Abstract
Context: Published studies indicate marked variability in plasma leptin levels among persons with similar body mass index (BMI). We tested the hypothesis that such variations in leptin levels reflect differences in insulin sensitivity.
Subjects and Methods: Using euglycemic clamp, we assessed insulin sensitivity (ISI) in 57 nondiabetic adults (36 women, 21 men), whose BMI ranged from 20 to 78 kg/m2. We identified 38 age-matched subjects, stratified by fasting leptin (normal, <15 ng/ml vs. high, ≥15 ng/ml) and BMI (nonobese, <27 kg/m2 vs. overweight/obese, BMI ≥ 27 kg/m2) and compared ISI across the four strata.
Results: Fasting leptin levels correlated with ISI (r = −0.66 in men and −0.60 in women). In a multivariate regression model, leptin emerged as a strong predictor of ISI (r = −0.41, P = 0.0002) after controlling for adiposity, whereas insulin weakened as a predictor (r = −0.32, P = 0.0116). From regression plots of ISI vs. BMI and leptin, a BMI greater than 27 kg/m2 and a leptin level greater than 15 ng/dl strongly predicted decreased ISI. A fasting leptin cutoff of 15 ng/ml for detection of insulin sensitivity has a sensitivity of 72.7%, specificity of 56.3%, and positive predictive value of 69.6%. Overweight/obese subjects with fasting leptin less than 15 ng/ml were 100% more insulin sensitive than control subjects with leptin greater than 15 ng/ml.
Conclusions: Insulin sensitivity explains about 40% of the variance in fasting leptin levels. Thus, fasting plasma leptin levels probably serve as an endogenous response to ambient insulin resistance and may provide a surrogate measure of insulin action.
Fasting leptin levels probably serve as an endogenous response to ambient insulin resistance and may provide a surrogate measure of insulin action.
Treatment with leptin results in profound weight loss in obese humans with congenital leptin deficiency (1). Similar but fewer dramatic benefits on weight reduction were observed after leptin treatment in a cohort of 54 lean and 73 obese men and women with normal leptin genotype (as indicated by baseline serum leptin levels >10 ng/ml) (2). Furthermore, replacement doses of recombinant leptin normalized glucose and insulin levels in leptin-deficient subjects with diabetes and insulin resistance (3). Similarly, administration of leptin, in doses (0.1–1.0 mg/kg) that had no effect on body weight or normalized hyperglycemia and hyperinsulinemia in diabetic ob/ob mice (4).
The pattern of glucose and insulin responses to recombinant leptin is consistent with amelioration of insulin resistance rather than a direct hypoglycemic effect (3,4). Studies in cultured human hepatocytes indicate that leptin has myriad effects on insulin signaling pathways. Some of these effects include modulation of tyrosine phosphorylation of the insulin receptor substrate-1, association of receptor substrate-1 with downstream signaling molecules, and potentiation of insulin’s ability to suppress phosphoenolpyruvate carboxykinase, the rate-limiting enzyme in gluconeogenesis (5).
Collectively the aforementioned effects of leptin indicate that leptin may be a naturally occurring insulin sensitizer. As such, persons with insulin resistance could elaborate more leptin as a compensatory response, whereas insulin-sensitive subjects would have no need for leptin augmentation. The widely reported hyperleptinemia in obese subjects lends facile support to this thesis because obesity is associated with insulin resistance. In further support of this notion, patients with congenital lipodystrophic diabetes, who lack sc fat tissue, are leptin deficient and severely insulin resistant. Treatment with recombinant leptin reversed insulin resistance in such patients (6).
Previous studies documented marked variability (7) in plasma leptin levels among persons of comparable adiposity. Because leptin exerts potent insulin-sensitizing effects in rodents (4) and humans (1,3,6), we hypothesized that the variations in fasting leptin levels in persons of similar body mass index (BMI) reflect differences in insulin sensitivity. To test this hypothesis, we analyzed fasting leptin levels and insulin sensitivity, measured with the hyperinsulinemic euglycemic clamp technique, in 57 nondiabetic subjects. Our studies show that fasting leptin levels are correlated directly with adiposity and fasting insulin levels and inversely with insulin sensitivity. After controlling for adiposity and insulin levels, we here show that fasting leptin status remains a strong predictor of insulin sensitivity.
Subjects and Methods
Study population
We studied 57 nondiabetic adult subjects (36 women, 21 men), whose mean (±sem) age was 36.4 ± 1.25 yr (range 18–62 yr) and BMI was 33.8 ± 1.62 kg/m2 (range 20–78 kg/m2). The study population comprised 42 Caucasians, 10 African-Americans, and five Asians. The subjects had no history of diabetes or current or previous use of glucocorticoids or other medications that alter appetite, body weight, insulin sensitivity, or glucoregulatory physiology. The study subjects had normal fasting (95.8 ± 2.23 mg/dl; 5.32 ± 0.12 mmol/liter) and 2-h postload plasma glucose (131.1 ± 7.37 mg/dl; 7.28 ± 0.41 mmol/liter) levels during a standard 75-g oral glucose tolerance test. No subject was enrolled in any active weight-loss program. All subjects gave written informed consent before enrollment in this study, which was approved by the Human Studies Committee and conducted in accordance with the principles of the Declaration of Helsinki.
After obtaining their informed consent, study subjects made a screening visit to the General Clinical Research Center after an overnight fast. During that visit, a fasting plasma specimen was obtained, the height and weight were recorded, and the BMI was calculated for each subject. Each subject then underwent a standard 75-g oral glucose tolerance test to rule out diabetes.
Hyperinsulinemic euglycemic clamp
We assessed insulin sensitivity using the hyperinsulinemic euglycemic clamp technique (8). In brief, study subjects were admitted to the General Clinical Research Center at 0700 h on the day of study, after an overnight fast. An indwelling iv catheter was placed in an antecubital vein for infusion of insulin and dextrose. Another catheter was inserted in a contralateral hand vein, and the hand was warmed in a thermoregulated (60 C) chamber to enable arterialized blood sampling. A continuous infusion (1 mU/kg · min−1) of regular human insulin (Eli Lilly, Indianapolis, IN) was administered for 180 min, and dextrose (20%) was infused at a variable rate to maintain euglycemia [plasma glucose target ∼100 mg/dl (5.6 mmol/liter)]. Blood sampling for glucose and insulin levels was performed approximately every 10 min. Insulin sensitivity index (ISI) was computed as the rate of total insulin-stimulated glucose disposal during the last 60 min of the hyperinsulinemic euglycemic clamp, corrected for ambient insulin concentrations during the same period (8).
Analytical methods
Plasma leptin was measured with an in-house RIA using a commercial kit (Linco Research, St. Louis, MO). Samples were assayed in duplicate and the limits of detection and linearity for the leptin RIA were 0.5 and 100 ng/ml, respectively; the intra- and interassay coefficients of variation were less than 7% (9). Insulin levels were measured by RIA (10). Plasma glucose was measured with a glucose oxidase method (Beckman Instruments, Fullerton, CA).
Statistical analysis
Results are expressed as mean ± sem. Data were analyzed using ANOVA, multivariable logistic regression, and linear regression analyses. Statistical analyses were run on an IBM StatView program for Windows (SAS Institute Inc., Cary, NC). P < 0.05 was accepted as significant.
Role of the funding source
This study was funded in part by grants from the American Diabetes Association and the National Institutes of Health. The funding sources had no role in study design; collection, analysis, and interpretation of data; or the writing of the report and the decision to submit the paper for publication.
Results
Correlates of plasma leptin and insulin sensitivity
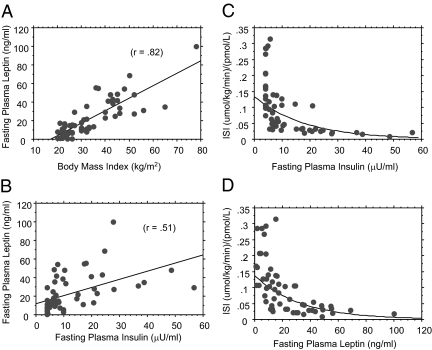
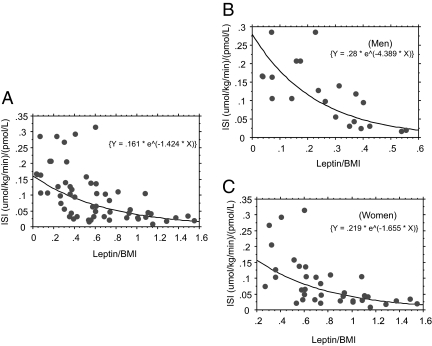
As has been reported previously (7), fasting plasma leptin levels were significantly correlated with BMI (r = 0.82) and fasting insulin levels (r = 0.51) (Fig. 1, A and B). In a multiple regression analysis, gender (P = 0.0004) and fasting insulin levels (P < 0.0001) but not age (P = 0.1827) or ethnicity (P = 0.8168) significantly predicted fasting plasma leptin levels. When BMI was added to the model, insulin was no longer a significant predictor of fasting leptin (P = 0.6478). Logarithmic regression analyses showed a strikingly similar exponential relationship between insulin sensitivity and fasting insulin or leptin levels (Fig. 1, C and D). Fasting leptin levels were inversely correlated with insulin sensitivity for the whole group (r = −0.59, P < 0.0001, as well as in men (r = −0.66, P = 0.0012) and women (r = −0.61, P < 0.0001). The inverse correlation between leptin and insulin sensitivity persisted after fasting plasma leptin levels were adjusted for BMI (r = −0.56) (Fig. 2A) and was again consistent across gender (Figs. 2, B and C). In a univariate ANOVA, the BMI-adjusted fasting plasma leptin level (F = 24.5, P < 0.0001) was a stronger predictor of insulin sensitivity than the BMI-adjusted fasting insulin level (F = 12.5, P = 0.0008). When both fasting leptin and insulin (both adjusted for BMI) were added in a multiple regression model that included terms for age, gender, and ethnicity, leptin remained a robust independent predictor of insulin sensitivity (r = −0.623, P = 0.0001), whereas insulin weakened as a predictor (r = −0.297, P = 0.0107) (Table 1).
Figure 1.
Regression of fasting plasma leptin levels vs. BMI (A) and fasting insulin levels (B) and of ISI vs. fasting insulin (C) or leptin levels (D). The logarithmic regression equation was Y = 0.134 * e^(−0.055 * X) for ISI vs. fasting insulin and Y = 0.138 * e^(−0.03 * X) for ISI vs. fasting leptin levels. To convert insulin from microunits per milliliter to picomoles per liter, multiply by 6.0.
Figure 2.
Logarithmic regression plots of ISI vs. fasting plasma leptin to BMI ratio for all subjects (A), men (B), and women (C). The regression equations are indicated in parentheses.
Table 1.
Multiple regression of insulin sensitivity vs. five independents
| Coefficient | se | Standard coefficient | t value | P value | |
|---|---|---|---|---|---|
| Intercept | 0.143 | 0.049 | 0.143 | 2.943 | 0.0049 |
| Leptin | −0.139 | 0.034 | −0.623 | −4.110 | 0.0001 |
| Insulin | −0.097 | 0.037 | −0.297 | −2.652 | 0.0107 |
| Gender | 0.049 | 0.023 | −0.297 | 2.097 | 0.0411 |
| Age | −0.001 | 0.001 | −0.128 | −1.132 | 0.2630 |
| Ethnicity | 0.020 | 0.013 | 0.162 | 1.545 | 0.1285 |
Insulin sensitivity in subgroups
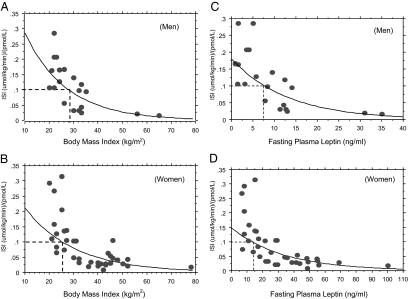
In a recent report, we observed that an ISI of 0.1 μmol/kg · min−1 per picomole per liter or greater, obtained from a hyperinsulinemic euglycemic clamp, characterized insulin-sensitive, glucose-tolerant healthy subjects (11). Regression analyses of ISI vs. BMI indicated that such normal insulin sensitivity was associated with a BMI cutoff point of about 26 kg/m2 and about 28 kg/m2 among male and female subjects, respectively, in the present study (Fig. 3, A and B). A similar analysis for ISI vs. fasting leptin levels revealed that normal insulin sensitivity (ISI ≥ 0.1 μmol/kg · min−1 per picomole per liter) was predicted by a fasting plasma leptin level of 7.5 ng/ml or less in men and 15 ng/ml or less in women (Fig. 3, C and D).
Figure 3.
A and B, Linear regression analyses of ISI vs. BMI indicating BMI cutoff points associated with a normal ISI (≥0.1 μmol/kg · min−1 per picomole per liter) in men (A) and women (B). C and D, Linear regression analyses of ISI vs. fasting leptin levels indicating cutoff points for leptin that are associated with a normal insulin sensitivity (ISI ≥ 0.1 μmol/kg · min−1 per picomole per liter) in men (C) and women (D).
To test the hypothesis that variations in fasting plasma leptin levels among individuals with comparable BMI reflect differences in insulin sensitivity, we used a fasting plasma leptin cutoff point of 15 ng/ml and a BMI cutoff of 27 kg/m2 to stratify the study subjects into four groups: 1) nonobese (BMI < 27 kg/m2)/normal fasting leptin (<15 ng/ml); 2) nonobese/hyperleptinemic (fasting leptin ≥ 15 ng/ml); 3) overweight or obese (BMI ≥ 27 kg/m2)/normal fasting leptin; and 4) overweight or obese/hyperleptinemic. Within each BMI stratum, subjects with fasting leptin less than 15 ng/ml were matched in age with subjects whose fasting leptin levels were 15 ng/ml or greater. Within the overweight/obese group (BMI ≥ 27 kg/m2), subjects in the two leptin strata were further matched by gender. The rarity of subjects with low BMI and elevated fasting leptin did not permit matching by gender among subjects with BMI less than 27 kg/m2. After matching the groups for age (and gender among the overweight/obese group), we identified 38 subjects that could be dichotomized into nonobese and overweight/obese subgroups, who were also discordant for fasting plasma leptin level (normoleptinemic vs. hyperleptinemic) (Table 2). The remainder of the 19 subjects could not be matched appropriately (11 subjects could not be matched for age or gender within their BMI strata; eight hyperleptinemic subjects with BMI > 45 kg/m2 did not have normoleptinemic matches).
Table 2.
Characteristics of subgroup of 38 subjects stratified by BMI and leptin status
| Group 1 nonobese/normoleptinemic | Group 2 nonobese/hyperleptinemic | Group 3 obese/normoleptinemic | Group 4 obese/hyperleptinemic | |
|---|---|---|---|---|
| n (male/female) | 14 (6/8) | 6 (3/3) | 9 (4/5) | 9 (4/5) |
| Age (yr) | 33.5 ± 2.27 | 35.5 ± 4.40 | 39.4 ± 2.92 | 39.2 ± 3.95 |
| BMI (kg/m2) | 23.5 ± 0.56 | 24.8 ± 0.98 | 32.6 ± 1.18 | 35.3 ± 1.44a |
| FPG (mg/dl) | 87.5 ± 2.67 | 87.6 ± 4.39 | 92.3 ± 2.89 | 104 ± 7.31b |
| Insulin (mU/ml) | 5.26 ± 0.56 | 7.08 ± 1.60 | 8.38 ± 2.02 | 18.7 ± 5.15c |
| Leptin (ng/ml) | 5.35 ± 0.87 | 18.2 ± 2.22 | 11.5 ± 0.60 | 33.5 ± 4.80 |
| ISI | 0.180 ± 0.023 | 0.149 ± 0.034 | 0.076 ± 0.011d | 0.041 ± 0.008e |
To convert insulin from microunits per milliliter to picomole per liter, multiply by 6.0; to convert glucose from milligrams per deciliter to millimole per liter, multiply by 0.05551. FPG, Fasting plasma glucose.
Significantly higher than groups 1 and 2 (P < 0.0001).
Significantly higher than group 1 (P = 0.005) and group 2 (P = 0.049).
Significantly higher than group 1 (P = 0.0039).
Significantly lower than group 1 (P = 0.0028) and group 2 (P = 0.04).
Significantly lower than group 1 (P = 0.0001), group 2 (P = 0.0025), and group 3 (P = 0.041).
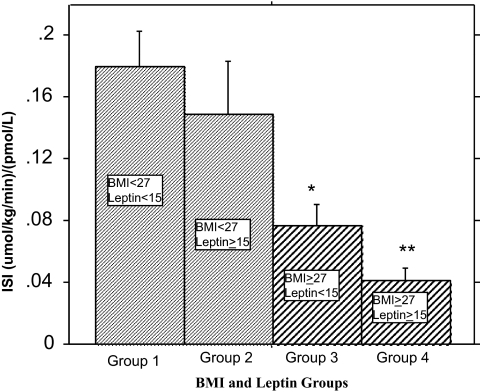
We then compared ISI values within each BMI stratum and across the four strata. The ISI (micromoles per kilogram per minute−1 per picomole per liter) was 0.180 ± 0.023 for group 1, 0.149 ± 0.034 for group 2, 0.076 ± 0.011 (P = 0.0028) for group 3, and 0.041 ± 0.008 (P = 0.0001) for group 2 (Fig. 4). Among the overweight/obese subjects (BMI ≥ 27 kg/m2), those with fasting leptin levels lower than 15 ng/ml had about 100% higher insulin sensitivity than persons with higher fasting leptin levels (Fig. 4). The leaner subjects showed a similar trend that was not statistically significant owing to the small number of subjects with low BMI and elevated leptin (a rare occurrence in nature). Based on receiver operating characteristics (ROC) analysis, a fasting leptin cutoff level of 15 ng/ml for the detection of insulin sensitivity had a true-positive rate (sensitivity) of 72.7%, specificity of 56.3%, accuracy of 65.8%, and a positive predictive value of 69.6%.
Figure 4.
Comparison of ISI in four groups of subjects stratified by BMI and fasting leptin levels. Within each BMI stratum, subjects with fasting leptin less than 15 ng/ml were compared with age-matched subjects with fasting leptin levels of 15 ng/ml or greater. Within the overweight/obese group (BMI ≥ 27 kg/m2), subjects in the two leptin strata were further matched by gender. The rarity of subjects with low BMI and elevated fasting leptin did not permit matching by gender among subjects with BMI less than 27 kg/m2. *, Significantly lower than group 1 (P = 0.0001), group 2 (P = 0.0025), and group 3 (P = 0.041); **, significantly lower than group 1 (P = 0.0028) and group 2 (P = 0.04).
Discussion
The fasting plasma insulin level is a widely used surrogate estimate of insulin sensitivity and a key component of the homeostasis model assessment of insulin resistance (12). However, the gold standard method for measuring insulin sensitivity is the hyperinsulinemic euglycemic clamp (8). In the present report, we have shown that the relationship between clamp-derived insulin sensitivity and fasting leptin levels actually is more robust than that between the former and fasting insulin levels. It must be noted though that isolated insulin levels are not a good marker of insulin sensitivity as are formulaic indices derived from the ratio fasting insulin to fasting glucose concentrations (such as homeostasis model assessment of insulin resistance, Matzsuda index, among others). Our findings indicate that about 40% of the variance in fasting plasma leptin levels among healthy, nondiabetic subjects can be explained by differences in insulin sensitivity. After adjustment for adiposity (BMI), insulin sensitivity still explained 25% of the variance in leptin levels. Our data show a strong inverse relationship between fasting leptin levels and insulin sensitivity in lean and obese subjects. Interestingly, among overweight/obese subjects who were discordant for fasting leptinemia, insulin sensitivity was considerably higher in those with lower fasting leptin levels than those with higher leptin levels.
In general, plasma leptin levels are strongly correlated with fat mass. Most obese persons have insulin resistance, hyperinsulinemia, and hyperleptinemia. The hyperleptinemia results from increased synthesis and secretion from the abundant adipose tissue (13). The crucial question is whether obesity-related hyperleptinemia is adaptive or maladaptive. The temporal course clearly indicates that hyperleptinemia is a consequence rather than a cause of obesity. Metabolic adaptations to obesity include hyperinsulinemia (a compensatory response by the pancreatic β-cells to obesity-induced insulin resistance), an often successful strategy for maintaining normoglycemia among obese persons who do not have a genetic risk for diabetes (14). We have argued that hyperleptinemia may be another metabolic adaptation to the obese state that serves as a counterregulatory (albeit ineffectual) attempt to restrain adiposity (15) and improve insulin sensitivity. In that sense, the hyperleptinemia of obesity may actually be an adaptive response. Consistent with that notion, we found in the present study that the overweight/obese subjects with hyperleptinemia (fasting plasma levels > 15 ng/ml) had a mean insulin sensitivity index that was only half that of subjects with lower leptin levels. We interpret the hyperleptinemia among those subjects as a response to their lower insulin sensitivity, rather than a cause of their insulin resistance. The preponderance of evidence indicates that leptin acts biologically as an insulin sensitizer (and a favorable glucoregulatory factor) in rodents and humans (1,2,3,4,5,6). Because leptin inhibits food intake and promotes weight loss (1,2,3,4), the near-universal hyperleptinemia observed in obese persons constitutes a physiological paradox that has generally been attributed to leptin resistance (16). The mechanism(s) of leptin resistance are not fully understood, but factors such as limited leptin delivery across the blood-brain barrier (17) or mutations in postreceptor signaling (16,18) have been proposed.
The findings of the present study, showing an inverse relationship between insulin sensitivity and plasma leptin levels (after controlling for BMI), suggest that circulating leptin levels may represent an endogenous response to ambient insulin sensitivity status. Based on this construct, interventions that improve insulin sensitivity should be associated with decreased leptin production, and those that induce insulin resistance should be associated with compensatory hyperleptinemia. Indeed, empirical support for this notion is provided by several reports in the literature indicating exactly the predicted relationship between leptin and interventions that alter insulin action. As shown in Table 3, behavioral and pharmacological intervention known to improve insulin sensitivity, such as fasting or caloric restriction (19), weight loss, exercise (20), and treatment with thiazolidinediones (21), all are associated with decreased plasma leptin levels. Furthermore, inositol phosphoglycan A, a second-messenger agonist along the insulin signaling pathway, has been shown to inhibit leptin release from cultured adipocytes (22). In contrast, interventions known to induce insulin resistance, such as overfeeding (23), weight gain, and treatment with glucocorticoids (24,25), estrogens (26), or GH (27) result in elevated leptin levels.
Table 3.
Interventions that alter insulin sensitivity and plasma leptin in opposite directions
| Increased insulin sensitivity/decreased leptin levels | Decreased insulin sensitivity/increased leptin levels |
|---|---|
| Caloric restriction | Overfeeding |
| Weight loss | Weight gain |
| Exercise | Glucocorticoids |
| Thiazolidinediones | Estrogen |
| Inositol phosphoglycans | GH |
Our findings suggest that fasting plasma leptin may be a more robust surrogate measure of insulin sensitivity than insulin. In a multiple regression model that included age, gender, ethnicity, leptin, and insulin, leptin emerged as a stronger independent predictor of insulin sensitivity than did insulin levels. In addition, plasma leptin levels have an excellent day-to-day reproducibility (28) and favorable kinetics (29) and are less susceptible to wide prandial swings, and the assay method (9) might be less subject to interference than that of insulin. Based on the foregoing premise, fasting plasma leptin concentration deserves to be evaluated further as a surrogate measure for insulin sensitivity. The major drawback to the use of leptin in that regard relates to the marked gender dimorphism in circulating leptin levels (7,30). Furthermore, linear regression analysis identified a gender split in the relationship between insulin sensitivity and fasting leptin levels among our study subjects: normal insulin sensitivity (ISI ≥ 0.1 μmol/kg · min−1 per picomole per liter) was predicted by a fasting plasma leptin level of 7.5 ng/ml or less in men and 15 ng/ml or less in women. Nonetheless, we selected a fasting plasma level of 15 ng/ml or lower as the cutoff point for differentiating insulin sensitive from insulin-resistant persons. We did so to accommodate all subjects (given the relatively small sample size), even though we realized that the 15-mg/dl cutoff point may lack specificity in women and sensitivity in men. Despite these foreseeable limitations, the ROC data showed fairly impressive sensitivity (72.7%), accuracy (65.8%), and positive predictive value (69.6%) for the provisional 15-ng/ml fasting plasma cutoff level for prediction of insulin sensitivity. In ongoing studies, we are expanding the sample size to enable the construction of ROC curves with multiple leptin cutoff points that can be analyzed in a gender-specific manner.
In conclusion, our data confirm and extend previous reports (31,32,33) showing an inverse correlation between insulin sensitivity and fasting plasma leptin concentration and suggest a role for leptin as a surrogate measure for insulin sensitivity. Importantly, we demonstrate for the first time that among persons of comparable adiposity, those with lower fasting plasma leptin levels (<15 ng/ml) generally have greater insulin sensitivity than age- and gender-matched persons with higher fasting leptin levels. Thus, hyperleptinemia is not merely a marker of obesity but may be a counterregulatory response to underlying insulin resistance.
Acknowledgments
Authors’ individual contributions were as follows: H.A., G.T., J.L., recruitment of subjects, performance of hyperinsulinemic euglycemic clamp, data entry, and review of manuscript, and S.D.-J., study concept and design, supervision of hyperinsulinemic euglycemic clamps and overall study, data analysis, and manuscript writing.
Footnotes
This work was supported by an American Diabetes Association Clinical Research Grant and National Institutes of Health Grants MO1 RR00036 and P60 DK20579. S.D.-J. is supported in part by National Institutes of Health Grants R01 DK067269 and MO1 RR00211.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 19, 2010
Abbreviations: BMI, Body mass index; ISI, insulin sensitivity index; ROC, receiver operating characteristic.
References
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S 1999 Effect of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341:879–884 [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M 1999 Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose escalation trial. JAMA 282:1568–1575 [DOI] [PubMed] [Google Scholar]
- Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML 2004 Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101:4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- Cohen B, Novick D, Rubinstein M 1996 Modulation of insulin activities by leptin. Science 274:1185–1188 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI 2002 Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109:1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M 1996 Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes 45:695–698 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M 1996 Radioimmunoassay of leptin in human plasma. Clin Chem 42:942–946 [PubMed] [Google Scholar]
- Kuzuya H, Blix PM, Horwitz DL, Steiner DF, Rubenstein AH 1977 Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes 26:22–29 [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack S, Askari H, Tykodi G 2009 Glucoregulatory physiology in subjects with low-normal, high-normal, or impaired fasting glucose. J Clin Endocrinol Metab 94:2031–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Lönnqvist F, Aener P, Nordfors L, Schalling M 1995 Overexpression of the obese (ob) gene in adipose tissue of obese subjects. Nat Med 1:950–953 [DOI] [PubMed] [Google Scholar]
- Polonsky KS, Sturis J, Bell GI 1996 Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus—a genetically programmed failure of the β cell to compensate for insulin resistance. N Engl J Med 334:777–783 [DOI] [PubMed] [Google Scholar]
- Askari H, Liu J, Dagogo-Jack S 2005 Energy adaptation to glucocorticoid-induced hyperleptinemia in human beings. Metabolism 54:876–880 [DOI] [PubMed] [Google Scholar]
- Bjørbaek C, El-Haschimi K, Fratz JD, Flier JS 1996 The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 274:30059–30065 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte Jr D 1996 Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2:589–593 [DOI] [PubMed] [Google Scholar]
- Steinberg GR, McAinch AJ, Chen MB, O'Brien PE, Dixon JB, Cameron-Smith D, Kemp BE 2006 The suppressor of cytokine signalling 3 (SOCS3) inhibits leptin activation of AMP-kinase in cultured skeletal muscle of obese humans. J Clin Endocrinol Metab 91:3592–3597 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M 1993. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 77:1287–1293 [DOI] [PubMed] [Google Scholar]
- Landt M, Lawson GM, Helgeson JM, Davila-Roman VG, Ladenson JH, Jaffe AS, Hickner RC 1997 Prolonged exercise decreases serum leptin concentrations. Metabolism 46:1109–1112 [DOI] [PubMed] [Google Scholar]
- Kallen CB, Lazar MA 1996 Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 93:5793–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunjara S, Wang DY, Mclean P, Greenbaum AL, Rademacher TW 2000 Inositol phosphoglycans and the regulation of the secretion of leptin: in vitro effects on leptin release from adipocytes and the relationship to obesity. Mol Genet Metab 70:61–68 [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF 1996 Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab 81:4162–4165 [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack S, Selke G, Melson AK, Newcomer JW 1997 Robust leptin secretory responses to dexamethasone in obese subjects. J Clin Endocrinol Metab 82:3230–3233 [DOI] [PubMed] [Google Scholar]
- Laferrère B, Fried SK, Hough K, Campbell SA, Thornton J, Pi-Sunyer FX 1998 Synergistic effects of feeding and dexamethasone on serum leptin levels. J Clin Endocrinol Metab 83:3742–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers JM, Asscheman H, Seidell JC, Frölich M, Meinders AE, Gooren LJ 1997 Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J Clin Endocrinol Metab 82:3267–3270 [DOI] [PubMed] [Google Scholar]
- Fouque D, Juillard L, Lasne Y, Tabakian A, Laville M, Joly MO, Laville M 1998 Acute leptin regulation in end-stage renal failure: the role of growth hormone and IGF-1. Kidney Int 54:932–937 [DOI] [PubMed] [Google Scholar]
- Liu J, Askari H, Dagogo-Jack S 1999 Reproducibility of fasting plasma leptin concentrations in lean and obese humans. Endocr Res 25:1–10 [DOI] [PubMed] [Google Scholar]
- Zeng J, Patterson BW, Klein S, Martin DR, Dagogo-Jack S, Kohrt WM, Miller S, Landt M 1997 Whole body leptin kinetics and renal metabolism in vivo. Am J Physiol 273:E1102–E1106 [DOI] [PubMed] [Google Scholar]
- Ostlund Jr RE, Yang JW, Klein S, Gingerich R 1996 Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 81:3909–3913 [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Walsh SA, Morgan DA, Thomas MJ, Haynes WG 1997 Effects of leptin on insulin sensitivity in normal rats. Endocrinology 138:3395–3401 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Karlsson C, Lönn L, Mårin P, Björntorp P, Sjöström L, Carlsson B, Carlsson LM, Bengtsson BA 1998 Serum leptin concentration and insulin sensitivity in men with abdominal obesity. Obes Res 6:416–421 [DOI] [PubMed] [Google Scholar]
- McIntyre HD, Chang AM, Callaway LK, Cowley DM, Dyer AR, Radaelli T, Farrell KA, Huston-Presley LP, Amini SB, Kirwan JP, Catalano PM 2010 Hormonal and metabolic factors associated with variations in insulin sensitivity in human pregnancy. Diabetes Care 33:356–360 [DOI] [PMC free article] [PubMed] [Google Scholar]