Abstract
Context: Adipose tissue in obese individuals is characterized by reduced capillary density and reduced oxygenation.
Objective: Our objective was to test whether hypoxia is associated with reduced antilipolytic effect of insulin.
Participants, Design, and Setting: Twenty-one lean and obese individuals participated in this cross-sectional study at a university-based clinical research center.
Intervention: In all subjects, in situ adipose tissue (AT) oxygenation [AT oxygen partial pressure (ATpO2)] was measured with a Clark electrode, insulin sensitivity as well as basal and insulin-suppressed lipolysis (continuous infusion of (2H5)glycerol) were measured during a euglycemic-hyperinsulinemic clamp, and abdominal sc AT biopsies were collected to assess fat cell size (Coulter counting of osmium-fixed cells), capillary density (by staining of histological sections), and gene expression (by quantitative RT-PCR).
Main Outcome Measure: In situ ATpO2 was evaluated.
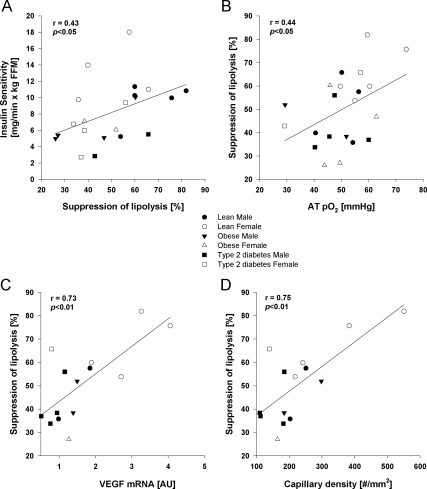
Results: The ability of insulin to suppress lipolysis (percent) was positively correlated with insulin sensitivity (r = 0.43; P < 0.05), ATpO2 (r = 0.44; P < 0.05), vascular endothelial growth factor mRNA (r = 0.73; P < 0.01), and capillary density (r = 0.75; P < 0.01).
Conclusion: These results indicate that low capillary density and ATpO2 in AT are potentially upstream causes of AT dysfunction.
Oxygenation in adipose tissue is associated with a reduced insulin suppression lipolysis, a hallmark of dysfunctional adipose tissue in insulin resistance.
Obesity is accompanied by insulin resistance in multiple organs including muscle, liver, and adipose tissue (AT) (1). There are multiple contributors to insulin resistance including AT hypoxia (2). Obese humans have reduced AT oxygen partial pressure (ATpO2), which is positively correlated with local inflammation (3). The reduced ATpO2 in obese adult humans may be due to decreased capillary density (rarefaction) in AT (3). Hypoxia interferes with insulin action in rodent models of obesity and cultured human adipocytes (4,5,6). Insulin, apart from its action in muscle and liver, inhibits lipolysis and efflux of nonesterified fatty acid in AT. Failure of insulin to suppress lipolysis has been proposed as one link between obesity and insulin resistance (7). In humans, however, reduced ATpO2 does not correlate with whole-body insulin sensitivity as measured during a high-dose hyperinsulinemic-euglycemic clamp (3) designed to measure skeletal muscle insulin sensitivity (8).
Given the role of insulin to suppress lipolysis in AT and that hypoxia leads to insulin resistance in vitro, we hypothesized that reduced ATpO2 in situ would be associated with reduced whole-body suppression of AT lipolysis by insulin.
Subjects and Methods
Twenty-one lean, obese, and type 2 diabetes mellitus subjects were recruited as previously described (3). Subjects were excluded for previous use of thiazolidinediones or drugs known to affect lipid metabolism or body weight. The protocol was approved by our Institutional Review Board, and all volunteers gave written informed consent.
Body composition was measured by dual-energy x-ray absorptiometry (QDR 4500; Hologic, Inc., Waltham, MA). As detailed previously, approximately 2 g sc AT was obtained by liposuction, 5 cm from the umbilicus (3). Mean fat cell size of sc abdominal AT was measured by Coulter counting of osmium-fixed cells (9), and ATpO2 was measured with a combined oxygen and temperature probe (micro Clark type electrode with a thermocouple, catalog item CC1.P1; Integra Lifesciences Corp., Plainsboro, NJ) inserted at 1 cm depth (3).
A euglycemic-hyperinsulinemic clamp and glycerol turnover were performed after an overnight fast and a 3-d standardized diet. Baseline blood samples were obtained for substrate and hormone concentrations and background isotope enrichments. A primed continuous infusion of [2H5]glycerol (0.1 mg/kg · min; Cambridge Isotope Laboratories, Andover, MA) was started and continued for 360 min. After 240 min, a euglycemic (∼90 mg/dl), hyperinsulinemic (80 mU/m2 · min) clamp was conducted for 120 min. Exogenous glucose disposal was calculated during the final 30 min of the clamp. The rate of appearance of glycerol (enrichment of [2H5]glycerol) was calculated from isotopic enrichment in four baseline and insulin-stimulated blood samples as previously described (10). Lipolysis was assessed as percentage change in rate of appearance of glycerol from the basal to insulin-stimulated state. The clinical characteristics, insulin sensitivity, ATpO2, and vascular endothelial growth factor (VEGF) have been previously reported (3).
Paraffin-embedded AT was incubated in tetraethylrhodamine isothiocyanate-conjugated lectin from Ulex europaeus (10 μg/ml) (catalog item L4889; Sigma-Aldrich, St. Louis, MO) and lectin-fluorescein isothiocyanate conjugate from Griffonia simplicifolia (25 μg/ml) (catalog item L2895; Sigma- Aldrich) for 30 min. Images were taken with a Zeiss Axioplan 2 upright microscope and microvessels counted using MBF ImageJ Bundle software (microvessel density = number of microvessels per square millimeter of section area, averaged across six to 10 images).
Human total RNA was isolated by column purification (QIAGEN, Valencia, CA). VEGF sequences were AGCCTTGCCTTGCTGCTCT (forward), ACCTCCACCATGCCAAGTGGTCCC (probe), and TCCTTCTGCCATGGGTGC (reverse). Quantitative RT-PCR was performed on a ABI PRISM 7900 with Cyclophilin-β as housekeeping gene.
Comparisons between lean, obese nondiabetic, and obese type 2 diabetic were tested with ANOVA. Relationships between lipolysis and measures of AT oxygenation were modeled with linear regression. Analyses were controlled for sex and race. If the sex and race interaction terms were not significant, these terms were removed. Analyses were conducted using JMP (version 5.0.1; SAS, Cary, NC).
Results
The clinical characteristics are presented in supplemental Table 1 (published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Mean fat cell size was negatively correlated with VEGF mRNA (r = −0.70; P < 0.008) and capillary density (r = −0.67; P < 0.01) but not with ATpO2. Glycerol turnover during fasting (glycerol appearance, micromoles per kilogram fat mass per minute) was positively correlated with ATpO2 (r = 0.48; P < 0.05) but not with VEGF or capillary density (r = 0.30; P value not significant). Insulin suppressed glycerol turnover (glycerol appearance, micromoles per kilogram fat mass per minute) was inversely correlated with VEGF (r = −0.55; P < 0.05) and only marginally with capillary density (r = −0.50; P = 0.06) but not with ATpO2 (r = 0.01; P value not significant). Insulin suppression of lipolysis was lower in obese vs. lean (44 ± 3 vs. 59 ± 15%, P < 0.05) and correlated with whole-body glucose disposal rate (r = 43; P < 0.05; Fig. 1A) and fat cell size (r = 0.53; P < 0.06). Insulin suppression of lipolysis was positively correlated with ATpO2 (r = 0.44; P < 0.05; Fig. 1B), VEGF mRNA (r = 0.73; P < 0.01; Fig. 1C), and capillary density (r = 0.75; P < 0.01; Fig. 1D). Sex and race were not significant contributors to suppression of lipolysis.
Figure 1.
Pearson correlation between suppression of lipolysis and insulin sensitivity for glucose disposal (A); AT oxygenation, ATpO2 (B); VEGF (C); and capillary density by image analysis (D). Males are represented by squares and females by circles: white for lean, gray for obese without type 2 diabetes, and black for obese with type 2 diabetes. AU, Arbitrary units.
Discussion
AT dysfunction consisting of infiltration of macrophages and inflammation (11), impaired regulation of lipolysis (12), and disordered secretion of adipokines (13) is recognized as a precursor to diabetes (14) and cardiovascular disease (15). Recent data in cell culture systems (4,5), animal models (4,6), and humans (3) suggest that AT hypoxia lies upstream of AT dysfunction. In this study, we explored the role of ATpO2 in the dysregulation of adipose lipolysis that occurs in diabetes as evidence of decreased sensitivity to insulin-suppressed lipolysis in human sc AT. Low ATpO2 was associated with decreased whole-body lipolysis as measured by glycerol turnover. The ability of insulin to suppress lipolysis was defective in subjects with a low ATpO2, and impaired lipolysis was associated with low AT capillary density and low VEGF mRNA. Taken together, these results implicate AT rarefaction and ATpO2 as potentially causative for AT dysfunction.
These results have implications for the treatment of AT inflammation and impaired lipolysis commonly described in obesity and insulin resistance. Strategies to increase AT blood flow by drugs blocking angiotensin II, a potent vasoconstrictor shown to be up-regulated in obesity (16), may improve AT oxygenation and possibly lipolysis. The observed reduction in AT VEGF and capillary rarefaction suggests that the reduced ATpO2 does not elicit an angiogenic response. Strategies that increase AT angiogenesis might be employed to increase ATpO2 and reverse the dysfunctional AT.
In summary, low ATpO2 is associated with a reduced insulin suppression of glycerol turnover, a hallmark of dysfunctional AT. These results provide new insight into the origins of AT dysfunction in obesity and suggest that defective angiogenesis might lie upstream of AT insulin resistance and therefore type 2 diabetes.
Acknowledgments
We acknowledge the research participants who contributed with their time to make this research possible.
Footnotes
This work was funded by a grant from the Health and Performance Enhancement Division of PBRC (M.P. and L.R.) and in part by an unrestricted grant from the Organization for the Study of Sex Differences (S.R.S.). This work used the facilities of the Cell Biology and Bioimaging Core that are supported in part by Pennington Center of Biomedical Research Excellence (COBRE) (NIH P20-RR021945) and the Nutrition Obesity Research Center (NIH 1P30-DK072476) grant from the National Institutes of Health.
This study is registered in www.Clinicaltrials.gov with ID: NCT00704197.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 13, 2010
Abbreviations: AT, Adipose tissue; ATpO2, AT oxygen partial pressure; VEGF, vascular endothelial growth factor.
References
- DeFronzo RA, Bonadonna RC, Ferrannini E 1992 Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15:318–368 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS 2004 Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355 [DOI] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR 2009 Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I 2007 Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911 [DOI] [PubMed] [Google Scholar]
- Wang B, Wood IS, Trayhurn P 2007 Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455:479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Gao Z, Yin J, He Q 2007 Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293:E1118–E1128 [DOI] [PubMed] [Google Scholar]
- Bays H, Mandarino L, DeFronzo RA 2004 Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 89:463–478 [DOI] [PubMed] [Google Scholar]
- Smith SR, Ravussin R 2006 Role of adipocyte in metabolism and endocrine function. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 5th ed. Philadelphia: Elsevier Saunders; 1045–1062 [Google Scholar]
- Hirsch J, Gallian E 1968 Methods for the determination of adipose cell size in man and animals. J Lipid Res 9:110–119 [PubMed] [Google Scholar]
- Wolfe RR, Chinkes DL 2005 Isotope tracers in metabolic research: principles and practice of kinetic analysis. Hoboken, NJ: John Wiley, Sons [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H 2003 Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsari C, Jensen MD 2006 Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. J Lipid Res 47:1643–1650 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE 2004 Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162 [DOI] [PubMed] [Google Scholar]
- De Costre P, Buhler U, DeGroot LJ, Refetoff S 1971 Diurnal rhythm in total serum thyroxine levels. Metabolism 20:782–791 [DOI] [PubMed] [Google Scholar]
- Smith SR, Wilson PW 2006 Free fatty acids and atherosclerosis: guilty or innocent? J Clin Endocrinol Metab 91:2506–2508 [DOI] [PubMed] [Google Scholar]
- Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiéra F, Sharma AM 2003 The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol 35:807–825 [DOI] [PubMed] [Google Scholar]