Abstract
Background: Neck circumference, a proxy for upper-body sc fat, may be a unique fat depot that confers additional cardiovascular risk above and beyond central body fat.
Methods and Results: Participants with neck circumference measures who underwent multidetector computed tomography to assess visceral adipose tissue (VAT) were included [n = 3307, 48% women; mean age = 51 yr; mean body mass index (BMI) = 27.8 kg/m2; mean neck circumference = 40.5 cm (men) and 34.2 cm (women)]. Sex-specific linear regression models were used to assess the association between sd increase in neck circumference and cardiovascular disease (CVD) risk factors (systolic and diastolic blood pressure; total, low-density lipoprotein, and high-density lipoprotein cholesterol and triglycerides; and fasting plasma glucose, insulin, proinsulin, and homeostasis model assessment of insulin resistance). Neck circumference was correlated with VAT [r = 0.63 (men); r = 0.74 (women); P < 0.001] and BMI [r = 0.79 (men); r = 0.80 (women); P < 0.001]. After further adjustment for VAT, neck circumference was positively associated with systolic blood pressure, diastolic blood pressure in men only, triglycerides, fasting plasma glucose in women only, insulin, proinsulin, and homeostasis model assessment of insulin resistance and was inversely associated with high-density lipoprotein (all P values <0.01). Similar results were observed in models that adjusted for both VAT and BMI. In a secondary analysis of incident CVD as an outcome, there was no statistically significant association observed for neck circumference in multivariable-adjusted models.
Conclusions: Neck circumference is associated with CVD risk factors even after adjustment for VAT and BMI. These findings suggest that upper-body sc fat may be a unique, pathogenic fat depot.
Neck circumference is associated with cardiometabolic risk factors even after adjustment for levels of visceral adipose tissue.
Visceral adipose tissue (VAT) is recognized as a unique, pathogenic fat depot, conferring metabolic risk above and beyond standard anthropometric measures, such as body mass index (BMI) and waist circumference (1). Individuals with large amounts of visceral fat are at increased risk of insulin resistance, type 2 diabetes, and atherosclerosis (2,3,4). However, VAT accounts for only modest correlations between cardiometabolic risk factors, suggesting that other mechanisms, or other fat depots, may also contribute to the development of cardiovascular disease (CVD) risk factors (1).
Upper body sc fat, as estimated by neck circumference, may confer risk above and beyond visceral abdominal fat. Anatomically, upper-body sc fat is a unique fat depot located in a separate compartment compared with VAT. Systemic free fatty acid concentrations are primarily determined by upper-body sc fat, suggesting that this fat depot may play an important role in risk factor pathogenesis (5). Elevated free fatty acid concentrations have been associated with insulin resistance, increased very-low-density lipoprotein cholesterol production, and endothelial cell dysfunction (6). Some studies have indicated that neck circumference may be an independent correlate of metabolic risk factors above and beyond BMI and waist circumference (7,8,9,10). In addition, a small study of men demonstrated that higher levels of upper-body sc fat, as measured by magnetic resonance imaging, were associated with higher low-density lipoprotein (LDL) and lower high-density lipoprotein (HDL) cholesterol levels (11). However, studies examining the joint impact of neck circumference and VAT have not as yet been reported.
Thus, the goal of this analysis is to characterize the cardiometabolic correlates of neck circumference and to ask the specific question of whether neck circumference is associated with cardiometabolic risk factors independently of VAT.
Subjects and Methods
Study sample
The study design of the Framingham Heart Study cohorts has been previously described (12,13,14,15). Briefly, in 1948, the Framingham Heart Study began enrollment of an original cohort of 5209 men and women aged 28–62 yr who subsequently underwent biennial examinations (12,13). In 1971, 5124 offspring of the original participants and their spouses were enrolled into the offspring cohort and underwent examinations approximately every 4 yr (14). In 2002, 4095 children of the offspring cohort participants and their spouses were enrolled in to the third-generation cohort and have completed their first examination (15). Offspring and third-generation cohort participants were invited to participate in the multidetector computed tomography (MDCT) substudy. Inclusion in this substudy was weighted toward individuals from larger Framingham Heart Study families who were residing in the New England area. To be eligible, participants had to be at least 35 yr old if male, at least 40 yr old if female, and nonpregnant and have a weight of less than 160 kg. A total of 3515 participants (1422 from offspring and 2093 from third generation) underwent MDCT scanning from 2002–2005. For the present analysis, we excluded individuals with type 1 diabetes or who had missing data on neck circumference, waist circumference, BMI, or VAT measurements, resulting in a final sample size of 3307. Individuals who were excluded were comparable to those included in the analysis with respect to age, sex, and levels of blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, fasting plasma glucose, insulin, proinsulin, and homeostasis model assessment of insulin resistance (HOMA-IR). The present analysis uses measurements from the seventh offspring examination (1998–2001) and the first third-generation examination (2002–2005).
Adiposity measurements
Neck circumference was measured to the nearest quarter inch using a tape measure.
Participants were asked to stand erect with their head positioned in the Frankfort horizontal plane. The superior border of a tape measure was placed just below the laryngeal prominence and applied perpendicular to the long axis of the neck. Waist circumference was measured at the level of the umbilicus and was recorded to the nearest quarter inch. Height and weight were measured using standardized protocols. BMI was calculated by dividing weight in kilograms by the square of height in meters.
Subcutaneous adipose tissue (SAT) and VAT were measured as previously described (16). Briefly, participants underwent MDCT scanning using an eight-slice scanner (LightSpeed Ultra; General Electric, Milwaukee, WI). Twenty-five contiguous 5-mm-thick slices (120 kVp, 400 mA, gantry rotation time 500 msec, table feed 3:1) were acquired covering 125 mm above the S1 level. SAT and VAT were assessed by experienced technicians using a dedicated offline workstation (Aquarius 3D Workstation; TeraRecon Inc., San Mateo, CA). The volume (in cubic centimeters) of SAT and VAT was determined by manually tracing the abdominal wall separating the SAT and VAT compartments.
Cardiometabolic risk factor measurements
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice by a study physician, and the average of the two measurements was taken. Hypertension was defined as a SBP of 140 mm Hg or higher or a DBP of 90 mm Hg or higher or current use of antihypertensive treatment. Total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were measured after an overnight fast. Low HDL was defined as an HDL less than 40 mg/dl for men and less than 50 mg/dl for women. A high triglyceride level was defined as a measurement of 150 mg/dl or higher or treatment with a lipid-lowering medication.
Participants underwent measurement of fasting plasma glucose, insulin, and proinsulin. Insulin and proinsulin were measured using RIA in the offspring cohort and ELISA in the third-generation cohort. Diabetes was defined as fasting plasma glucose of 126 mg/dl or higher or treatment with insulin or a hypoglycemic agent. The HOMA-IR measurement was calculated as previously described (17). A participant was considered to have insulin resistance if they had a HOMA-IR value in the top quartile of the distribution in the nondiabetic study sample. The presence of metabolic syndrome was defined according to modified National Cholesterol Education Program criteria (18).
Participants were defined as current cigarette smokers if they reported smoking at least one cigarette per day over the previous year. Regular alcohol consumption was defined as more than 14 drinks per week for men or more than seven drinks per week for women.
Statistical analysis
Neck circumference and VAT were standardized within each sex to a mean of zero and a sd of one to facilitate comparisons of the regression coefficients across different fat depots. Triglycerides, insulin, proinsulin, and HOMA-IR were log (ln) transformed to improve normality of their distributions. All analyses were sex specific. All analyses involving insulin measures (insulin, proinsulin, and HOMA-IR) were restricted to participants without diabetes. Age-adjusted Pearson correlation coefficients were calculated between neck circumference and continuous cardiometabolic risk factors. Linear regression models were constructed to assess the association between sd increase in neck circumference and cardiometabolic risk factors. A logistic regression model was constructed to assess the association between neck circumference and dichotomous cardiometabolic risk factors. In model 1, we adjusted for age (years), current cigarette smoking (yes vs. no), alcohol consumption (men, >14 vs. ≤14 drinks/wk; women, >7 vs. ≤7 drinks/wk), menopausal status (yes vs. no), and hormone replacement therapy use (yes vs. no). In model 2, we adjusted for the covariates in model 1 as well as VAT. In model 3, we adjusted for the covariates in model 1 and for BMI and waist circumference. In model 4, we adjusted for the covariates in model 1 and for BMI and VAT. Because previous work in the Framingham Heart Study has demonstrated that VAT is much more strongly associated with cardiometabolic risk factors than SAT, we chose to focus the comparison of neck circumference with VAT only (1). The interaction between neck circumference and sex was assessed by including a cross-product term and determining its significance using a one-degree-of-freedom Wald test.
In a secondary analysis, we used a Cox proportional hazards model to examine the association between neck circumference and incident cardiovascular disease (defined as myocardial infarction, atherothrombotic infarction, cerebral embolism, intracerebral hemorrhage, subarachnoid hemorrhage, and CVD death) and incident coronary heart disease (CHD) (defined as myocardial infarction and CHD death) occurring between the date of the seventh offspring examination cycle and December 31, 2007. The third-generation participants were not included in this analysis because they have insufficient numbers of events. Additionally, we expanded the study sample to include all participants who attended the seventh offspring examination cycle rather than only those who were participants in the MDCT substudy. Individuals with prevalent CVD at the time of seventh examination were excluded as well as those with missing data on neck circumference, BMI, or waist circumference, resulting in a final sample size of 3086 for the analysis. We constructed an age- and sex-adjusted model as well as a multivariable model that adjusted for age, sex, alcohol use, smoking, diabetes, SBP, hypertension treatment, total cholesterol to HDL cholesterol ratio, triglycerides, lipid treatment, menopausal status, and hormone replacement therapy use.
All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). A P value <0.05 was considered statistically significant.
Results
Study sample characteristics of the 3307 study participants are presented in Table 1. The sample was 48% women, and the mean age was 49.8 yr among men and 52.1 yr among women.
Table 1.
Study sample characteristics
| Men (n = 1718) | Women (n = 1589) | |
|---|---|---|
| Continuous characteristics, mean (sd) | ||
| Age (yr) | 49.8 (10.7) | 52.1 (9.9) |
| Neck circumference (cm) | 40.5 (2.9) | 34.2 (2.8) |
| Waist circumference (cm) | 100.8 (11.7) | 93.2 (15.5) |
| BMI (kg/m2) | 28.4 (4.5) | 27.0 (5.8) |
| Abdominal SAT (cm3) | 2632 (1202) | 3144 (1516) |
| VAT (cm3) | 2240 (1021) | 1362 (833) |
| Insulin (pmol/liter)a | 91.6 (42.8) | 82.3 (35.1) |
| Proinsulin (pmol/liter)a | 14.4 (10.3) | 11.5 (7.3) |
| HOMA-IRa | 3.2 (1.5) | 2.7 (1.3) |
| Fasting plasma glucose (mg/dl) | 102 (21) | 96 (18) |
| SBP (mm Hg) | 123 (15) | 120 (18) |
| DBP (mm Hg) | 78 (9) | 74 (9) |
| Total cholesterol (mg/dl) | 195 (34) | 198 (36) |
| LDL cholesterol (mg/dl) | 122 (30) | 114 (33) |
| HDL cholesterol (mg/dl) | 46 (12) | 61 (17) |
| Triglycerides (mg/dl) | 142 (105) | 113 (69) |
| Categorical characteristics, n (%) | ||
| Hypertension | 551 (32.1) | 421 (26.6) |
| Diabetes | 113 (6.6) | 80 (5.0) |
| High triglycerides | 749 (43.7) | 427 (26.9) |
| Low HDL cholesterol | 559 (32.6) | 408 (25.7) |
| Hypertension treatment | 340 (19.8) | 294 (18.5) |
| Diabetes treatment | 54 (3.2) | 43 (2.7) |
| Lipid treatment | 303 (17.6) | 166 (10.5) |
| Metabolic syndrome | 575 (35.8) | 384 (25.5) |
| HOMA-IR ≥75th percentilea | 465 (30.8) | 259 (18.7) |
| Current cigarette smoker | 229 (13.3) | 199 (12.5) |
| Regular alcohol consumptionb | 225 (13.3) | 191 (12.2) |
| Postmenopausal | 808 (50.9) | |
| Current hormone replacement therapy use | 319 (20.2) |
Excludes individuals with diabetes.
More than 14 drinks/wk for men or more than seven drinks/wk for women.
Pearson correlation coefficients for neck circumference
Neck circumference was correlated with all cardiometabolic risk factors (Table 2), with the exception of total and LDL cholesterol. Among men, neck circumference was not correlated with either total or LDL cholesterol, whereas in women, there were statistically significant, although weak, correlations for total (r = 0.09) and LDL (r = 0.14) cholesterol.
Table 2.
Age-adjusted Pearson correlation coefficients between neck circumference and cardiometabolic risk factors, by sex
| Men | Women | |
|---|---|---|
| Waist circumference (cm) | 0.75b | 0.78b |
| Body mass index (kg/m2) | 0.79b | 0.80b |
| Abdominal SAT (cm3) | 0.60b | 0.69b |
| VAT (cm3) | 0.63b | 0.74b |
| Log insulina | 0.48b | 0.47b |
| Log proinsulina | 0.46b | 0.47b |
| Log HOMA-IRa | 0.49b | 0.51b |
| Fasting plasma glucose (mg/dl) | 0.25b | 0.34b |
| SBP (mm Hg) | 0.18b | 0.27b |
| DBP (mm Hg) | 0.22b | 0.26b |
| Total cholesterol (mg/dl) | 0.00 | 0.09c |
| LDL cholesterol (mg/dl) | −0.01 | 0.14b |
| HDL cholesterol (mg/dl) | −0.29b | −0.34b |
| Log triglycerides (mg/dl) | 0.31b | 0.39b |
Excludes individuals with diabetes.
P value <0.0001.
P value <0.001.
Linear regression of continuous risk factors on neck circumference
Table 3 presents β-coefficients [95% confidence intervals (CI)] representing the association of risk factor levels per sd increment in neck circumference (men, 1 sd = 2.9 cm; women, 1 sd = 2.8 cm). Among men, neck circumference was correlated with all risk factors, except total and LDL cholesterol. After further adjustment for VAT, effect sizes were substantially attenuated but still remained strongly associated with all risk factors. For example, among men, an increment in neck circumference of 1 sd (2.9 cm) was associated with a 2.4 mm Hg increase (P < 0.0001) in SBP in the primary model. Upon further adjustment for VAT, the increase in SBP was 1.3 mm Hg (P = 0.002) per sd increment in neck circumference. In models that adjusted for BMI and waist circumference instead of VAT, the β-coefficients were also attenuated but remained statistically significant for all risk factors except for SBP, total and LDL cholesterol, and fasting plasma glucose. In models that adjusted for both BMI and VAT, neck circumference was strongly correlated with DBP in men only, HDL, triglycerides, fasting plasma glucose in women only, insulin, proinsulin, and HOMA-IR. Details of the predictive power of models, shown as adjusted model R-squares are presented in Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Table 3.
Multivariable linear regression of continuous cardiometabolic risk factors on neck circumference
| Men
|
Women
|
P value for sex interactiona | |||
|---|---|---|---|---|---|
| β (se) | P value | β (se) | P value | ||
| SBP (mm Hg)b | |||||
| Multivariable | 2.42 (0.34) | <0.0001 | 3.99 (0.40) | <0.0001 | <0.0001 |
| Multivariable + VAT | 1.29 (0.42) | 0.002 | 1.83 (0.58) | 0.002 | |
| Multivariable + BMI, waist | 0.65 (0.54) | 0.23 | 1.25 (0.66) | 0.06 | |
| Multivariable + BMI, VAT | 0.43 (0.55) | 0.43 | 0.69 (0.69) | 0.31 | |
| DBP (mm Hg)b | |||||
| Multivariable | 2.00 (0.22) | <0.0001 | 2.20 (0.23) | <0.0001 | 0.19 |
| Multivariable + VAT | 1.09 (0.27) | <0.0001 | 1.15 (0.33) | 0.0006 | |
| Multivariable + BMI, waist | 0.91 (0.35) | 0.01 | 0.74 (0.38) | 0.05 | |
| Multivariable + BMI, VAT | 0.75 (0.35) | 0.03 | 0.52 (0.40) | 0.19 | |
| Total cholesterol (mg/dl)c | |||||
| Multivariable | 0.58 (0.83) | 0.49 | 3.73 (0.89) | <0.0001 | 0.002 |
| Multivariable + VAT | −1.56 (1.06) | 0.14 | −0.60 (1.30) | 0.65 | |
| Multivariable + BMI, waist | −0.69 (1.36) | 0.61 | 0.81 (1.49) | 0.59 | |
| Multivariable + BMI, VAT | −1.47 (1.37) | 0.28 | −0.60 (1.55) | 0.70 | |
| LDL cholesterol (mg/dl)c | |||||
| Multivariable | 0.38 (0.74) | 0.61 | 4.86 (0.80) | <0.0001 | <0.0001 |
| Multivariable + VAT | −1.30 (0.95) | 0.17 | 0.38 (1.17) | 0.74 | |
| Multivariable + BMI, waist | −1.41 (1.21) | 0.24 | 1.33 (1.34) | 0.32 | |
| Multivariable + BMI, VAT | −1.95 (1.21) | 0.11 | −0.13 (1.39) | 0.92 | |
| HDL cholesterol (mg/dl)c | |||||
| Multivariable | −3.85 (0.28) | <0.0001 | −5.52 (0.39) | <0.0001 | 0.0002 |
| Multivariable + VAT | −2.20 (0.35) | <0.0001 | −2.75 (0.57) | <0.0001 | |
| Multivariable + BMI, waist | −2.90 (0.46) | <0.0001 | −3.73 (0.66) | <0.0001 | |
| Multivariable + BMI, VAT | −2.42 (0.46) | <0.0001 | −2.54 (0.68) | 0.0002 | |
| Log triglycerides (mg/dl)c | |||||
| Multivariable | 0.18 (0.01) | <0.0001 | 0.19 (0.01) | <0.0001 | 0.17 |
| Multivariable + VAT | 0.09 (0.02) | <0.0001 | 0.07 (0.02) | <0.0001 | |
| Multivariable + BMI, waist | 0.14 (0.02) | <0.0001 | 0.12 (0.02) | <0.0001 | |
| Multivariable + BMI, VAT | 0.12 (0.02) | <0.0001 | 0.07 (0.02) | 0.0001 | |
| Fasting plasma glucose (mg/dl)d | |||||
| Multivariable | 3.63 (0.45) | <0.0001 | 4.66 (0.34) | <0.0001 | 0.03 |
| Multivariable + VAT | 2.98 (0.57) | <0.0001 | 2.13 (0.50) | <0.0001 | |
| Multivariable + BMI, waist | 1.12 (0.72) | 0.12 | 2.64 (0.57) | <0.0001 | |
| Multivariable + BMI, VAT | 1.16 (0.73) | 0.11 | 1.78 (0.59) | 0.003 | |
| Log insulin (pmol/liter)e | |||||
| Multivariable | 0.19 (0.01) | <0.0001 | 0.18 (0.01) | <0.0001 | 0.20 |
| Multivariable + VAT | 0.11 (0.01) | <0.0001 | 0.08 (0.01) | <0.0001 | |
| Multivariable + BMI, waist | 0.08 (0.01) | <0.0001 | 0.07 (0.01) | <0.0001 | |
| Multivariable + BMI, VAT | 0.06 (0.01) | <0.0001 | 0.05 (0.02) | 0.001 | |
| Log proinsulin (pmol/liter)e | |||||
| Multivariable | 0.25 (0.01) | <0.0001 | 0.22 (0.01) | <0.0001 | 0.08 |
| Multivariable + VAT | 0.16 (0.01) | <0.0001 | 0.10 (0.02) | <0.0001 | |
| Multivariable + BMI, waist | 0.13 (0.02) | <0.0001 | 0.10 (0.02) | <0.0001 | |
| Multivariable + BMI, VAT | 0.11 (0.02) | <0.0001 | 0.06 (0.02) | 0.0007 | |
| Log HOMA-IRe | |||||
| Multivariable | 0.21 (0.01) | <0.0001 | 0.21 (0.01) | <0.0001 | 0.79 |
| Multivariable + VAT | 0.12 (0.01) | <0.0001 | 0.10 (0.01) | <0.0001 | |
| Multivariable + BMI, waist | 0.09 (0.02) | <0.0001 | 0.09 (0.02) | <0.0001 | |
| Multivariable + BMI, VAT | 0.07 (0.02) | <0.0001 | 0.06 (0.02) | 0.0001 | |
Neck circumference and VAT are standardized to a mean of 1 and sd of 0. β represents the change in risk factor level per sd of neck circumference. Multivariable means adjusted for age, smoking, alcohol, menopausal status, and hormone replacement therapy use.
Interaction between neck circumference and sex.
Additionally adjusted for hypertension treatment.
Additionally adjusted for cholesterol treatment.
Additionally adjusted for diabetes treatment.
Excludes individuals with diabetes.
Overall, the results for women were similar to those for men, with the exception that women had an association of neck circumference with total and LDL cholesterol. There was evidence of an interaction between sex and neck circumference for SBP (P < 0.0001), total cholesterol (P = 0.002), LDL cholesterol (P < 0.0001), HDL cholesterol (P = 0.0002), and fasting plasma glucose (P = 0.03). For all risk factors, women exhibited a larger effect size in risk factor levels per sd increment in neck circumference than men.
Logistic regression of dichotomous risk factors on neck circumference
Table 4 presents the odds ratios (OR) for categorized cardiometabolic risk factors per sd increase in neck circumference. Among both men and women, neck circumference was associated with increased odds of hypertension (P < 0.0001), low HDL cholesterol (P < 0.0001), high triglycerides (P < 0.0001), diabetes (P < 0.0001), metabolic syndrome (P < 0.0001), and HOMA-IR of 75th or higher percentile (P < 0.0001). In models that further adjusted for VAT or for BMI and waist circumference, neck circumference remained a significant correlate of all categorical cardiometabolic risk factors. In models that adjusted for both BMI and VAT, neck circumference was no longer a statistically significant correlate of hypertension and of diabetes in women but remained associated with low HDL, high triglycerides, metabolic syndrome, HOMA-IR of 75th or higher percentile, and diabetes in men.
Table 4.
Multivariablea logistic regression of cardiometabolic risk factors on neck circumference
| Men
|
Women
|
P interactionb | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Hypertension | |||||
| Multivariable | 1.62 (1.44–1.81) | <0.0001 | 1.90 (1.67–2.16) | <0.0001 | 0.07 |
| Multivariable + VAT | 1.25 (1.08–1.44) | 0.003 | 1.37 (1.14–1.66) | 0.0009 | |
| Multivariable + BMI, waist | 1.20 (1.00–1.44) | 0.05 | 1.36 (1.11–1.68) | 0.004 | |
| Multivariable + BMI, VAT | 1.12 (0.93–1.35) | 0.23 | 1.23 (0.98–1.53) | 0.07 | |
| Low HDL | |||||
| Multivariable | 1.72 (1.54–1.93) | <0.0001 | 1.90 (1.68–2.14) | <0.0001 | 0.23 |
| Multivariable + VAT | 1.11 (1.06–1.17) | <0.0001 | 1.39 (1.16–1.66) | 0.0003 | |
| Multivariable + BMI, waist | 1.65 (1.38–1.97) | <0.0001 | 1.61 (1.32–1.97) | <0.0001 | |
| Multivariable + BMI, VAT | 1.53 (1.28–1.83) | <0.0001 | 1.40 (1.14–1.73) | 0.002 | |
| High triglycerides | |||||
| Multivariable | 1.75 (1.57–1.95) | <0.0001 | 2.01 (1.77–2.29) | <0.0001 | 0.09 |
| Multivariable + VAT | 1.32 (1.16–1.51) | <0.0001 | 1.37 (1.14–1.65) | 0.0008 | |
| Multivariable + BMI, waist | 1.62 (1.37–1.92) | <0.0001 | 1.76 (1.44–2.17) | <0.0001 | |
| Multivariable + BMI, VAT | 1.48 (1.24–1.76) | <0.0001 | 1.48 (1.19–1.84) | 0.0004 | |
| Diabetes | |||||
| Multivariable | 2.28 (1.88–2.76) | <0.0001 | 2.26 (1.80–2.82) | <0.0001 | 0.85 |
| Multivariable + VAT | 2.16 (1.71–2.72) | <0.0001 | 1.52 (1.10–2.10) | 0.01 | |
| Multivariable + BMI, waist | 1.72 (1.28–2.30) | 0.0003 | 1.67 (1.17–2.40) | 0.005 | |
| Multivariable + BMI, VAT | 1.73 (1.29–2.33) | 0.0003 | 1.40 (0.95–2.06) | 0.09 | |
| Metabolic syndrome | |||||
| Multivariable | 3.38 (2.90–3.95) | <0.00001 | 4.33 (3.61–5.19) | <0.0001 | 0.04 |
| Multivariable + VAT | 2.06 (1.73–2.46) | <0.0001 | 2.34 (1.86–2.94) | <0.0001 | |
| Multivariable + BMI, waist | 1.63 (1.32–2.02) | <0.0001 | 2.52 (1.96–3.23) | <0.0001 | |
| Multivariable + BMI, VAT | 1.50 (1.20–1.86) | <0.0001 | 2.05 (1.57–2.67) | <0.0001 | |
| HOMA-IR ≥75th percentilec | |||||
| Multivariable | 3.20 (2.74–3.74) | <0.0001 | 3.32 (2.79–3.95) | <0.0001 | 0.80 |
| Multivariable + VAT | 2.10 (1.76–2.51) | <0.0001 | 1.91 (1.52–2.40) | <0.0001 | |
| Multivariable + BMI, waist | 1.75 (1.41–2.18) | <0.0001 | 1.88 (1.46–2.43) | <0.0001 | |
| Multivariable + BMI, VAT | 1.58 (1.26–1.97) | <0.0001 | 1.53 (1.17–2.01) | <0.0001 | |
Multivariable means adjusted for age, smoking, alcohol, menopausal status, and hormone replacement therapy use. Neck circumference and VAT are standardized to a mean of 0 and a sd of 1. OR is of risk factor per sd increase in neck circumference.
Interaction between neck circumference and sex.
Excludes individuals with diabetes.
Interaction between neck circumference and VAT
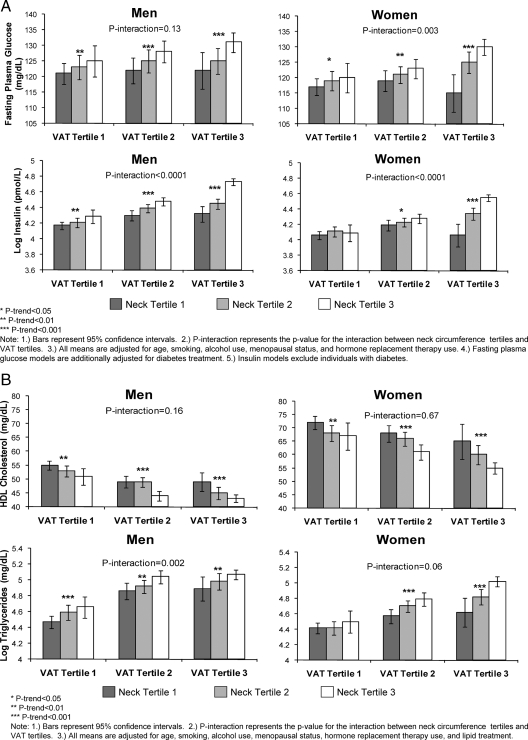
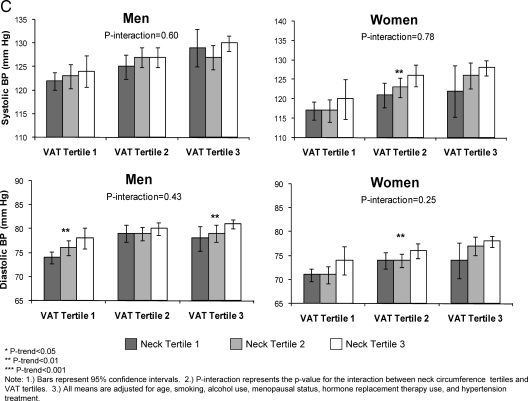
Figure 1, A–C, presents an analysis of the interaction between tertiles of neck circumference and tertiles of VAT on cardiometabolic risk factor levels. Within each tertile of VAT, there was a stepwise increase in risk factor levels by tertile of neck circumference. Among men, there was a significant interaction between neck circumference and VAT for insulin (P < 0.0001), proinsulin (P = 0.0009), HOMA-IR (P < 0.0001), total cholesterol (P = 0.0004), LDL cholesterol (P = 0.0002), and triglycerides (P = 0.008) (Fig. 1, A and B). Among women, there was a significant interaction between neck circumference and VAT for fasting plasma glucose (P = 0.003), insulin (P < 0.0001), proinsulin (P < 0.0001), HOMA-IR (P < 0.0001), and LDL cholesterol (P = 0.004) (Fig. 1, A and B).
Figure 1.
A, Multivariable adjusted fasting plasma glucose and log insulin levels by neck circumference and VAT tertiles; B, multivariable adjusted HDL cholesterol and log triglyceride levels by neck circumference and VAT tertiles; C (on next page), multivariable adjusted SBP and DBP by neck circumference and VAT tertiles.
Secondary analysis of incident CVD and CHD as an outcome
After a mean of 6.7 yr of follow-up, 178 incident CVD events (110 in men, 68 in women) and 109 CHD events (70 in men, 39 in women) occurred among the 3086 eligible participants at baseline. The hazard ratio for CVD per sd increase in neck circumference was 1.20 (95% CI = 1.04–1.38; P = 0.01) in the age- and sex-adjusted model and 1.05 (95% CI = 0.89–1.23; P = 0.56) in the multivariable-adjusted model. After further adjustment of the multivariable model for BMI and waist circumference, the hazard ratio for CVD was 1.10 (95% CI = 0.87–1.39; P = 0.45). For CHD, the hazard ratio was 1.13 (95% CI = 0.94–1.36; P = 0.19) in the age- and sex-adjusted model and 0.97 (95% CI = 0.79–1.19; P = 0.75) in the multivariable-adjusted model. After additional adjustment of the multivariable model for BMI and waist circumference, the hazard ratio for CHD was 0.90 (95% CI = 0.66–1.23; P = 0.52). There was no statistically significant interaction between neck circumference and sex for either CVD or CHD.
Discussion
In this study, we examined the association between neck circumference and cardiometabolic risk factors among participants in the Framingham Heart Study. First, neck circumference is associated with cardiometabolic risk factors. Second, neck circumference was more strongly associated with adverse risk factor levels in women compared with men. Third, neck circumference, VAT, and BMI independently contribute to cardiometabolic risk. Fourth, we observed an interaction between neck circumference and VAT for several cardiometabolic risk factors, where individuals who had both large neck circumference and high levels of VAT had the most adverse risk factor levels. Finally, there was no association between neck circumference and risk of incident CVD or CHD.
Recent research has focused extensively on body composition and CVD risk. Emphasis has been placed on whether an individual has an upper-body or lower-body fat distribution or what proportion of fat is stored in visceral vs. sc fat depots. Typically, central obesity, particularly high levels of upper-body visceral fat, is associated with adverse metabolic outcomes such as insulin resistance, diabetes, hypertension, and elevated triglycerides, whereas individuals with lower-body obesity tend to have lower levels of these adverse metabolic outcomes (19). Now, in the current study, we show that neck circumference, as a proxy of upper-body sc fat, is a novel, discrete, and pathogenic fat depot both independent of and synergistic with VAT. Although we observed that adjustment for VAT attenuates the association between neck circumference and cardiometabolic risk factors, it is important to note that most associations remained statistically significant.
In the context of the current literature
Several previous studies have examined the association between neck circumference and cardiometabolic risk factors. However, none of these previous studies have compared neck circumference directly with VAT with respect to their association with cardiometabolic risk factors. In a cross-sectional study of 43,595 women participating in the Take Off Pounds Sensibly (TOPS) Club, those with a self-reported neck circumference in the top tertile were found to have a 2-fold increased risk of diabetes relative to those in the bottom tertile, even after adjustment for multiple other measures of adiposity (9). In a cross-sectional analysis of 541 Finnish individuals, neck circumference in the highest quintile was associated with nearly a 5-fold increased risk of impaired fasting glucose in women after adjustment for BMI (10). No association was seen for men. Additionally, neck circumference was associated with approximately a 3-fold increased OR of hypertension, after adjustment for BMI, in both men and women. Although neck circumference is a proxy measure for upper-body sc fat, only one study has examined the association of upper-body sc fat as measured by MRI (11,20). Among 258 men from the control group of the Fat Redistribution and Metabolic Change in HIV Infection study, upper-body sc fat was shown to be independently associated with insulin resistance even after adjustment for VAT (20). Additional analyses of 145 control participants from the Fat Redistribution and Metabolic study showed that increased levels of upper-body sc fat were positively associated with LDL cholesterol and inversely associated with HDL cholesterol levels, after adjustment for demographic and lifestyle factors (11). In contrast, in our study, we did not observe any association between neck circumference and LDL cholesterol level among men.
One interesting finding from the present study was a greater association of neck circumference with cardiometabolic risk factors in women compared with men. This differential effect of neck circumference by sex has previously been observed (7). Previous analyses in the Framingham Heart Study have also shown that fat depots, especially VAT, are more strongly associated with an adverse risk factor profile in women compared with men (1). The mechanisms by which there is a stronger adverse effect associated with increased body fat in women are unknown. However, it has been suggested that in women, there is a greater proportion of free fatty acid delivery to the liver from VAT than in men (5).
It is possible that the association we observed between neck circumference and cardiometabolic risk factors may be mediated by its relationship to sleep-disordered breathing, which often occurs among individuals with larger neck circumferences. Previous studies have shown that sleep apnea is associated with hypertension, high total cholesterol, low HDL cholesterol, diabetes, and insulin resistance (21,22,23,24,25,26). However, the mechanisms to explain this association are unclear. Furthermore, it is unknown whether sleep apnea is a causal factor in the development of metabolic risk factors or if it is merely a correlate due to its strong association with obesity.
Potential biological mechanisms
Obesity and elevated levels of plasma free fatty acids are associated with insulin resistance and increased very-low-density lipoprotein triglyceride production (27,28,29). Increased levels of free fatty acids have also been correlated with markers of oxidative stress and vascular injury and are associated with the development of hypertension (29). Much of the literature has focused on the adverse effects of visceral fat; however, whereas visceral fat may be a marker for excess free fatty acids, it is not the primary source of circulating levels (30). It has been demonstrated that upper-body sc fat is responsible for a much larger proportion of systemic free fatty acid release than visceral fat, particularly in obese individuals (5). Obese men and women have a 2- to 3-fold larger fraction of fatty acids stored in sc fat compared with normal-weight men and women (6). The excess free fatty acid release associated with upper-body sc fat may be one mechanism to explain the association between neck circumference and cardiometabolic risk. Although free fatty acid release from upper-body sc fat is the primary contributor of abnormal free fatty acid metabolism in obese individuals, lipolysis of VAT is also an important contributor to hepatic free fatty acid delivery, which may explain why we observed an interaction between neck circumference and VAT (5).
Differences in free fatty acid metabolism between men and women may explain the sex differences we observed in the relationship between neck circumference and cardiometabolic risk factors. It has been shown that women store a much larger proportion of free fatty acids in sc tissue than do men (6,29,31). This difference in free fatty acid storage between men and women may account for the stronger association we found between neck circumference and cardiometabolic risk factors among women.
Implications for further research
Upper-body sc fat is a novel, easily measured fat depot, which may be an important predictor of cardiometabolic risk. This fat depot may lead to a better understanding of the differential effects of adiposity in men and women. Further studies are needed to examine the relationship between neck circumference and cardiometabolic risk factors in a longitudinal setting.
Strengths and limitations
Our study adds to the current literature by showing that neck circumference is a correlate of cardiometabolic risk after adjusting for levels of VAT. We were able to compare the effects of neck circumference and VAT in a large, well-defined cohort. One of the limitations of our study is that our results may not be generalizable to other racial or ethnic groups because the Framingham Heart Study is predominantly white. Additionally, this study was a cross-sectional, observation design so we cannot infer causality from our results. A final limitation is that neck circumference is a proxy for upper-body sc fat; we did not have radiographic measures to directly quantify this fat depot.
Conclusions
Neck circumference is associated with cardiometabolic risk factors even after adjustment for VAT. Further study of the role of upper-body sc fat in cardiometabolic risk is warranted.
Footnotes
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (N01-HC-25195) and by an American Diabetes Association Career Development Award (to J.B.M.).
This manuscript was presented as a poster at the 49th Cardiovascular Disease Epidemiology and Prevention Annual Conference, Palm Harbor, FL, 2009.
S.R.P. and C.S.F. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: J.B.M. was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant K24 DK080140, is the current recipient of research grants from GlaxoSmithKline and Sanofi-Aventis, and serves on consultancy boards for GlaxoSmithKline, Sanofi-Aventis, Interleukin Genetics, Kalypsis, and Outcomes Sciences. R.S.V. is supported in part by 2K24HL04334 [National Heart, Lung, and Blood Institute, National Institutes of Health (NIH)] and RO1DK080739 (NIDDK/NIH). The additional authors have nothing to disclose.
First Published Online May 19, 2010
Abbreviations: BMI, Body mass index; CI, confidence interval; CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; MDCT, multidetector computed tomography; OR, odds ratio; SAT, sc adipose tissue; SBP, systolic blood pressure; VAT, visceral adipose tissue.
References
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino Sr RB, O'Donnell CJ 2007 Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB 2005 Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 165:777–783 [DOI] [PubMed] [Google Scholar]
- Ding J, Kritchevsky SB, Hsu FC, Harris TB, Burke GL, Detrano RC, Szklo M, Criqui MH, Allison M, Ouyang P, Brown ER, Carr JJ 2008 Association between non-subcutaneous adiposity and calcified coronary plaque: a substudy of the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 88:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrini S, Leonardini A, Laviola L, Giorgino F 2008 Biological specificity of visceral adipose tissue and therapeutic intervention. Arch Physiol Biochem 114:277–286 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD 2004 Splanchnic lipolysis in human obesity. J Clin Invest 113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsari C, Snozek CL, Jensen MD 2008 Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 51:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Noun L, Laor A 2003 Relationship of neck circumference to cardiovascular risk factors. Obes Res 11:226–231 [DOI] [PubMed] [Google Scholar]
- Ben-Noun LL, Laor A 2006 Relationship between changes in neck circumference and cardiovascular risk factors. Exp Clin Cardol 11:14–20 [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Rimm AA 1989 The relation of body fat distribution, as assessed by six girth measurements, to diabetes mellitus in women. Am J Public Health 79:715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M, Matilainen V, Keinänen-Kiukaanniemi S 2002 Association of neck circumference with insulin resistance-related factors. Int J Obes Relat Metab Disord 26:873–875 [DOI] [PubMed] [Google Scholar]
- Wohl D, Scherzer R, Heymsfield S, Simberkoff M, Sidney S, Bacchetti P, Grunfeld C 2008 The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr 48:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore Jr FE 1951 Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 41:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber TR, Kannel WB, Lyell LP 1963 An approach to longitudinal studies in a community: the Framingham Study. Ann NY Acad Sci 107:539–556 [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP 1979 An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110:281–290 [DOI] [PubMed] [Google Scholar]
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino Sr RB, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D 2007 The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 165:1328–1335 [DOI] [PubMed] [Google Scholar]
- Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U 2007 Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 31:500–506 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults 2001 Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Jensen MD 2008 Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93(11 Suppl 1):S57–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C, Rimland D, Gibert CL, Powderly WG, Sidney S, Shlipak MG, Bacchetti P, Scherzer R, Haffner S, Heymsfield SB 2007 Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr 46:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora RN, Punjabi NM 2007 Sleep apnea and metabolic dysfunction: cause or co-relation? Sleep Med Clin 2:237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG 2000 Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283:1829–1836 [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J 2000 Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384 [DOI] [PubMed] [Google Scholar]
- Reichmuth KJ, Austin D, Skatrud JB, Young T 2005 Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 172:1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche F, Sforza E, Pichot V, Maudoux D, Garcin A, Celle S, Picard-Kossovsky M, Gaspoz JM, Barthélémy JC 2009 Obstructive sleep apnoea/hypopnea influences high-density lipoprotein cholesterol in the elderly. Sleep Med 10:882–886 [DOI] [PubMed] [Google Scholar]
- Tishler PV, Larkin EK, Schluchter MD, Redline S 2003 Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA 289:2230–2237 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mokan M, Simoneau JA, Mandarino LJ 1993 Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah AH, Alfarsi S, Adams PW, Wynn V 1976 Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 12:563–571 [DOI] [PubMed] [Google Scholar]
- Santosa S, Jensen MD 2008 Why are we shaped differently, and why does it matter? Am J Physiol Endocrinol Metab 295:E531–E535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Hensrud DD, Johnson CM, Jensen MD 1999 Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 48:1586–1592 [DOI] [PubMed] [Google Scholar]
- Santosa S, Hensrud DD, Votruba SB, Jensen MD 2008 The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr 88:1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]