ± 16.2 pmol/liter; P = 0.025) after GBP but not after diet. The peak of OXM after glucose was significantly correlated with glucagon-like peptide-1 and peptide YY3-36.
Conclusions: Our data suggest that the observed changes in OXM primarily occur in response to GBP and not as a consequence of weight loss. These changes were observed early after surgery and occurred in parallel with previously reported increases in incretins and peptide YY. We speculate that the combination of gut hormone changes is essential for the improved glucose homeostasis and may partially explain the success of this surgery on diabetes resolution and weight loss.
Oxyntomodulin levels rise in response to oral glucose after gastric bypass surgery but not after diet-induced weight loss in patients with type 2 diabetes.
Roux-en-Y gastric bypass surgery (GBP) typically results in a reduction of body weight by 40% with resolution and/or improvement of most comorbidities, including diabetes, in 50–80% of cases (1). The mechanisms of weight loss and appetite control after GBP are not fully understood but may be related to the decrease of ghrelin (2) and/or the postprandial increase in glucagon-like peptide-1 (GLP-1) (3,4) and peptide YY (PYY) (3,5) observed after this surgery. Although the improvement of glucose homeostasis is largely due to calorie restriction and weight loss, changes of incretins and other related hormones may also be important factors (4,6).
Oxyntomodulin (OXM) is secreted postprandially by the L-cells in the small intestine (7,8) together with GLP-1 and PYY. It acts as a dual agonist on GLP-1 receptors and glucagon receptors (9). In rodents and human studies, OXM has been shown to reduce food intake and body weight (10,11) and to improve glucose homeostasis (10,12,13,14). In addition, a synthetic glucagon and GLP-1 agonist was recently shown to decrease adiposity and improved glucose tolerance in diet-induced obese (DIO) rodents (15).
Given the postoperative changes of GLP-1 and PYY after GBP and the known dual effect of OXM on both weight control and as an incretin, we hypothesized that an increase in endogenous OXM may occur after GBP and contribute to sustained weight loss and diabetes remission. A matched cohort of women with type 2 diabetes who achieved an equivalent diet-induced weight loss served as controls.
Subjects and Methods
Study participants eligible for GBP (surgical group) had body mass index (BMI) higher than 35 kg/m2; were age 60 yr or older, both genders, and all ethnic groups; had type 2 diabetes for <5 yr; were not on insulin, thiazolidinedione, exenatide, or dipeptidyl-peptidase IV inhibitors; and had a glycated hemoglobin less than 8%. The control group was a cohort of women with type 2 diabetes who achieved equivalent diet-induced weight loss, fulfilled the same criteria, and was similar for age, weight, BMI, and diabetes duration and control. All participants signed an informed consent before enrollment. The surgical group was studied before and 1 month after GBP, whereas the control group was studied before and after a 10-kg diet-induced weight loss (5,6). Partial data from two patients from the surgical group and from the 10 patients of the diet group were previously published (5,6).
The diet consisted of a meal replacement (Robard Corp., Mt. Laurel, NJ) of 1000 kcal/d, with a 1-wk supply given to each patient during an individual weekly visit at the General Clinical Research Center. Body weight was measured weekly and the diet adjusted when necessary. If no weight loss or if weight gain occurred at two consecutive weekly visits, the patients were excluded from the study. Patients were kept on the 1000-kcal diet and in negative energy balance (active weight loss) while they were retested for incretin levels and effect after a 10-kg weight loss. Although there was no time limit, the expectation was that patients would lose 10 kg in 4–8 wk. Diabetes management as well as surgical procedures were described previously (6).
All patients underwent two 3-h oral glucose tolerance tests (OGTT) containing 50 g glucose in 200 ml total volume. Blood samples were collected in chilled EDTA tubes with aprotinin (500 kallikrein inhibitory units/ml blood) and dipeptidyl-peptidase IV inhibitor (Millipore, Billerica, MA) (10 μl/ml blood) and were centrifuged at 4 C before storage at −70 C. To assess the incretin effect, we also performed isoglycemic iv glucose tests as previously described (4).
OXM was measured by RIA (Phoenix Pharmaceuticals Inc., Belmont, CA). The cross-reactivity is 100% with human, rat, mouse, and porcine OXM, 0% with human GLP-1 (all forms), and 0% with human glucagon. The interassay coefficient of variation was 11.6% with a limit of detection of 0.165 ng/ml. Total GLP-1, PYY3-36, total ghrelin, insulin, and leptin were measured by RIA and GIP by ELISA (Millipore).
Total areas under the curve (AUC) and incremental areas 0–180 min for outcome variables were calculated using the trapezoidal method. ANOVA with repeated measures was used to detect hormonal changes over time during the OGTT within each condition and for comparison before and after GBP and before and after diet or between diet and surgical groups. Paired t tests were used to compare data between before and after GBP or diet. Partial correlations adjusting for group and adjusting for fasting values (for PYY3-36) were performed to explore relationships between OXM and outcome variables.
Data are expressed as the mean ± sd except in the figures where mean ± sem were used. Statistical significance was set at P < 0.05. Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL).
Results
Subjects characteristics are shown in Table 1. The duration of weight loss was shorter for the GBP group compared with the diet group (P < 0.001).
Table 1.
Subject characteristics
| Diet | GBP | P | |
|---|---|---|---|
| Age (yr) | 46.8 ± 7.2 | 49.7 ± 8.2 | 0.431 |
| Type 2 diabetes duration (months) | 18.2 ± 14.7 | 38.1 ± 39.3 | 0.183 |
| HbA1c (%) | 6.5 ± 0.6 | 6.8 ± 0.4 | 0.177 |
| Baseline weight (kg) | 110.8 ± 10.1 | 109.7 ± 14.5 | 0.945 |
| Follow-up weight (kg) | 101.1 ± 9.5 | 99.0 ± 12.2 | 0.674 |
| Baseline BMI (kg/m2) | 43.3 ± 3.6 | 44.4 ± 4.8 | 0.583 |
| Follow-up BMI (kg/m2) | 39.5 ± 3.5 | 39.7 ± 4.2 | 0.909 |
| Weight loss duration (wk) | 8.0 ± 1.4 | 4.8 ± 1.2 | <0.001 |
| Weight loss rate (kg/wk) | −1.26 ± 0.47 | −2.19 ± 0.46 | <0.001 |
HbA1c, Glycated hemoglobin.
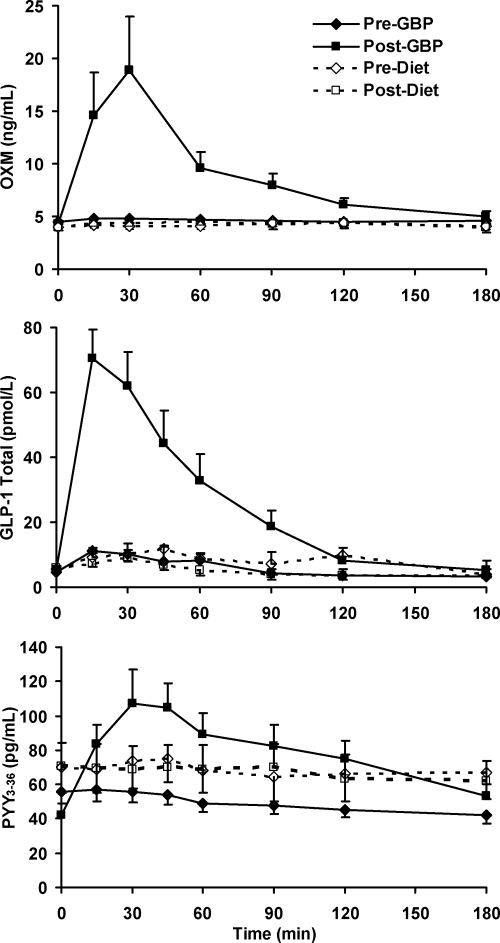
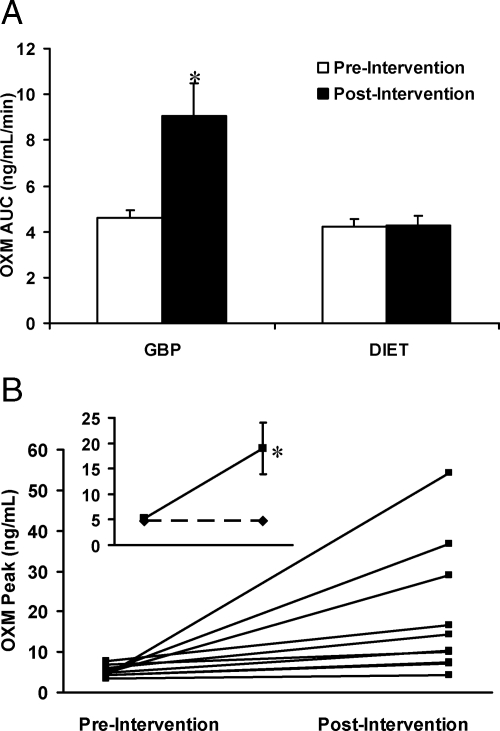
Before weight loss, there were no significant differences in OXM levels (P = 0.152) between the surgical and diet groups, and OXM levels did not respond to the oral glucose load (time effect, P = 0.555; time × group effect P = 0.481). After weight loss, there was a significant time × group effect (P = 0.043) largely due to the change of OXM after GBP, with OXM levels higher after GBP than after diet starting from 15 until 120 min after glucose challenge (Fig. 1). Peak OXM levels and OXM AUC after oral glucose increased by a factor of 3 (P = 0.025) and 2 (P = 0.012) after GBP (Fig. 2 and Table 2) but did not change after diet-induced weight loss.
Figure 1.
Changes in plasma OXM, GLP-1, and PYY3-36 concentrations during a 3-h OGTT after either GBP or diet-induced weight loss. Data are presented as mean ± sem.
Figure 2.
A, OXM AUC during OGTT; B, individual changes in peak OXM concentrations during OGTT after GBP; inset represents mean change of peak OXM after GBP (solid lines) or after diet-induced weight loss (dotted lines). Data are presented as mean ± sem. *, P ≤ 0.05.
Table 2.
Changes after GBP or diet-induced weight loss
| Change with diet | P | Change with GBP | P | P of the change | |
|---|---|---|---|---|---|
| Weight (kg) | −9.71 ± 2.37 | 0.000 | −10.71 ± 3.75 | 0.000 | 0.488 |
| BMI (kg/m2) | −3.79 ± 0.92 | 0.000 | −4.26 ± 1.35 | 0.000 | 0.374 |
| Fasting OXM (ng/ml) | 0.047 ± 0.63 | 0.820 | −0.15 ± 0.49 | 0.351 | 0.438 |
| OXM peak (ng/ml) | −0.018 ± 1.56 | 0.972 | 13.8 ± 16.2 | 0.025 | 0.025 |
| OXM AUC (ng/ml · min) | 0.058 ± 0.98 | 0.856 | 4.43 ± 4.44 | 0.012 | 0.013 |
| Change in 30-min insulin (pmol/liter) | 54.6 ± 280.2 | 0.553 | 322.7 ± 314.6 | 0.015 | 0.066 |
| Glucagon AUC (ng/liter · min) | −8.89 ± 11.81 | 0.041 | 16.27 ± 14.92 | 0.007 | 0.001 |
| PYY3-36 peak (pg/ml) | −0.94 ± 29.9 | 0.923 | 54.1 ± 63.0 | 0.024 | 0.022 |
| PYY3-36 AUC (pg/ml · min) | −5.71 ± 30.9 | 0.573 | 30.2 ± 28.7 | 0.009 | 0.015 |
| Total GLP-1 peak (pmol/liter) | −8.40 ± 18.4 | 0.183 | 75.0 ± 51.5 | 0.001 | 0.000 |
| Total GLP-1 AUC (pmol/liter · min) | −3.27 ± 7.83 | 0.220 | 19.57 ± 10.75 | 0.000 | 0.001 |
| Fasting total ghrelin (pg/ml) | 124.4 ± 157.2 | 0.034 | −44.8 ± 215.2 | 0.527 | 0.060 |
| Fasting leptin (ng/ml) | −10.4 ± 7.99 | 0.003 | −13.3 ± 7.97 | 0.001 | 0.423 |
| HOMA-IR | −3.92 ± 2.85 | 0.002 | −2.93 ± 3.12 | 0.023 | 0.477 |
| Incretin effect on insulin (%) | 7.15 ± 18.1 | 0.244 | 24.5 ± 19.9 | 0.010 | 0.071 |
HOMA-IR, Homeostasis model assessment of insulin resistance.
The marked increase in postprandial OXM levels after GBP parallels the previously reported increase in GLP-1 and PYY3-36 levels (Fig. 1). Although OXM and PYY3-36 peak at 30 min, GLP-1 peak is earlier at 15 min. GLP-1 AUC increased by a factor of 5 (P < 0.001), and PYY3-36 AUC increased by a factor of 2 (P = 0.024) after GBP but not after diet-induced weight loss (Fig. 1 and Table 2). The incretin effect on insulin increased by 25% after GBP (P = 0.010) but did not change significantly after diet (Table 2).
At baseline, in all patients (n = 20), glucose-stimulated OXM correlated with GLP-1 and GIP and weakly with PYY3-36 postprandial responses. After intervention, glucose-stimulated OXM strongly correlated with GLP-1 and PYY levels but not with insulin response, incretin effect, or glucose levels, adjusting for group (Table 3). The change of OXM AUC with weight loss was highly correlated with the change of GLP-1 AUC (r = 0.808; P < 0.001) and of PYY3-36 AUC (r = 0.524; P = 0.021), adjusting for group.
Table 3.
Correlations at baseline (n = 20) and after weight loss (n = 20), partial correlations adjusted for group: gastric bypass or diet
| GIP peaka | GIP AUCb | GLP-1 peaka | GLP-1 AUCb | PYY3–36 peaka | PYY3–36 AUCb | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| r | 0.515 | 0.377 | 0.617 | 0.331 | 0.398 | 0.332 |
| P | 0.020 | 0.146 | 0.004 | 0.155 | 0.083 | 0.152 |
| After weight loss | ||||||
| r | 0.028 | 0.084 | 0.687 | 0.887 | 0.614 | 0.775 |
| P | 0.909 | 0.733 | 0.001 | <0.001 | 0.007 | <0.001 |
Correlates with OXM peak.
Correlates with OXM AUC.
Discussion
We observed a marked increase of OXM concentrations in response to an oral glucose load early after GBP surgery in morbidly obese women with type 2 diabetes. This response was not found in patients who achieved an equivalent weight loss by diet. The increase of OXM was strongly correlated with total GLP-1 and PYY3-36 after GBP. This is not surprising because the OXM peptide is generated by posttranslational processing of preproglucagon and is secreted postprandially from L-cells of the jejuno-ileum together with GLP-1 and PYY3-36 (16).
Similarly to GLP-1, OXM has been shown to enhance insulin secretion in vitro in perfused rat pancreas (17,18) and in vivo (13,14,15) and can thus be considered an incretin, albeit with an effect of lesser magnitude. A specific OXM receptor has not yet been identified, but OXM is known to act as dual GLP-1 and glucagon receptor agonist. The effects of OXM on glucose and energy homeostasis are thought to be mostly mediated by GLP-1 receptors, because they are not observed in GLP-1−/− mice (14,19). OXM enhances β-cell function in mice and prevents β-cell apoptosis in vitro and in vivo (14), an effect that appears again to be GLP-1 receptor dependent. OXM ameliorates glucose intolerance in mice fed a high-fat diet by increasing glucose-induced insulin concentrations but not by increasing insulin action (13). Day et al. (15) recently reported that they synthesized various glucagon and GLP-1 coagonists with differing activity at each receptor. Their preclinical results showed that once per week administration of these agents reduced adiposity by increasing energy expenditure and decreased food intake and improved glucose tolerance in DIO mice. We observed that the changes of OXM correlated with other incretins but not with glucose, insulin, or incretin effect, suggesting an indirect contribution of OXM to the amelioration of glucose homeostasis after GBP. However, our data remain associative and only suggestive of a possible role of OXM. The effect of exogenous OXM on glucose homeostasis in diabetes has not been tested.
Some of the metabolic changes observed after GBP appear to be weight loss independent, especially in the early period after surgery, before significant weight loss. OXM has been shown to have metabolic effects, in addition to its effect on glucose and insulin, independently of weight loss. Acute treatment of DIO mice with OXM reduced triglycerides, increased ketone bodies, up-regulated gluconeogenic and fatty acid oxidation genes, and down-regulated lipogenic genes in the liver, without weight changes (20).
Many questions remain including whether the rise of OXM is a long-term effect of GBP or vanishes after a certain follow-up period. Future studies with peptide agonists and antagonists will need to be done to clarify the role of each peptide in the decreased appetite and spectacular metabolic improvement after GBP.
Acknowledgments
We thank Antonia Colarusso and Betty Kovack for recruitment and diet supervision and Mary Walter for her assistance with the OXM assay.
Footnotes
This work was supported by grants from the American Diabetes Association CR-7-05 and CR-18, and National Institutes of Health (NIH), R01-DK67561, NYONRC DK-26687, DERC DK-63068-05, and UL1 RR024156 from the National Center for Research Resources, a component of the NIH and NIH Roadmap for Medical Research, and in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
Clinical Trial no. NCT00571220. Disclosure Summary: The authors have nothing to declare.
First Published Online May 25, 2010
Abbreviations: BMI, Body mass index; DIO, diet-induced obese; GBP, gastric bypass surgery; GLR-1, glucagon-like peptide-1; OGTT, oral glucose tolerance test; OXM, oxyntomodulin; PYY, peptide YY.
References
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I 2009 Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122:248–256.e5 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ 2002 Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630 [DOI] [PubMed] [Google Scholar]
- Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J 2006 Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 91:1735–1740 [DOI] [PubMed] [Google Scholar]
- Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B 2007 Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliván B, Teixeira J, Bose M, Bawa B, Chang T, Summe H, Lee H, Laferrère B 2009 Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg 249:948–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B 2008 Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Quellec A, Kervran A, Blache P, Ciurana AJ, Bataille D 1992 Oxyntomodulin-like immunoreactivity: diurnal profile of a new potential enterogastrone. J Clin Endocrinol Metab 74:1405–1409 [DOI] [PubMed] [Google Scholar]
- Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR 1983 Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab 57:488–495 [DOI] [PubMed] [Google Scholar]
- Gros L, Thorens B, Bataille D, Kervran A 1993 Glucagon-like peptide-1-(7-36) amide, oxyntomodulin, and glucagon interact with a common receptor in a somatostatin-secreting cell line. Endocrinology 133:631–638 [DOI] [PubMed] [Google Scholar]
- Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR 2003 Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 88:4696–4701 [DOI] [PubMed] [Google Scholar]
- Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, Bloom SR 2005 Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 54:2390–2395 [DOI] [PubMed] [Google Scholar]
- Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR 2001 Oxyntomodulin inhibits food intake in the rat. Endocrinology 142:4244–4250 [DOI] [PubMed] [Google Scholar]
- Parlevliet ET, Heijboer AC, Schröder-van der Elst JP, Havekes LM, Romijn JA, Pijl H, Corssmit EP 2008 Oxyntomodulin ameliorates glucose intolerance in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 294:E142–E147 [DOI] [PubMed] [Google Scholar]
- Maida A, Lovshin JA, Baggio LL, Drucker DJ 2008 The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances β-cell function but does not inhibit gastric emptying in mice. Endocrinology 149:5670–5678 [DOI] [PubMed] [Google Scholar]
- Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, Holland J, Hembree J, Abplanalp W, Grant E, Ruehl J, Wilson H, Kirchner H, Lockie SH, Hofmann S, Woods SC, Nogueiras R, Pfluger PT, Perez-Tilve D, DiMarchi R, Tschöp MH 2009 A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 5:749–757 [DOI] [PubMed] [Google Scholar]
- Drucker DJ 2005 Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab 1:22–31 [DOI] [PubMed] [Google Scholar]
- Jarrousse C, Bataille D, Jeanrenaud B 1984 A pure enteroglucagon, oxyntomodulin (glucagon 37), stimulates insulin release in perfused rat pancreas. Endocrinology 115:102–105 [DOI] [PubMed] [Google Scholar]
- Baldissera FG, Holst JJ, Knuhtsen S, Hilsted L, Nielsen OV 1988 Oxyntomodulin (glicentin-(33-69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul Pept 21:151–166 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ 2004 Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 127:546–558 [DOI] [PubMed] [Google Scholar]
- Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G, Liu F, Miller C, Tota LM, Zhou G, Zhang X, Sountis MM, Santoprete A, Capito' E, Chicchi GG, Thornberry N, Bianchi E, Pessi A, Marsh DJ, SinhaRoy R 2009 Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 58:2258–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]