Abstract
Background: Type B insulin resistance belongs to a class of diseases caused by an autoantibody to a cell surface receptor. Blockade of insulin action results in hyperglycemia, hypercatabolism, severe acanthosis nigricans, and hyperandrogenism in women. This rare autoimmune disorder has been treated with various forms of immunosuppression with mixed success.
Methods: We describe 14 patients with type B insulin resistance referred to the National Institutes of Health, adding to an existing cohort of 24 patients. This report focuses on seven patients who were treated with an intensive combination protocol of rituximab, cyclophosphamide, and pulse corticosteroids aimed at control of pathogenic autoantibody production. Hematological, metabolic, and endocrine parameters, including fasting glucose, glycated hemoglobin, insulin dose, lipids, and testosterone, were monitored before and after treatment.
Results: All seven treated patients achieved remission, defined as amelioration of hyperglycemia, discontinuation of insulin therapy, and resolution of hyperandrogenism. Glycated hemoglobin has normalized in all seven treated patients. Remission was achieved on average in 8 months from initiation of treatment. The medication regimen was well tolerated, with no serious adverse events.
Conclusions: In seven patients with type B insulin resistance, standardized treatment with rituximab, cyclophosphamide, and pulse steroids results in remission of the disease. Future studies will determine whether this treatment protocol can be applied to other autoantibody/cell surface receptor disease states.
A standardized therapeutic protocol of rituximab, cyclophosphamide, and pulse steroids results in remission from type B insulin resistance.
Type B insulin resistance due to autoantibodies to the insulin receptor is characterized by extreme insulin resistance, diabetes refractory to massive doses of insulin, dramatic weight loss, severe hyperandrogenism, and unusually widespread acanthosis nigricans. The syndrome usually occurs against the background of a rheumatologic illness (e.g. lupus, Sjogren, mixed connective tissue disease) or is a paraneoplastic manifestation of malignancy (1,2,3). This condition is representative of a class of diseases in which an autoantibody is produced against a cell surface protein. Other targets of such pathogenic antibodies include the TSH receptor (Graves disease), acetylcholine receptor (myasthenia gravis) (4), and gonadotropin receptor (ovarian failure) (5), with phenotypic manifestations of the receptor blockade relating to the biological role of the receptor affected. Recently inhibitory antibodies to the melanocortin-4 receptor were described in overweight and obese individuals (6).
In the type B syndrome, a highly specific autoantibody is produced against the cell surface insulin receptor and the interaction between the autoantibody and the receptor is the basis for the pathophysiology (7,8,9). The syndrome usually presents with extreme hyperglycemia, which may be followed by either fasting or reactive hypoglycemia. Rarely hypoglycemia in isolation may be seen (1). These different manifestations of the antibody receptor interaction can broadly be explained by quantitative differences in antibody titers. The autoantibody acts as a partial agonist, and so at low concentration it elicits a hypoglycemia response. At higher concentrations it chronically down-regulates cellular responsiveness to insulin, a phenomenon that most commonly dominates in vivo (1,10,11).
Whereas the etiological basis and the critical pathogenic role of the antireceptor antibodies in type B insulin resistance are reasonably well established, the therapy has generally been unsatisfactory. Treatment has consisted of generalized forms of immunosuppression, which may be toxic and generally disappointing (1). Accordingly, we have been motivated to find a more specific and safer form of therapy by a very severe presentation of this disease in a young woman. In the present paper, we describe our most recent experience with this treatment regimen in seven patients with type B insulin resistance.
Patients and Methods
We previously described 24 patients with the type B insulin resistance syndrome (1), and in this report we add 14 additional patients. All patients were evaluated at the Clinical Center of the National Institutes of Health (NIH), and informed consent was obtained from each patient or his/her legal guardian. Seven of these patients have been treated with the new therapeutic protocol, which was initiated with patient B-30. All subsequent patients with active disease received treatment.
Laboratory testing
After an overnight fast, the patients underwent routine hematological, immunological, and biochemical testing. Glucose and lipid values were determined by a Vista analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). Insulin was analyzed by a chemiluminescence immunoassay on a Siemens Immulite 2500 analyzer. Hemoglobin A1c was obtained by HPLC. Complete blood count and differential was determined by Cell-Dyn Sapphire (Abbott Diagnostics, Santa Clara, CA). Rheumatological antibodies were determined by ELISA. Total testosterone was obtained through chemiluminescence immunoassay on a Siemens Immulite 2500 analyzer. Free testosterone was calculated using the equation supplied by The Endocrine Society (12). Adiponectin was assayed using a customized autoDELFIA assay (PerkinElmer Lifesciences, Boston, MA) as described previously (13).
Insulin receptor antibody
The presence of the insulin receptor antibody was confirmed by measuring the ability of patients’ sera to immunoprecipitate recombinant human insulin receptors as described previously (14). Titers were based on semiquantitative assessment of Western blot band intensities compared with a positive control and were scored by one observer.
Medication protocol
A treatment protocol aimed at antibody production and general immunosuppression was chosen, consisting of rituximab, cyclophosphamide, and pulse steroids. Rituximab, an anti-CD-20 monoclonal antibody, was given iv, 750 mg/meter (2) body surface area in two consecutive doses 2 wk apart, which constituted one cycle. Subsequent doses of rituximab were given every 3–4 months if the disease persisted and CD-19 B lymphocyte levels were detectable. Cyclophosphamide was initiated with rituximab and given 100 mg daily by mouth continuously until remission was achieved. Pulse steroids, in the form of dexamethasone 40 mg daily by mouth for 4 d or methylprednisone 1 g iv for 2 d, were given concomitantly with each rituximab dose and then every 4–6 wk if the disease remained active.
In the most severe case (B-30), iv cyclophosphamide 500 mg was used in two cycles. In one patient with severe lupus-related neutropenia (B-34), cyclosporine 200 mg daily, with dose adjusted based on levels, was used instead of cyclophosphamide. Once patients achieved remission, they were placed on a maintenance regimen of azathioprine 100 mg daily, with patient B-34 continuing on a lower dose of cyclosporine 75 mg daily.
Remission
Remission was defined as the amelioration of the hyperglycemia and discontinuation of insulin therapy or, in the case of one patient who was not diabetic, normalization of testosterone levels.
Results
Patient demographics
Table 1 shows the patient demographics. Since our original case series of 24 patients with type B insulin resistance seen at the NIH (1), an additional 14 have been added to the cohort, resulting in a total of 38 patients. Consistent with our previous findings, 86% of the patients were female and the remaining two patients were male (14%). The ages range from 17 to 64 yr of age. Seventy-nine percent of the patients were African-American, 14% were Hispanic, and one was a First-Nation Canadian. Five patients were diagnosed with systemic lupus erythematosus (SLE) and two with mixed connective tissue disorder. Whereas none of the other patients carried an autoimmune diagnosis, they had multiple positive autoantibodies. Patient B-30 was the first patient in the case series to be treated, and patients B-33 through B-38 have all undergone treatment. Patients B-31 and B-32 were not treated as they presented to the NIH in remission. All patients were confirmed to have autoantibodies to the insulin receptor. Their titers are listed in Table 1.
Table 1.
Demographics of patients
| Patient | Age at NIH presentation | Gender | Race | Titerb | Underlying disease | Antibodies |
|---|---|---|---|---|---|---|
| B-30a | 20 | F | Black | ++++ | Mixed connective tissue disorder | ANA, ENA, anti-SmRNP |
| B-33a | 50 | F | Black | +++ | SLE | ANA, anti-Sm, anti-SmRNP, ENA |
| B-34a | 62 | M | Black | +++(+) | SLE | ANA, anti-SmRNP, anticardiolipin antibody IgG, ENA |
| B-35a | 17 | F | Black | + | Unknown | ANA, anti-Smith RNP, anti-Sm, ENA |
| B-36a | 64 | M | 1st Nation Canadian | ++++ | SLE | ENA, ANA, anti-SSA, SSB, anti-ds-DNA |
| B-37a | 58 | F | Black | +++ | Unknown | ANA, anti-TPO |
| B-38a | 21 | F | Black | +++ | Unknown | ANA, anti-SSA, anti-TG |
| B-25 | 53 | F | Hispanic | +++ | Mixed connective tissue disorder | anti-TPO, anti-TG, ENA |
| B-26 | 53 | F | Black | +++ | Unknown | ANA, ENA |
| B-27 | 44 | F | Black | ++ | SLE | ENA, ANA, anti-SmRNP, anti-Smith, anti-SSA, SSB |
| B-28 | 51 | F | Black | +++ | Unknown | RF, ANA, anti-SSA, anti-JKA, anticardiolipin IgM |
| B-29 | 53 | F | Hispanic | ++ | Unknown | ANA, ENA, anti-SSA, anti-SSB |
| B-31 | 40 | F | Black | + | Unknown | ANA, ENA, anti-Sm RNP, anti-SSA&B, anti-cardiolipin IgM |
| B-32 | 28 | F | Black | +++ | SLE | ENA, ANA, anti-ds-DNA, anti-SmRNP, anti-Smith, anti-SSA |
ANA, Antinuclear antibody; ENA, extractable nuclear antigen; ds-DNA, double-stranded DNA; TPO, thyroid perioxidase antibody; TG, thyroglobulin; SSA, Sjögren’s syndrome A; SSB, Sjögren’s syndrome B; JKA, kidd; RF, rheumatoid factor; Sm, Smith antigen; SmRNP, Smith ribonucleoprotein.
Patients are the seven who were treated under the described protocol.
Description of insulin receptor antibody titers were based on semiquantitative assessment of Western blot band intensities compared with a positive control and were scored by one observer.
Case reports
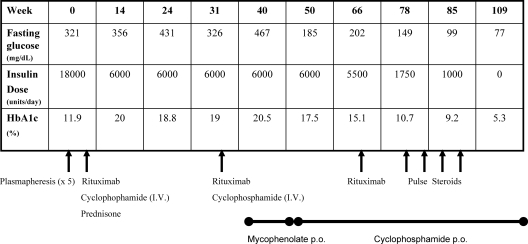
Figure 1 summarizes the treatment course of patient B-30, a 20-yr-old African-American woman. She was admitted to the NIH in 2006 after 6 months of uncontrolled hyperglycemia despite 18,000 U of U-500 insulin daily. She had lost 35 lb despite consuming approximately 3500 kcal daily. She was polyuric with 10 liters of urine daily, and amenorrheic for 1 yr. In addition to her severe catabolic state, she presented to the NIH with a unilateral facial nerve palsy, left eye uveitis, and disfiguring acanthosis nigricans. The severity of her skin involvement had resulted in multiple skin infections with methicillin-resistant Staphylococcus aureus requiring both surgical and antimicrobial intervention. During her evaluation, she developed bilateral otitis media requiring tympanic tube placement. Her daily insulin dose was reduced to 6,000 U due to discomfort from the large injection volumes required to administer 18,000 U/d; nevertheless, the high insulin doses were ineffective at providing glycemic control, with glycated hemoglobin levels ranging from 12 to 20%. Her complete blood count revealed leucopenia, anemia, and neutropenia. A diagnosis of mixed connective tissue disorder was made on clinical and immunological grounds.
Figure 1.
B-30 disease and treatment course.
Given the severity of the disease in this patient, specifically her catabolic state and multiple infections despite high-dose insulin therapy, we created a stepwise and targeted therapeutic protocol for her. We initially administered five cycles of plasmapheresis, but her insulin requirements did not change. We proceeded to a more aggressive immunosuppressive regimen, combining iv rituximab and cyclophosphamide. In her first cycle, we used low-dose prednisone therapy, 40 mg daily, with a prolonged taper. In the second dose of her first cycle, she developed diabetic ketoacidosis, requiring hospitalization for management. Low-dose prednisone therapy was discontinued. Two cycles of iv rituximab and cyclophosphamide were administered. This treatment did not result in any change in her clinical status: she continued to require high-dose insulin therapy with uncontrolled hyperglycemia. At wk 40, she was placed on mycophenolate mofetil 500 mg twice daily for 6 wk; however, with no change in her symptoms, she was placed on daily oral cyclophosphamide. The patient showed marked improvement in glycemic control, with a reduction of her insulin dose after her third cycle of solo rituximab therapy but was not yet in remission. Pulse steroids were added at wk 78. By wk 109 she had complete resolution of her symptoms, including a normal fasting blood glucose, normal oral glucose tolerance test, normal glycated hemoglobin, and no requirement for insulin therapy.
Figure 2 shows the immunoprecipitation of the insulin receptor in patient B-30. By wk 66, there was an appreciable decrease in her antiinsulin receptor antibodies, with her antibodies undetectable by wk 85.
Figure 2.
Immunoprecipitation of insulin receptors: NIH B-30.
Since patient B-30, we have standardized the therapeutic protocol as described in Patients and Methods, and it has been applied to patients B-33 through B-38. Unlike patient B-30, in whom the therapeutic regimen was applied in a stepwise manner, all three medications, rituximab, pulse steroids, and oral cyclophosphamide/cyclosporine, were started simultaneously in patients B-33 through B-38.
Metabolic parameters: response to therapy
Table 2 shows the metabolic parameters of the patients before and after treatment. All but one patient (B-35) presented with hyperglycemia, with fasting glucose levels of approximately 200–500 mg/dl. After treatment and induction of remission, they are all exhibiting normal fasting glucose levels. Of the patients on insulin therapy, all were taking more than several hundred to thousands of units daily, with the largest daily dose of 18,000 U/d. None of the patients currently require insulin therapy for maintenance of euglycemia. The glycated hemoglobin values have decreased accordingly in our patients, ranging from 5 to 6.5%. Triglyceride values have ranged from 42 to102 mg/dl, with an average of 58 mg/dl. Adiponectin levels measured at baseline showed a mean of 25.7 mg/liter (range 8.3–54.4 mg/liter). The low triglycerides (15) and high adiponectin (16) are a characteristic finding in insulin resistance mediated by insulin receptor dysfunction, in contrast to the more common forms of insulin resistance, such as type 2 diabetes, in which triglycerides are high and adiponectin is low (17).
Table 2.
Metabolic parameters and response to therapy
| Patients | Adiponectin (mg/liter) | Fasting glucose (mg/dl)
|
Insulin dose (U/d)
|
Triglycerides (mg/dl)
|
HDL (mg/dl)
|
HbA1c (%)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| B-30 | 54.4 | 321 | 77 | 18000 | 0 | 42 | 88 | 70 | 30 | 11.9 | 5.3 |
| B-33 | 21.3 | 277 | 79 | 1300 | 0 | 68 | 43 | 29 | 47 | 9 | 5.5 |
| B-34 | 12.5 | 306 | 88 | 1250 | 0 | 36 | 47 | 62 | 79 | 9.2 | 6.5 |
| B-35 | 8.3 | 71 | 78 | 0 | 0 | 41 | 87 | 51 | 33 | 6.8 | 5 |
| B-36 | 15.2 | 212 | 72 | 1800 | 0 | 102 | 80 | 36 | 67 | 11.7 | 6.4 |
| B-37 | 24.8 | 496 | 80 | 7000 | 0 | 92 | 55 | 49 | 88 | 10.2 | 5.6 |
| B-38 | 43.4 | 191 | 95 | 750 | 0 | 41 | 65 | 72 | 40 | 13.5 | 6.5 |
All (Pre) laboratory tests were obtained during the patient’s initial visit to the NIH Clinical Center. All (Post) laboratory tests were obtained during the patient after the patient discontinued insulin therapy and were readmitted to the clinical center for evaluation and initiation of maintenance therapy. Adiponectin levels were measured at the initial visit to the clinical center. HDL, High-density lipoprotein; HbA1c, glycated hemoglobin.
Patient B-35 did not present with hyperglycemia but rather with acanthosis nigricans, hyperandrogenism, and hyperinsulinemia with plasma insulin levels greater than 800 μU/ml. Her serum confirmed the presence of the antiinsulin receptor antibody, and with treatment her fasting insulin dropped from 871 to 26 μU/ml.
Endocrine parameters: response to therapy
Table 3 shows the total and free testosterone levels of the three premenopausal females. The three women exhibited markedly elevated total and free testosterone. This is driven by high endogenous insulin levels, which appear to act as a pathologic growth factor on the ovary, directing ovarian steroid production toward androgens (18). The patients had amenorrhea, increased acne, and hirsutism. After treatment, testosterone levels normalized. The women had relief of their symptoms of androgen excess, including resumption of their menstrual cycles.
Table 3.
Testosterone levels
| Patients | Total testosterone (ng/dl)
|
Free testosterone (ng/dl)
|
||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| B-30 | 623 | 25.9 | 9.3 | 0.3 |
| B-35 | 686 | <20 | 10.6 | <0.1 |
| B-38 | 334 | <20 | 2.4 | <0.1 |
Total testosterone reference range for tanner stage V women: 8–60 ng/dl; free testosterone reference range for tanner stage V women: 0.1–2.4 ng/dl.
Hematologic parameters: response to therapy
All patients presented with leucopenia, with an average white blood cell count of 2.7 K/μl (range 2.05–3.31 K/μl). Four of the 7 patients had neutropenia. Five of the 7 patients exhibited anemia. Four patients also exhibited lymphopenia, and 2 patients had thrombocytopenia. After therapy and induction of remission, neutropenia improved in 3 of the affected patients. Anemia improved in all of the affected patients. Absolute lymphocyte counts were lower in all patients as a result of the immunosuppressive therapy.
Remission
Table 4 summarizes the treatment courses, time to remission, and duration of remission in all seven patients. Four of the patients required one cycle of rituximab, and the remaining three required two or more. Pulse steroids were administered as described in Patients and Methods with patients receiving an average of three doses. Unlike patient B-30, our first and most severely affected patient, patients B-33 through B-38, when treated with the standardized protocol reached remission much faster, within 2.5–9 months after initiation of therapy. Although the average time to remission in these six patients was 5 months, most patients had a gradual reduction in their insulin requirements and improvement in their clinical symptoms that started after the first cycle of treatment. Three of the seven treated patients have been in remission for more than 1 yr. The most recently treated patients have been in remission 2–9 months.
Table 4.
Summary of treatment and remission
| Patients | Number of rituximab cycles | Number of pulse steroid doses | Time to remission (months)a | Duration of remission (months)b |
|---|---|---|---|---|
| B-30 | 2.5 | 5 | 27.25 | 15 |
| B-33 | 1 | 3 | 6.5 | 16 |
| B-34 | 2 | 4 | 9 | 12 |
| B-35 | 1 | 2 | 4.5 | 13 |
| B-36 | 2 | 5 | 6 | 3 |
| B-37 | 1 | 1 | 2.5 | 9 |
| B-38 | 1 | 2 | 2.5 | 2 |
Calculated from day treatment protocol initiated until cessation of insulin therapy or normalization of androgen levels.
Time point is calculated up until February 1, 2010.
Once patients were euglycemic and off insulin therapy, they were placed on maintenance therapy with either azathioprine or cyclosporine. In the case of the one nondiabetic patient, azathioprine was initiated once her androgen levels normalized. The patients receive maintenance therapy for 1 yr. Three patients have recently come off maintenance therapy and remain euglycemic.
Discussion
Patients with type B insulin resistance have posed many therapeutic challenges, from insulin dosing to diabetic complications to interdiction of pathogenic autoantibody production. Furthermore, the mortality rate in this rare disease is high: in our original case series, 54% of the patients had died less than 10 yr after diagnosis (1). Most present with a dramatic clinical picture, but the various therapeutic approaches tried in the past have been unsatisfactory (1). These have included a variety of steroid preparations, plasmapheresis, azathioprine, mycophenolate mofetil, and cyclophosphamide (19,20,21,22,23,24,25,26). A group at King’s Hospital in London reported using rituximab with success in one patient with SLE and type B insulin resistance (27). After more than 30 yr of experience managing what we believe is the largest cohort of patients with this rare disease, we have tried and been disappointed with many of these therapeutic approaches. With this report, we propose a standardized therapeutic regimen that uses complementary strategies to eliminate the pathogenic antibody. To target antibody-producing B lymphocytes, we used rituximab, an antibody against CD-20, a cell surface molecule expressed by B-cell progenitors of antibody-producing cells. Pulse steroids were used to suppress the activity of preexisting antibody producing plasma cells. Adjunctive immunosuppressive drugs were used to bolster the suppression of both B- and T-cell functions that were contributing to the related autoimmune processes. Cyclophosphamide was used as a first-line agent, but in one case with severe neutropenia, we chose cyclosporine. After entering remission, the immunosuppressive regimen was changed to azathioprine, with the exception of one patient who remains on cyclosporine. Although the patients will remain on maintenance for 1 yr, we do not know whether type B insulin resistance will recur once patients are taken off all immunosuppression.
Our patients have tolerated the standardized medication protocol with no serious adverse events or side effects. Rituximab infusions were given with acetaminophen and diphenhydramine pretreatment, and none of the patients experienced infusion-related reactions. We noted no increase in infections, including progressive multifocal leukoencephalopathy with rituximab. Cyclophosphamide can cause hemorrhagic cystitis; however, this was not seen in this cohort. Our patients are likely protected by the polyuria that results from their hyperglycemia, and they were instructed to maintain good hydration and void every 4 h. The pulse steroid cycles did result in transient worsening of hyperglycemia, but insulin doses were adjusted accordingly. Pulse steroids were better tolerated than continuous low-dose steroid therapy, which, when used with patient B-30, resulted in a hospital admission for diabetic ketoacidosis.
Since initiating this treatment protocol, we have not encountered patients presenting with hypoglycemia as their main manifestation of the syndrome. In our initial case series, patients presenting with hypoglycemia had a worse prognosis (1). It is not clear whether they would experience the same benefit from the present combination therapy. Several of our patients have experienced reactive hypoglycemia after hyperglycemia remission; however, this has been easily managed by dietary modifications.
A weakness of the current trial is the small sample size. However, type B insulin resistance is an extremely rare disease. The unknown, but very low, prevalence of this disorder would not allow for a randomized, placebo-controlled trial. In our previously published cohort of 24 patients, there was sufficient long-term follow-up on 18 patients. Six of these patients entered remission spontaneously within a 1- to 4-yr period. The 12 other patients were treated with a variety of immunosuppressive therapies. Six patients had clinical remission (within 6 wk of treatment) and six patients had persistent disease (1). Without the benefit of a placebo-controlled trial, we cannot state with certainty that spontaneous remission did not occur in any or all seven patients.
Type B insulin resistance falls into a class of cell surface receptor autoantibody syndromes. The proposed regimen targets circulating antibodies as well as antibody production. Whereas the therapeutic protocol is specific to the underlying pathophysiology of the disease, it is not specific to type B insulin resistance and could potentially be used in other autoantibody/cell surface receptor disease states, such as severe Graves’ ophthalmopathy or refractory myasthenia gravis. Further study is needed to see whether treatment for other diseases in the same class would result in a similar benefit.
Dedication
This manuscript is dedicated to Dr. Jeffrey S. Flier (Dean of Harvard Medical School, Boston, MA), who described the syndrome of type B insulin resistance when he was an intramural NIH research fellow (8). This dedication represents the dependence of academic leadership on creative fellows and the need to provide an environment for their growth.
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; the Wellcome Trust (Intermediate Clinical Fellowship 080952/Z/06/Z, to R.K.S.); and the U.K. National Institute for Health Research Cambridge Biomedical Research Centre.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 19, 2010
Abbreviation: SLE, Systemic lupus erythematosus.
References
- Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P 2002 Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 81:87–100 [DOI] [PubMed] [Google Scholar]
- Braund WJ, Naylor BA, Williamson DH, Buley ID, Clark A, Chapel HM, Turner RC 1987 Autoimmunity to insulin receptor and hypoglycaemia in patient with Hodgkin’s disease. Lancet 1:237–240 [DOI] [PubMed] [Google Scholar]
- Chan JC, Zhu SQ, Ho SK, Cockram CS 1990 Hypoglycaemia and Hodgkin’s disease. Br J Haematol 76:434–436 [DOI] [PubMed] [Google Scholar]
- Gorden P, Collier E, Roach P 1993 Autoimmune mechanisms of insulin resistance and hypoglycemia. In: Moley DE, ed. Insulin resistance. London: John Wiley; 123–142 [Google Scholar]
- Sluss PM, Schneyer AL 1992 Low molecular weight follicle-stimulating hormone receptor binding inhibitor in sera from premature ovarian failure patients. J Clin Endocrinol Metab 74:1242–1246 [DOI] [PubMed] [Google Scholar]
- Peter JC, Bekel A, Lecourt AC, Zipfel G, Eftekhari P, Nesslinger M, Breidert M, Muller S, Kessler L, Hofbauer KG 2009 Anti-melanocortin-4 receptor autoantibodies in obesity. J Clin Endocrinol Metab 94:793–800 [DOI] [PubMed] [Google Scholar]
- Flier JS, Kahn CR, Jarrett DB, Roth J 1976 Characterization of antibodies to the insulin receptor: a cause of insulin-resistant diabetes in man. J Clin Invest 58:1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier JS, Kahn CR, Roth J, Bar RS 1975 Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science 190:63–65 [DOI] [PubMed] [Google Scholar]
- Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J 1976 The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 294:739–745 [DOI] [PubMed] [Google Scholar]
- De Pirro R, Roth RA, Rossetti L, Goldfine ID 1984 Characterization of the serum from a patient with insulin resistance and hypoglycemia. Evidence for multiple populations of insulin receptor antibodies with different receptor binding and insulin-mimicking activities. Diabetes 33:301–304 [DOI] [PubMed] [Google Scholar]
- Di Paolo S, Giorgino R 1991 Insulin resistance and hypoglycemia in a patient with systemic lupus erythematosus: description of antiinsulin receptor antibodies that enhance insulin binding and inhibit insulin action. J Clin Endocrinol Metab 73:650–657 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Semple RK, Soos MA, Luan J, Mitchell CS, Wilson JC, Gurnell M, Cochran EK, Gorden P, Chatterjee VK, Wareham NJ, O'Rahilly S 2006 Elevated plasma adiponectin in humans with genetically defective insulin receptors. J Clin Endocrinol Metab 91:3219–3223 [DOI] [PubMed] [Google Scholar]
- Coll AP, Morganstein D, Jayne D, Soos MA, O'Rahilly S, Burke J 2005 Successful treatment of type B insulin resistance in a patient with otherwise quiescent systemic lupus erythematosus. Diabet Med 22:814–815 [DOI] [PubMed] [Google Scholar]
- Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, Soos MA, Carpenter TA, Lomas DJ, Cochran EK, Gorden P, O'Rahilly S, Savage DB 2009 Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 119:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Halberg NH, Burling K, Soos MA, Schraw T, Luan J, Cochran EK, Dunger DB, Wareham NJ, Scherer PE, Gorden P, O'Rahilly S 2007 Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes 56:1712–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J 2002 Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360:57–58 [DOI] [PubMed] [Google Scholar]
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Bao S, Root C, Jagasia S 2007 Type B insulin resistance syndrome associated with systemic lupus erythematosus. Endocr Pract 13:51–55 [DOI] [PubMed] [Google Scholar]
- Bloise W, Wajchenberg BL, Moncada VY, Marcus-Samuels B, Taylor SI 1989 Atypical antiinsulin receptor antibodies in a patient with type B insulin resistance and scleroderma. J Clin Endocrinol Metab 68:227–231 [DOI] [PubMed] [Google Scholar]
- Eriksson JW, Bremell T, Eliasson B, Fowelin J, Fredriksson L, Yu ZW 1998 Successful treatment with plasmapheresis, cyclophosphamide, and cyclosporin A in type B syndrome of insulin resistance. Case report. Diabetes Care 21:1217–1220 [DOI] [PubMed] [Google Scholar]
- Gehi A, Webb A, Nolte M, Davis Jr J 2003 Treatment of systemic lupus erythematosus-associated type B insulin resistance syndrome with cyclophosphamide and mycophenolate mofetil. Arthritis Rheum 48:1067–1070 [DOI] [PubMed] [Google Scholar]
- Kramer N, Rosenstein ED, Schneider G 1998 Refractory hyperglycemia complicating an evolving connective tissue disease: response to cyclosporine. J Rheumatol 25:816–818 [PubMed] [Google Scholar]
- Page KA, Dejardin S, Kahn CR, Kulkarni RN, Herold KC, Inzucchi SE 2007 A patient with type B insulin resistance syndrome, responsive to immune therapy. Nat Clin Pract Endocrinol Metab 3:835–840 [DOI] [PubMed] [Google Scholar]
- Sims RE, Rushford FE, Huston DP, Cunningham GR 1987 Successful immunosuppressive therapy in a patient with autoantibodies to insulin receptors and immune complex glomerulonephritis. South Med J 80:903–906 [DOI] [PubMed] [Google Scholar]
- Tran HA, Reeves GE 2009 Treatment of type B insulin resistance with immunoglobulin: novel use of an old therapy. Med J Aust 190:168 [DOI] [PubMed] [Google Scholar]
- Coll AP, Thomas S, Mufti GJ 2004 Rituximab therapy for the type B syndrome of severe insulin resistance. N Engl J Med 350:310–311 [DOI] [PubMed] [Google Scholar]