Abstract
Context: GNAS encodes the α-subunit of the stimulatory G protein as well as additional imprinted transcripts including the maternally expressed NESP55 and the paternally expressed XLαs, antisense, and A/B transcripts. Most patients with pseudohypoparathyroidism type Ib (PHP-Ib) exhibit imprinting defects affecting the maternal GNAS allele, which are thought to reduce/abolish Gsα expression in renal proximal tubules and thereby cause resistance to PTH.
Objective: Our objective was to define the genetic defect in a previously unreported family with autosomal dominant PHP-Ib.
Design and Setting: Analyses of serum and urine chemistries and of genomic DNA and lymphoblastoid-derived RNA were conducted at a tertiary hospital and research laboratory.
Patients: Affected individuals presented with muscle weakness and/or paresthesia and showed hypocalcemia, hyperphosphatemia, and elevated serum PTH. Obligate carriers were healthy and revealed no obvious abnormality in mineral ion homeostasis.
Results: A novel 4.2-kb microdeletion was discovered in the affected individuals and the obligate carriers, ablating two noncoding GNAS antisense exons while preserving the NESP55 exon. On maternal transmission, the deletion causes loss of all maternal GNAS imprints, partial gain of NESP55 methylation, and PTH resistance. Paternal transmission of the mutation leads to epigenetic alterations in cis, including a partial loss of NESP55 methylation and a partial gain of A/B methylation.
Conclusions: The identified deletion points to a unique cis-acting element located telomeric of the NESP55 exon that is critical for imprinting both GNAS alleles. These findings provide novel insights into the molecular mechanisms underlying PHP and GNAS imprinting.
A mutation deleting GNAS antisense exons 3 and 4 but not NESP55 causes pseudohypoparathyroidism-Ib upon maternal inheritance and GNAS imprinting defects upon both maternal and paternal inheritance.
A small but significant number of mammalian genes are regulated in such an intricate manner that the expression occurs from a single allele in a parent-of-origin specific manner. Referred to as genomic imprinting, this type of gene regulation involves epigenetic modifications and often the expression of noncoding RNAs, but the underlying mechanisms have yet to be precisely deciphered (1,2,3). An imprinted locus on chromosome 20q13.3 comprises GNAS, which encodes the α-subunit of the stimulatory G protein (Gsα), a ubiquitous signaling protein essential for many biological processes (4,5). Using distinct promoters and first exons, at least four additional, imprinted gene products are derived from GNAS: XLαs, A/B (also referred to as 1A), and antisense transcripts are expressed paternally, whereas the NESP55 transcript is expressed maternally (6,7,8,9,10,11,12) (Fig. 1A). In contrast, Gsα expression is biallelic in most tissues; however, paternal Gsα expression is silenced, at least partially, in certain tissues, including proximal renal tubules, pituitary, and thyroid. The promoters for the imprinted GNAS transcripts, but not the promoter for Gsα, are located within differentially methylated regions (DMRs), and transcription occurs from the nonmethylated allele. In addition to these products, a distinct amino-terminally extended XLαs variant, termed XXLαs (13,14), and alternative translational products of XLαs and XXLαs transcripts, termed ALEX (15) and ALEXX (13), respectively, have been described, adding further complexity to this imprinted gene cluster.
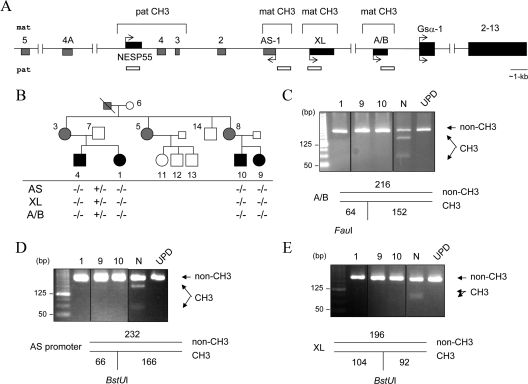
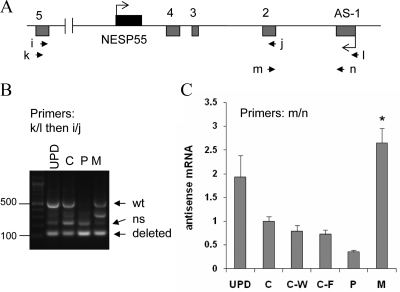
Figure 1.
The novel AD-PHP-Ib kindred and GNAS methylation status. Panel A, Schematic representation of the GNAS locus. Maternal (mat) and paternal (pat) alleles and DMRs are indicated. Boxes and connecting lines represent exons and introns, respectively; exons of the antisense (AS) transcripts are in gray. Arrows indicate the direction and parental origin of transcription. White horizontal bars indicate the regions analyzed for methylation. Panel B, The AD-PHP-Ib kindred and the GNAS methylation status. White, black, and gray symbols indicate unaffected, affected, and unaffected carriers, respectively. The presence (+) or absence (−) of methylation was determined by COBRA. Results for individuals 1, 9, and 10, as well as a normal control (N) and a patient with patUPD20q (UPD) are shown in panels C (exon A/B), D (antisense promoter), and E (exon XL). The size of each amplicon and the digestion products are depicted below the images of ethidium bromide-stained agarose gels; non-CH3 is the product derived from the nonmethylated allele, and CH3 is the product from the methylated allele. All samples for a given product were analyzed within the same experiment and digestion products were separated on the same gel; vertical black lines indicate intervening lanes with unrelated samples, which were digitally removed; some images are overexposed to ensure the presence or the absence of digestion products.
At least two noncoding RNAs are derived from GNAS, including the antisense (termed Nespas in mice) and the A/B transcript. Mouse studies have identified female germline imprint marks at the promoters of these two transcripts (16,17). The genomic region comprising the nonmethylated paternal promoter of the 1A transcript is required for the tissue-specific silencing of Gsα expression in cis, as determined by the findings in mice with paternal deletion of the 1A DMR (18,19). On the other hand, deletion of the paternal Nespas promoter in mice abolishes this transcript and relaxes Nesp55 imprinting in cis (20). The mutant mice also show additional changes on the paternal Gnas allele, including hypermethylation of the exon 1A DMR and reduced 1A transcription, which is associated with derepressed Gsα expression in tissues where expression of this gene product is normally silenced. It remains unclear whether these additional alterations result from loss of the genomic region comprising the Nespas promoter, lack of the antisense transcript, or derepressed Nesp55 transcription.
Pseudohypoparathyroidism type-Ib (PHP-Ib) (MIM 603233) is a rare genetic disorder characterized by end-organ resistance to the proximal tubular actions of PTH and, in some cases, to the actions of TSH. Most patients with this disorder show epigenetic alterations within GNAS, and a loss of exon A/B methylation on the maternal allele is the most consistent defect (21,22,23). Biallelic absence of exon A/B methylation is thought to silence Gsα expression on both parental alleles in the proximal tubules, thereby leading to little or no Gsα protein in this tissue and, thus, to PTH resistance. Microdeletions within the gene encoding syntaxin-16 (STX16), a distinct locus located approximately 200 kb upstream of GNAS, are found in patients with autosomal dominant (AD)-PHP-Ib, who show an isolated loss of exon A/B imprinting (24,25,26,27,28,29,30,31). The identified STX16 deletions define a long-range cis-acting element regulating the establishment and/or maintenance of the methylation imprint at GNAS exon A/B. Other PHP-Ib patients display broad GNAS imprinting defects, which include loss of methylation at exon A/B, exon XL, and the antisense promoter as well as gain of exon NESP55 methylation (21,22,24,26,27,32). In two AD-PHP-Ib kindreds displaying such broad epigenetic abnormalities, microdeletions comprising the entire NESP55 DMR have been identified (33). These approximately 4-kb deletions are nearly identical and include, in addition to exon NESP55, exons 3 and 4 of the GNAS antisense transcript (delNESP55/delAS3-4). Thus, although another cis-acting imprinting regulatory element of GNAS is predicted to be disrupted by these deletions, it remains unknown whether the observed imprinting changes and PTH resistance in these patients result from the loss of NESP55 expression or the loss of the deleted genomic region.
We have now investigated a new AD-PHP-Ib kindred with broad epigenetic defects at the GNAS locus and identified a novel deletion that exclusively affects exons encoding the antisense transcript (delAS3-4). Overlapping with the previously identified deletions by approximately 1.5 kb, the novel deletion reduces the size of the critical interval predicted to harbor the cis-acting element controlling imprinting of the entire maternal GNAS allele. Moreover, unlike the previously identified deletions associated with AD-PHP-Ib, the novel deletion not only disrupts methylation of three GNAS DMRs (A/B, AS, and XL) after maternal transmission but also appears to partially alter methylation of the NESP55 and the A/B DMRs after paternal transmission, revealing a novel cis-acting mechanism that governs imprinting on both parental alleles.
Materials and Methods
Kindred with AD-PHP-Ib
The proband (individual 1) is now a 29-yr-old Chinese female, who presented to the Nephrology Department at the Kaohsiung Medical University Hospital with hypocalcemia of 5.7 mg/dl. She had been investigated at another hospital for intermittent muscle weakness and paresthesias that had started about 5 yr ago; there was no history of seizures. It was also noted that she had complained of muscle weakness while in junior high school, especially during/after physical exercise. She had delayed tooth eruption, but development of her teeth was normal. At the age of 19 yr, she was furthermore diagnosed with Hashimoto’s thyroiditis. She has a college degree and works as a registered nurse. Her height was 158.75 cm, but her physical examination revealed no signs of Albright’s hereditary osteodystrophy. Her calcium was found to be low, whereas her phosphorus and PTH levels were elevated (Table 1 and Fig. 1); serum TSH was 7.1 mU/liter (normal, 0.4–5 mU/liter). A thyroid scan showed diffuse goiter and brain magnetic resonance imaging revealed basal ganglion calcifications. She was treated with calcium supplements, calcitriol, and levothyroxine. Two years later, her total calcium was 7.8 mg/dl, and her serum PTH had improved slightly to 381.1 pg/dl.
Table 1.
Biochemical features of the individuals from the AD-PHP-Ib kindred
| Affected, carrier
|
Unaffected, carrier
|
Unaffected, not carrier
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4a | 9a | 10 | 3 | 5 | 8 | 6 | 11 | 12 | 13 | 14 | |
| Age at diagnosis/investigation (yr) | 20 | 18 | 8 | 20 | 54 | 57 | 51 | 84 | 35 | 33 | 30 | 60 |
| Calcium (8.6–10.3 mg/dl) | 5.7 | 6.3 | 8.7 | 5.1 | 8.4 | 8.9 | 9.0 | 8.9 | 9.1 | 8.7 | 8.3 | 9.0 |
| Phosphorus (2.5–4.5 mg/dl) | 5.2 | 5.8 | 4.0 | 6.6 | 4.4 | 3.7 | 4.0 | 2.9 | 3.2 | 3.5 | 3.8 | |
| PTH (10–65 pg/dl) | 480 | 181 | 50.4 | 204 | 9 | 33 | 32 | |||||
| Urinary Ca/creatinine (<0.2 mg/mg) | 0.2 | 0.06 | 0.04 | 0.04 | 0.08 | 0.08 | 0.01 | 0.19 | ||||
| 25(OH)D (9.7–41.7 ng/ml) | 27.9 | 31.3 | ||||||||||
| 1,25(OH)2D (15.9–55.6 pg/ml) | 75.5 | 41.3 | ||||||||||
For conversion to SI units multiply with the following factor: calcium, 0.25; phosphorus, 0.323; 25(OH)D, 2.496; 1,25(OH)2D, 2.6.
Blood samples were collected while under treatment.
The proband’s brother (individual 4), who is now 27 yr old, was also found to have hypocalcemia (Table 1) but less severe muscle weakness than his sister. Brain computed tomography showed diffuse calcifications. Her 18-yr-old cousin (individual 9) also had a history of muscle weakness since childhood, and she was previously noted to have low calcium levels, for which she received oral calcium supplements. At the age of 8 yr, she was also diagnosed with Hashimoto’s thyroiditis. The proband’s 22-yr-old cousin (individual 10) was found to have only mild symptoms of muscle weakness, but laboratory investigations revealed low serum calcium, high phosphate, and high PTH (Table 1); serum TSH level was elevated (value not available). The proband’s mother (individual 3) was noted to have low serum PTH and elevated 1,25 dihydroxyvitamin D, but no other biochemical or clinical abnormalities were present.
These clinical/laboratory findings and inspection of the pedigree, which suggested an autosomal dominant mode of inheritance, were consistent with the diagnosis of AD-PHP-Ib. Informed consent was obtained from each individual, and the study was approved by the Institutional Review Board of Massachusetts General Hospital.
Bisulfite-modified genomic DNA sequencing and combined bisulfite restriction enzymatic analysis (COBRA)
Genomic DNA (1 μg) was digested overnight with restriction enzyme XbaI and then purified using QIAquick PCR purification kit (QIAGEN, Valencia, CA). The digested DNA was denatured by adding 5.5 μl 3 m NaOH and incubating at 37 C for 15 min. Subsequently, 30 μl 10 mm hydroquinone (Sigma) and 520 μl sodium bisufite (Sigma) was added, and the mixture was incubated at 55 C overnight. After cleaning with the Geneclean II kit (Qbiogene, Irvine, CA), modified products were deaminated by the addition of 5.5 μl NaOH (Sigma) and incubation for 15 min at 37 C. The products were then ethanol precipitated and reconstituted in 15 μl Tris-EDTA (10 mm/1 mm) buffer. Two microliters of the modified product were used in 50 μl PCR (primary and nested PCRs were performed). For COBRA, nested PCR products were digested with restriction enzyme AciI (NESP55), FauI (A/B), or BstUI (AS promoter and A/B) and separated on 3% MetaPhor agarose gel. Restriction enzymes were purchased from New England Biolabs (Beverly, MA). For sequencing, 1 μl of each undigested nested PCR product was subcloned by using the TOPO cloning kit for sequencing (Invitrogen, Carlsbad, CA). Cloned products were sequenced at the Massachusetts General Hospital DNA Core Facility to identify the number of modified (nonmethylated) colonies in comparison with the unmodified (methylated) colonies. The proportion of methylated vs. nonmethylated clones derived from genomic DNA of patients or unaffected carriers was compared with the proportion obtained from genomic DNA of an unaffected individual in the same experiment. Amplification primers, in forward and reverse order, were as follows: NESP55 DMR primary, 5′-GAGGATAAAGATTTAAGGGATTT-3′ and 5′-CTCAAACTCCCCAATTTAAC-3′, and nested, 5′-GAAGGAGTTTAAGGAGGAGAAGTAG-3′ and 5′-CCATAAAAACAAAAAAAATCTAAAC-3′; XL DMR primary, 5′-GGTAGTTTATTTTAAGAGGTTGTTAGATTT-3′ and 5′-AAAAAAATACTTTTCCTCCCTCC-3′, and nested, 5′-GGGTAGTAGTTTTTGGATGGAGAT-3′ and 5′-CATCTCTACTACTTCCTCCTCAACTAAA-3′; A/B DMR primary, 5′-TTTTGTTTTTTTTTTGTTTGTTTAT-3′ and 5′-ACAACTTCAACAACCACCTCAACAAC-3′, and nested, 5′-TAAACTTCATAACCATCTTCAACATAA-3′ and 5′-TTAATTTTTAGGTAGTTAGTTTAGTAGTT-3′; and AS promoter DMR primary, 5′-TGTGTATATATTAAGGTTATTAGGTG-3′ and 5′-AAAAATTTTAATTAAAATTTAATACC-3′, and nested, 5′-GGTGTGGGTATTTATTTTTGGTTAGT-3′ and 5′-TAATCAATCAACTCCTTTAACCCC-3′.
Southern blot analysis and PCR amplification across the heterozygous deletion
Genomic DNA was digested with restriction enzymes BamHI or XmnI, separated on a 0.8% agarose gel, and transferred onto a nitrocellulose membrane. Probe P1 (106195–107583, according to GenBank accession number AL132655) was from the NESP55 region. Probe P2 (113720–115315 according to AL132655) was located upstream of exon XL. The mutant allele was amplified by PCR using primers flanking the deletion breakpoint. Forward primer (e) was 5′-GGCAGACCTTGAGCTGTCC-3′, and reverse primer (f) was 5′-GCATGTATGATGGAGGCAAA-3′. The mutant allele was also amplified by duplex PCR using primer pairs flanking each of the deletion breakpoints. Primers used for the duplex PCR were as follows: primer a, 5′-TTAGTTGCCCACCGCTAAAC-3′; primer b, 5′-CCAGGACGTCTCCTGCTAAG-3′; primer c, 5′-TCAAGTGGCCTTAGGTCAGA-3′; and primer d, 5′-AGCTATTGGAGGCGTTTGAA-3′. Conditions are available upon request.
Methylation analysis of the overlapping segment
The methylation status of two small segments within the 1519-bp region of overlap between the two previously identified deletions and the deletion identified in this study was analyzed by direct sequencing of PCR products amplified from bisulfite-modified genomic DNA of a noncarrier individual, an affected individual, an unaffected carrier, and a patient with paternal uniparental isodisomy of chromosome 20q (patUPD20q). The following primary PCR primers, designed by using MethPrimer (34), were used for the amplification of both regions together: 5′-TTTAGGTTTTAGAGTTGATAATTAAGT-3′ and 5′-AAACAAATTCCCCTCCAAATTAC-3′ (108095-108121 and 108739–108761, respectively, according to AL132655). CpG island 1 was then amplified by using the same forward primer and the following nested reverse primer: 5′-TAAAAACATCCCCAAAAATCC-3′ (108313–108333 according to AL132655). CpG island 2 was amplified by using the following nested forward and reverse primers: 5′-GGATTTTTGGGGATGTTTTTA-3′ and 5′-AATTCCCCTCCAAATTACAAATTA-3′ (108313–108333 and 108733–108756, respectively, according to AL132655).
RT-PCR amplification of the GNAS antisense transcript
Total RNA was extracted from lymphoblastoid cell lines (LCLs) of controls (healthy individuals), a patient with patUPD20q, an unaffected carrier, and an affected individual by using the Trizol reagent (Invitrogen). Total RNA was also isolated from the whole blood by using the PAXgene kit (QIAGEN). Superscript III (Invitrogen) was used to reverse transcribe 1 μg total RNA according to the manufacturer’s instructions. Nonquantitative RT-PCR was done by previously described primers 88F3 (primer k) and 88R8 (primer l) (6). The products were reamplified by using primer 88F5 (primer i; see Ref. 6) and the following reverse primer: 5′-ACTGGCTACCAGACTCTGAAAAAC-3′ (primer j). Real-time RT-PCR was performed by using the Quantitect SYBR Green PCR kit (QIAGEN), and the data were analyzed as previously described (35) by using the Q-gene module (36). Primer m was 5′-GGTTTTTCAGAGTCTGGTAGCC-3′, and primer n was 5′-TGAGGAGCAAGAAGATTTCCA-3′.
Statistical analyses
Data from quantitative real-time RT-PCR experiments were analyzed by first calculating the mean ± sem of four to six independent experiments for each sample and then performing one-way ANOVA followed by Tukey’s multiple-comparison test. To determine the statistical significance of differences obtained from the methylation analysis of the NESP55 DMR in affected individuals and of the A/B DMR in unaffected carriers, Fisher’s exact test was performed. P values <0.05 were considered significant. The GraphPad Prism software was used for the statistical analyses.
Results
We investigated a previously unreported AD-PHP-Ib kindred in which affected individuals presented with hypocalcemia, hyperphosphatemia, and elevated serum PTH (Table 1). Data from microsatellite analyses, although only partially informative, were consistent with linkage to the chromosomal region comprising GNAS (Supplemental Data, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). The mutations within or upstream of GNAS that are known to cause AD-PHP-Ib (30,33) were excluded in the affected index case of this family. Methylation analysis of the four GNAS DMRs by using COBRA showed that affected individuals had a loss of all maternal imprints, i.e. loss of methylation at exon A/B, the antisense promoter, and exon XL (Fig. 1, B–E). The methylation of the NESP55 DMR of these individuals appeared to differ from the methylation observed for a normal individual at this site; i.e. there was increased abundance of the digested products indicating methylated DNA. Although this finding suggested gain of methylation at the NESP55 DMR, the banding pattern was markedly different from that observed in a previously described patient with paternal uniparental isodisomy of chromosome 20q (patUPD20q) (32) (Fig. 2A). The amplicons derived from bisulfite-treated DNA were therefore cloned, and nucleotide sequence analysis was performed on individual clones. Compared with a normal control, DNA from affected individuals yielded significantly more clones representing the methylated allele (containing C) than the nonmethylated allele (containing T). Consistent with COBRA, the results were different from those obtained from the patient with patUPD20q, which revealed only clones from the methylated allele (Fig. 2B). Thus, the affected individuals of this new family had incomplete gain of methylation at the NESP55 DMR.
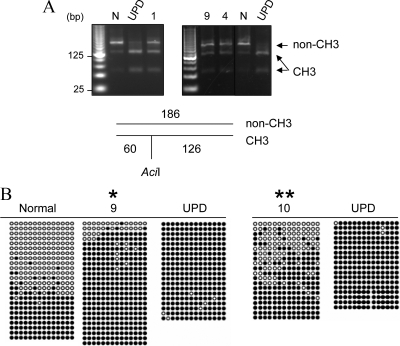
Figure 2.
Methylation analysis of the NESP55 DMR. A, Results of COBRA are shown for individuals 1, 9, and 4, as well as a normal control (N) and a patient with patUPD20q (UPD). The size of the amplicon and the AciI digestion products are depicted below the gel image; non-CH3 is the product derived from the nonmethylated allele, and CH3 is the product from the methylated allele. Samples investigated at the same time are separated on the same gel; the vertical line in the image on the left indicates intervening lanes with unrelated samples, which were digitally removed. B, Each row of circles represents cloned amplicons from bisulfite-treated genomic DNA of individual 7 or 9 or the individual with patUPD20q (UPD). In a separate experiment, the analysis was also performed by using DNA from individual 10 and patUPD20q. Black circles represent methylated CpG; white circles represent nonmethylated CpG. The segment for the methylation analysis is indicated in Fig. 1A. Significantly different from normal (*, for individual 9) or UPD (**, for individual 10) according to Fisher’s exact test (P < 0.05; two-tailed).
The incomplete nature of the methylation changes at the NESP55 DMR suggested that this region was not deleted in our kindred. We thus performed Southern blot analyses using a probe from the NESP55 region, which detected the expected wild-type fragments (∼7.3 kb with BamHI and ∼8.9 kb with XmnI) in genomic DNA from both the healthy control and affected individual 9 (Fig. 3A). However, the affected individual 9 showed additional hybridizing fragments upon both BamHI (∼11 kb) and XmnI (∼4.7 kb) digestion. These data suggested the existence of a novel microdeletion in this region, and this was confirmed by using another probe located upstream of exon XL (Fig. 3A). The length of the additional hybridizing bands suggested that the microdeletion was approximately 4.2 kb in size and that it removed the BamHI site located within antisense exon 4 (Fig. 3A). Using primers that were predicted to flank the breakpoints of this microdeletion (primers e and f), we were able to amplify the deleted allele by PCR from genomic DNA of an affected individual, and the same primers were used to assess the presence or absence of the mutation in all members of the family (Fig. 3B). Nucleotide sequence analysis of the PCR product revealed that the mutation deleted precisely 4174 bp (nucleotides 107922–112095 according to AL132655 and 56849752–56853925 according to NCBI36/hg18), which was combined with insertion of a CTTT tetranucleotide at the deletion site (Fig. 3C). Based on these data, the deletion removes antisense exons 3 and 4 but not exon NESP55 (Fig. 3A). The identified deletion could also be detected in genomic DNA from an affected individual, but not from a noncarrier, through the use of duplex PCR, which used two separate primer pairs designed to amplify the wild-type allele at each breakpoint (Fig. 3D). The novel deletion overlaps with the previously reported delNESP55/delAS3-4 by an approximately 1.5-kb DNA fragment, which harbors two large and one small CpG island (Fig. 4A). Using bisulfite-modified genomic sequencing and DNA from an individual with no deletion, an unaffected carrier (paternal deletion), an affected individual (maternal deletion), and the aforementioned patient with patUPD20q (32), we analyzed the methylation status in the two larger CpG islands, showing that the overlapping region is methylated in healthy individuals on the paternal but not on the maternal allele; i.e. this region has the same differential methylation pattern as the rest of the NESP55 DMR (Fig. 4B).
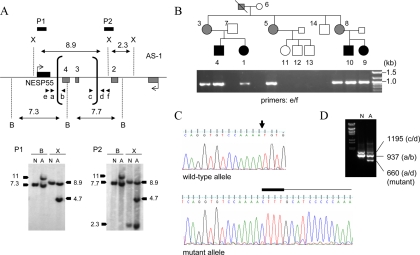
Figure 3.
Identification and analysis of the novel GNAS deletion causing AD-PHP-Ib. Panel A, Southern blot analysis of genomic DNA from a normal control (N) and the affected individual 9 (A), indicating the novel antisense deletion. Horizontal bars represent locations of the probes P1 and P2; vertical brackets, deletion break points (determined precisely by PCR shown in panel B); arrowheads, primers used for the amplification of the deleted allele; B, BamHI; X, XmnI. Note that the slight size differences between the wild-type hybridizing bands from the normal and the affected individual reflect a gel running artifact due possibly to differences in the salt concentration. Panel B, PCR amplification of the deleted allele using primers located upstream and downstream of the deletion breakpoints. Pedigree is shown above the ethidium bromide-stained agarose gel showing the amplicons. White, black, and gray symbols indicate unaffected, affected, and unaffected carriers, respectively. The expected wild-type product of approximately 5 kb did not amplify, and hence an approximately 1-kb band is seen in all the individuals of this AD-PHP-Ib kindred who are heterozygous for the deletion. Panel C, Nucleotide sequence analysis of the PCR products derived from the mutant allele. In the wild-type sequence, the deletion site is indicated by an arrow. In the mutant sequence, the CTTT insertion and the sequence from the telomeric end of the deletion are indicated above the sequence chromatogram by thick and thin horizontal lines, respectively. Panel D, Duplex PCR amplification of the wild-type and deleted alleles by using primer pairs at each of the deletion breakpoints (arrowheads in panel A).
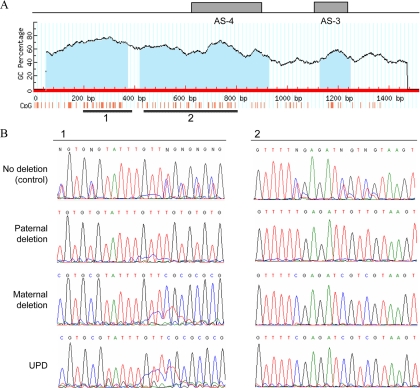
Figure 4.
Methylation status of the region that overlaps the previously identified deletions (AL132655 nucleotides 107922–109440). A, CpG island prediction revealed three CpG islands (blue shading) based on the following criteria: island size over 100, GC percent over 50.0, and observed/expected CpG ratio over 0.6. Regions within CpG island 1 and CpG island 2 analyzed by bisulfate-modified genomic sequencing are depicted by thick horizontal lines. The locations of antisense exons 3 and 4 are indicated. B, Direct nucleotide sequence analysis was performed on PCR-amplified bisulfite-modified genomic DNA from the noncarrier healthy individual 12 (no deletion control), the unaffected carrier 5 (paternal deletion), the affected individual 9 (maternal deletion), and the patient with patUPD20q (UPD).
To analyze the antisense transcript derived from the GNAS region comprising the novel deletion, we established lymphoblastoid cells (LCL) from one of the patients (individual 1) and her unaffected carrier mother (individual 3). Nested RT-PCR using primers located on either side of the deletion (exons 5 and 1 for primers k and l; exons 5 and 2 for primers i and j) amplified a product of roughly 500 bp (predicted size is 527 bp according to Ref. 6) when using total RNA obtained from the LCLs of the affected individual but not the unaffected carrier (Fig. 5A, B). This product was also amplified when we used, as controls, LCL total RNA from the patient with patUPD20q (32) and a healthy individual (Fig. 5B). The deletion of exons 3 and 4 is predicted to reduce the size of the antisense transcript by 406 bp. Indeed, in both the affected patient and the unaffected carrier, we observed a novel amplification product that is almost 100 bp in size. However, a PCR product of similar size was also amplified from control RNA (Fig. 5B), which is consistent with previously identified splice variants that are derived from the antisense pre-mRNA (6). However, compared with the band intensity of the wild-type 527-bp product in the same lane, the short product obtained from the affected individual (lane 4) appeared to be more abundant than that obtained from the controls (lanes 1 and 2), thus suggesting that the antisense transcript comprising the novel deletion could be amplified (Fig. 5B). To provide more definitive evidence for this conclusion, we performed real-time RT-PCR using primers located on exon 2 and exon 1 (primers m and n); note that these primers yielded only a single product of 159 bp from both the affected individual and the unaffected carrier (data not shown). Quantitative analysis of the data showed that the affected individual had significantly elevated levels of the antisense transcript in LCLs than controls (Fig. 5C), which was consistent with the loss of imprinting at the antisense promoter. The unaffected carrier tended to express lower levels of the antisense transcript than the controls, although this reduction did not reach statistical significance (Fig. 5C).
Figure 5.
Amplification of the antisense transcript by RT-PCR using total RNA extracted from LCLs. Panel A, Depiction of GNAS antisense and NESP55 exons. Boxes represent exons; connecting lines, introns; arrowheads, locations of the PCR primers. Panel B, Amplicons generated by nested RT-PCR by using primers k and l followed by primers i and j. Multiple amplicons are due to alternative splicing, as described (6); arrows indicate expected positions of the longest amplicons from the wild-type (wt; 527 bp) and deleted alleles (121 bp). ns, Nonspecific; UPD, patient with patUPD20q; C, healthy normal control; P, unaffected carrier with paternal deletion (individual 3); M, affected individual with maternal deletion (individual 1). Panel C, Real-time RT-PCR results using total RNA from LCLs and primers m and n (mean ± sem of four to six experiments). C-W and C-F are unrelated patients with AD-PHP-Ib, who have STX16 deletions and a loss of exon A/B methylation but show normal differential methylation at the antisense promoter; the methylation status of the antisense promoter was normal in these controls, as reported (28,30,32). Data were normalized to C; *, Value significantly different from C, C-W, and C-F by one-way ANOVA/Tukey’s multiple-comparison test.
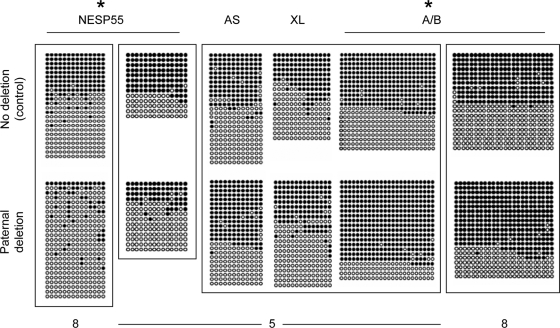
To determine whether any GNAS methylation abnormalities existed in the genomic DNA of unaffected carriers, we performed nucleotide sequence analysis of bisulfite-modified genomic DNA and revealed that paternal inheritance of the deletion causes a partial loss of NESP55 methylation (Fig. 6). Nucleotide sequence analysis of cloned products derived from the bisulfite-treated DNA also indicated a modest gain of A/B methylation in unaffected carriers, who displayed normal methylation at the antisense promoter and exon XL (Fig. 6).
Figure 6.
Methylation analysis of GNAS DMRs using DNA from healthy controls (no deletion; upper panels) and from individuals with paternal inheritance of delAS3–4 (paternal deletion; lower panels). Amplicons obtained from bisulfite-modified genomic DNA were cloned and sequenced. Results are shown for individual 7, who has no deletion, and individuals 5 (NESP55, AS, XL, and A/B) or 8 (NESP55, A/B), who have paternal deletions. DNA samples that were bisulfite treated in the same experiment are placed in the same frame. Each row of circles represents a single clone containing multiple CpG dinucleotides; nonmethylated CpG (white circles); methylated CpG (black circles). *, Pooled data from the analyzed unaffected carriers are significantly different from no deletion controls according to Fisher’s exact test (P < 0.05; two-tailed).
Discussion
By investigating a new AD-PHP-Ib kindred, we discovered a novel deletion that exclusively involves GNAS antisense exons but not exon NESP55. Indistinguishable from the two previously identified deletions of the NESP55 DMR, maternal inheritance of the novel deletion results in loss of all maternal GNAS imprints and in PTH resistance in the renal proximal tubule. Chotalia et al. (37) have recently shown that premature truncation of the Nesp55 transcript in mice causes defects in the establishment of maternal Gnas imprints, making it possible that the deletion in our patients has a similar effect on the NESP55 transcript. Alternatively, a cis-acting genomic element controls imprinting throughout the maternal GNAS allele, which is now predicted to be located within the 1.5-kb region defined by the overlap between the previously reported deletions and the deletion reported herein. Besides the loss of all three maternal methylation imprints, the affected individuals in our new AD-PHP-Ib kindred demonstrate a partial gain of NESP55 methylation. Consistent with the finding in mice, namely that the Nespas transcript silences Nesp55 expression in cis (20), this epigenetic change at exon NESP55 likely results from maternal derepression of the antisense transcript. It remains unclear, however, why the gain of NESP55 methylation is incomplete.
Unlike the previously identified delNESP55/delAS3-4, the novel deletion partially enhances GNAS exon A/B methylation and partially reduces exon NESP55 methylation on paternal transmission. We can offer no clear explanation for these findings at this time, although it is possible that the identified deletion disrupts a hitherto unrecognized cis-acting element on the paternal allele. For example, this element may be required for establishing or maintaining paternal NESP55 methylation, and the observed gain of A/B methylation may be secondary to the methylation changes at exon NESP55 and/or in NESP55 transcription. Because similar imprinting changes were not observed in patients with the previously described NESP55 DMR deletions (33), this putative cis-acting element is likely located within the region that is unique to the novel deletion described herein. It should be noted, however, that A/B methylation, at least in the mouse, is established during gametogenesis long before the establishment of NESP55 imprinting (16), thus making it unlikely that the methylation changes at exon A/B occur secondarily to changes in NESP55 methylation and/or NESP55 transcription.
On the other hand, it is interesting to note that the methylation defects observed in the unaffected carriers, who carry the deletion on the paternal allele, are similar to those observed in mice in which the paternal Nespas promoter is ablated (20). This similarity may implicate the mutant antisense transcript in the methylation changes observed upon the paternal inheritance of the deletion. In fact, antisense transcript levels in the unaffected carrier, as assessed by quantitative RT-PCR using primers in antisense exons 1 and 2, tended to be lower than in the controls (see Fig. 5C), and when ANOVA was limited to data from controls and the unaffected carrier only (four instead of six data sets in total), this difference was found to be statistically significant. It is thus possible that the deletion might lead to a slight reduction of the antisense transcript levels on the paternal allele. The paternal deletion of the Nespas promoter in mice results in a complete loss of the antisense transcript and is associated, on the paternal allele, with a complete loss of Nesp55 methylation and a partial gain of exon 1A methylation (20). It is therefore tempting to speculate that the NESP55 and exon A/B methylation changes observed in our unaffected carriers result from the slightly diminished antisense transcript expression. Nevertheless, the quantitative RT-PCR analysis of the antisense transcript in the affected individual did not provide evidence for reduced expression of the deleted antisense transcript from the maternal allele (see Fig. 5C). Although this finding may reflect a technical limitation resulting from the coamplification of the wild-type transcript in that individual, it may also suggest that the deletion affects the antisense transcript expression only after paternal inheritance.
In mice, partial gain of paternal exon 1A methylation is associated with derepression, in cis, of Gsα transcription in the renal proximal tubules and, thus, augmented Gsα levels in this tissue (20). It is thus possible that the healthy obligate carriers in our kindred, who display a modest gain of exon A/B methylation, have similar increases in Gsα levels and, therefore, increased PTH sensitivity in the proximal renal tubules. Consistent with this prediction, one of three available unaffected carriers showed diminished serum PTH levels with elevated 1,25(OH)2 vitamin D concentrations despite having serum calcium concentrations that were slightly below the normal range (see Table 1). This finding suggests enhancement of PTH sensitivity, which may have implications for the regulation of mineral ion homeostasis. The lack of such changes in the other unaffected carriers may be related to dietary and environmental factors and/or differences in other genetic loci.
In summary, we identified a novel GNAS deletion that causes AD-PHP-Ib upon maternal transmission and methylation defects throughout this locus upon both maternal and paternal transmission. Our findings provide novel insights into the mechanisms involved in the development of hormone resistance and the regulation of imprinting within the complex GNAS locus.
Acknowledgments
We thank the members of the investigated AD-PHP-Ib kindred for participating in this research study.
Footnotes
This work was supported by research grants from National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK073911 to M.B. and R37 DK46718 to H.J.). S.T. was the recipient of a fellowship from the Scientific and Technical Research Council of Turkey (TÜBITAK), Scientific Human Resources Development (BAYG) within the framework of the Postdoctoral Research Fellowship program.
Disclosure Summary: The authors declare no conflict of interest.
First Published Online May 5, 2010
Abbreviations: AD, Autosomal dominant; COBRA, combined bisulfite restriction enzymatic analysis; DMR, differentially methylated region; Gsα, α-subunit of the stimulatory G protein; LCL, lymphoblastoid cell line; PHP-Ib, pseudohypoparathyroidism type-Ib.
References
- Ideraabdullah FY, Vigneau S, Bartolomei MS 2008 Genomic imprinting mechanisms in mammals. Mutat Res 647:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey G 2007 Genomic imprinting: roles and regulation in development. Endocr Dev 12:99–112 [DOI] [PubMed] [Google Scholar]
- Edwards CA, Ferguson-Smith AC 2007 Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol 19:281–289 [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M 2004 GNAS: normal and abnormal functions. Endocrinology 145:5459–5464 [DOI] [PubMed] [Google Scholar]
- Peters J, Williamson CM 2008 Control of imprinting at the Gnas cluster. Adv Exp Med Biol 626:16–26 [DOI] [PubMed] [Google Scholar]
- Hayward BE, Bonthron DT 2000 An imprinted antisense transcript at the human GNAS1 locus. Hum Mol Genet 9:835–841 [DOI] [PubMed] [Google Scholar]
- Hayward BE, Kamiya M, Strain L, Moran V, Campbell R, Hayashizaki Y, Bonthron DT 1998 The human GNAS1 gene is imprinted and encodes distinct paternally and biallelically expressed G proteins. Proc Natl Acad Sci USA 95:10038–10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward BE, Moran V, Strain L, Bonthron DT 1998 Bidirectional imprinting of a single gene: GNAS1 encodes maternally, paternally, and biallelically derived proteins. Proc Natl Acad Sci USA 95:15475–15480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Wroe SF, Wells CA, Miller HJ, Bodle D, Beechey CV, Williamson CM, Kelsey G 1999 A cluster of oppositely imprinted transcripts at the Gnas locus in the distal imprinting region of mouse chromosome 2. Proc Natl Acad Sci USA 96:3830–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischia R, Lovisetti-Scamihorn P, Hogue-Angeletti R, Wolkersdorfer M, Winkler H, Fischer-Colbrie R 1997 Molecular cloning and characterization of NESP55, a novel chromogranin-like precursor of a peptide with 5-HT1B receptor antagonist activity. J Biol Chem 272:11657–11662 [DOI] [PubMed] [Google Scholar]
- Kehlenbach RH, Matthey J, Huttner WB 1994 XLαs is a new type of G protein. Nature [Erratum (1995) 375:253] 372:804–809 [DOI] [PubMed] [Google Scholar]
- Wroe SF, Kelsey G, Skinner JA, Bodle D, Ball ST, Beechey CV, Peters J, Williamson CM 2000 An imprinted transcript, antisense to Nesp, adds complexity to the cluster of imprinted genes at the mouse Gnas locus. Proc Natl Acad Sci USA 97:3342–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramowitz J, Grenet D, Birnbaumer M, Torres HN, Birnbaumer L 2004 XLαs, the extra-long form of the α-subunit of the Gs G protein, is significantly longer than suspected, and so is its companion Alex. Proc Natl Acad Sci USA 101:8366–8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Aytan N, Mahon MJ, Tawfeek HA, Kowall NW, Dedeoglu A, Bastepe M 2009 Extralarge XLαs (XXLαs), a variant of stimulatory G protein α-subunit (Gsα), is a distinct, membrane-anchored GNAS product that can mimic Gsα. Endocrinology 150:3567–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke M, Kehlenbach RH, Huttner WB 2001 Two overlapping reading frames in a single exon encode interacting proteins: a novel way of gene usage. EMBO J 20:3849–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu S, Litman D, Chen W, Weinstein LS 2000 Identification of a methylation imprint mark within the mouse gnas locus. Mol Cell Biol 20:5808–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes C, Arnaud P, Gordon E, Dean W, Coar EA, Williamson CM, Feil R, Peters J, Kelsey G 2003 Epigenetic properties and identification of an imprint mark in the Nesp-Gnasxl domain of the mouse Gnas imprinted locus. Mol Cell Biol 23:5475–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CM, Ball ST, Nottingham WT, Skinner JA, Plagge A, Turner MD, Powles N, Hough T, Papworth D, Fraser WD, Maconochie M, Peters J 2004 A cis-acting control region is required exclusively for the tissue-specific imprinting of Gnas. Nat Genet 36:894–899 [DOI] [PubMed] [Google Scholar]
- Liu J, Chen M, Deng C, Bourc'his D, Nealon JG, Erlichman B, Bestor TH, Weinstein LS 2005 Identification of the control region for tissue-specific imprinting of the stimulatory G protein α-subunit. Proc Natl Acad Sci USA 102:5513–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CM, Turner MD, Ball ST, Nottingham WT, Glenister P, Fray M, Tymowska-Lalanne Z, Plagge A, Powles-Glover N, Kelsey G, Maconochie M, Peters J 2006 Identification of an imprinting control region affecting the expression of all transcripts in the Gnas cluster. Nat Genet 38:350–355 [DOI] [PubMed] [Google Scholar]
- Liu J, Litman D, Rosenberg MJ, Yu S, Biesecker LG, Weinstein LS 2000 A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin Invest 106:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastepe M, Pincus JE, Sugimoto T, Tojo K, Kanatani M, Azuma Y, Kruse K, Rosenbloom AL, Koshiyama H, Jüppner H 2001 Positional dissociation between the genetic mutation responsible for pseudohypoparathyroidism type Ib and the associated methylation defect at exon A/B: evidence for a long-range regulatory element within the imprinted GNAS1 locus. Hum Mol Genet 10:1231–1241 [DOI] [PubMed] [Google Scholar]
- Jan de Beur S, Ding C, Germain-Lee E, Cho J, Maret A, Levine MA 2003 Discordance between genetic and epigenetic defects in pseudohypoparathyroidism type 1b revealed by inconsistent loss of maternal imprinting at GNAS1. Am J Hum Genet 73:314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani G, Bondioni S, Linglart A, Maghnie M, Cisternino M, Corbetta S, Lania AG, Beck-Peccoz P, Spada A 2007 Genetic analysis and evaluation of resistance to thyrotropin and growth hormone-releasing hormone in pseudohypoparathyroidism type Ib. J Clin Endocrinol Metab 92:3738–3742 [DOI] [PubMed] [Google Scholar]
- de Nanclares GP, Fernández-Rebollo E, Santin I, García-Cuartero B, Gaztambide S, Menéndez E, Morales MJ, Pombo M, Bilbao JR, Barros F, Zazo N, Ahrens W, Jüppner H, Hiort O, Castaño L, Bastepe M 2007 Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright’s hereditary osteodystrophy. J Clin Endocrinol Metab 92:2370–2373 [DOI] [PubMed] [Google Scholar]
- Linglart A, Bastepe M, Jüppner H 2007 Similar clinical and laboratory findings in patients with symptomatic autosomal dominant and sporadic pseudohypoparathyroidism type Ib despite different epigenetic changes at the GNAS locus. Clin Endocrinol (Oxf) 67:822–831 [DOI] [PubMed] [Google Scholar]
- Liu J, Nealon JG, Weinstein LS 2005 Distinct patterns of abnormal GNAS imprinting in familial and sporadic pseudohypoparathyroidism type IB. Hum Mol Genet 14:95–102 [DOI] [PubMed] [Google Scholar]
- Linglart A, Gensure RC, Olney RC, Jüppner H, Bastepe M 2005 A Novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet 76:804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laspa E, Bastepe M, Jüppner H, Tsatsoulis A 2004 Phenotypic and molecular genetic aspects of pseudohypoparathyroidism type Ib in a Greek kindred: evidence for enhanced uric acid excretion due to parathyroid hormone resistance. J Clin Endocrinol Metab 89:5942–5947 [DOI] [PubMed] [Google Scholar]
- Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, Körkkö J, Nakamoto JM, Rosenbloom AL, Slyper AH, Sugimoto T, Tsatsoulis A, Crawford JD, Jüppner H 2003 Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest 112:1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud FH, Linglart A, Bastepe M, Jüppner H, Lteif AN 2005 Molecular diagnosis of pseudohypoparathyroidism type Ib in a family with presumed paroxysmal dyskinesia. Pediatrics 115:e242–e244 [DOI] [PubMed] [Google Scholar]
- Bastepe M, Lane AH, Jüppner H 2001 Paternal uniparental isodisomy of chromosome 20q (patUPD20q)—and the resulting changes in GNAS1 methylation—as a plausible cause of pseudohypoparathyroidism. Am J Hum Genet 68:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastepe M, Fröhlich LF, Linglart A, Abu-Zahra HS, Tojo K, Ward LM, Jüppner H 2005 Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type-Ib. Nat Genet 37:25–37 [DOI] [PubMed] [Google Scholar]
- Li LC, Dahiya R 2002 MethPrimer: designing primers for methylation PCRs. Bioinformatics 18:1427–1431 [DOI] [PubMed] [Google Scholar]
- Bastepe M, Weinstein LS, Ogata N, Kawaguchi H, Jüppner H, Kronenberg HM, Chung UI 2004 Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci USA 101:14794–14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z 2002 Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32:1372–1374, 1376, 1378–1379 [PubMed] [Google Scholar]
- Chotalia M, Smallwood SA, Ruf N, Dawson C, Lucifero D, Frontera M, James K, Dean W, Kelsey G 2009 Transcription is required for establishment of germline methylation marks at imprinted genes. Genes Dev 23:105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]