Abstract
Aims
To identify distinct trajectories of fatigue over a 12-month period and to examine their impact on mortality in chronic heart failure (CHF).
Methods and results
Consecutive CHF patients (n = 310) were assessed at baseline and at 2- and 12-month follow-up for symptoms of exertion and general fatigue. Latent growth mixture modelling was used to examine the course of fatigue over time. The endpoint was mortality following the 12-month assessment of fatigue. Over the initial 12-month follow-up, six distinct trajectories for exertion fatigue and four distinct trajectories for general fatigue were identified. Beyond the 12-month follow-up (mean follow-up period, 693 days), 50 patients (17%) had died. After controlling for standard risk factors and disease severity, both severe exertion fatigue [hazards ratio (HR) = 2.59, 95% confidence interval (CI): 1.09–6.16, P = 0.03] and severe general fatigue (HR = 3.20, 95% CI: 1.62–6.31, P = 0.001) trajectories predicted an increased mortality rate (29 vs. 19% and 28 vs. 14%, respectively). The low exertion fatigue trajectory was associated with a decreased mortality risk (3 vs. 19%, HR = 0.12, 95% CI: 0.02–0.93, P = 0.04).
Conclusion
Fatigue trajectories varied across CHF patients and had a differential effect on mortality. Persistent severe fatigue was a predictor of poor prognosis. These results may help identify distinct groups of CHF patients with potentially differential risks of adverse health outcomes.
Keywords: Chronic heart failure, Fatigue, Mortality, Latent class mixture modelling
Introduction
Fatigue is considered one of the key symptoms in chronic heart failure (CHF), as symptoms of fatigue to a large extent determine the patient's quality of life.1 Moreover, it has also been suggested that symptoms of fatigue may be associated with poor cardiovascular outcomes in CHF.2–5
Research on fatigue in CHF has primarily focused on its pathophysiological underpinnings6,7 and its determinants.3,8–13 It has been suggested that chronic, low-grade haemodynamic stress as seen in CHF may lead to dominance of catabolic processes, which in turn leads to skeletal myopathy, causing the sensation of fatigue.6,7 Other studies have identified symptoms of dyspnoea, symptoms of depression, and personality factors as important determinants of fatigue in CHF.11–14 Nonetheless, fatigue is still beyond complete comprehension, and it is unlikely that the factors mentioned above fully explain individual differences in fatigue.
Fatigue is highly prevalent among CHF patients, and progression of CHF is on par with an increase in symptoms of fatigue.1,3,4,11 However, the evolution of fatigue over time is not the same for all patients with CHF. It may be important to distinguish distinct developmental trajectories of fatigue in CHF, as knowledge of fatigue trajectories, their clinical and psychological determinants, and their prognostic impact allow for the identification of high-risk CHF patients who may need additional clinical care above and beyond the standard medical management of the disease.
Since the course of fatigue has not been studied in CHF patients, the current study's objective was to examine (i) the course and predictors of fatigue in CHF during a 12-month period and (ii) their prognostic impact beyond the 12-month follow-up.
Methods
Patients
This study included 310 CHF patients with systolic heart failure and a left ventricular ejection fraction (LVEF) ≤40%, attending the heart failure outpatient clinic of the tertiary TweeSteden hospital, Tilburg, The Netherlands. Of the 424 patients who were initially identified as eligible to participate in the study between October 2003 and June 2006, 46 refused to participate. In total, 44 patients died during the first year of the study, and 24 patients had missing questionnaire data on two or more measurement points. This left 310 patients for inclusion in the present study. The study exclusion criteria were: patients aged ≥80 years, myocardial infarction (MI) in the month prior to inclusion, other life-threatening diseases, and insufficient understanding of the spoken and written Dutch language. Patients with diastolic heart failure were also excluded from the study because of their hearts' intact pump function during systole and diminished filling capacity during diastole.
Patients completed a take-home questionnaire at baseline and then at 2- and 12-month follow-up. The study protocol was approved by the local medical Ethics Committee in Tilburg, The Netherlands. The study was conducted to conform to the Helsinki Declaration and every patient provided written informed consent.
Symptoms of fatigue
Previous research suggests that it is important to differentiate between exertion and general fatigue,12 the former referring to fatigue directly related to the performance of activities of daily living and the latter to an overwhelming, sustained sense of exhaustion that does not necessarily relate to exertion. The Dutch Exertion Fatigue Scale (DEFS) was used to assess exertion fatigue.15 The DEFS consists of nine items which are answered with five response alternatives ranging from 0 (no) to 4 (yes). Cronbach's α for this scale is high (α = 91). The Fatigue Assessment Scale (FAS) was used to assess symptoms of general fatigue.16 This questionnaire consists of 10 items, which are answered on a five-point Likert scale, ranging from 0 (never) to 4 (always). The reliability of this instrument is high (α = 0.90).
Demographic and clinical variables
Demographic variables included sex, age, marital status, and educational level. Clinical variables comprised LVEF, NYHA functional class, CHF aetiology, diabetes mellitus, hypertension, hypercholesterolaemia, co-morbidities [stroke, chronic obstructive pulmonary disease, peripheral arterial disease (PAD), and renal insufficiency], cardiac history [MI, percutaneous coronary intervention (PCI), and coronary artery bypass graft], smoking status, physical inactivity, body mass index (BMI), cardiac medication, and psychotropic medication. Exercise capacity was measured by means of the 6 min walking test (walking small circuits of 52 m), which was carried out within the hospital as part of this study. Patients were instructed to walk at a normal pace and to continue walking until they were told to stop or until they experienced too many adverse symptoms. Patients were not encouraged to walk as far as possible because the test was meant to reflect daily life exercise capacity. Information on clinical variables was obtained from the medical records and from the treating cardiologist or heart failure nurse.
Mortality beyond 12-month follow-up
Patients' hospital real-time medical records were used to assess cardiovascular mortality since the 12-month follow-up. The mean duration of follow-up counting from 12 months after baseline was 693 days (range 74–1516 days).
Statistical analyses
Prior to statistical analyses, age (<60 vs. ≥60 years), educational level (low vs. higher), marital status (partner vs. no partner), LVEF (<30 vs. ≥30%), NYHA functional class (I/II vs. III/IV), CHF aetiology (ischaemic vs. non-ischaemic), co-morbidities (present vs. not present), cardiac history (present vs. not present), and exercise capacity (<300 vs. ≥300 m) were re-coded into dichotomous variables.
Latent class (LC) regression analysis was employed to examine trajectories of fatigue symptoms in CHF patients over a 12-month period.17 Although the general approach has been to study the course of symptoms in a single group, LC regression allows us to study subgroups of patients with varying courses of fatigue over time. Therefore, a finite mixture model was fitted to identify classes of individuals following similar patterns of behaviour over time. The model assumes unobserved latent variables to explain the associations among observed scores and can be seen as a categorical equivalent of factor analysis. One of the problems with fitting these types of LC models is that the categorization into classes is dominated by the overall symptom levels, which makes it less likely that the model picks up symptom changes. A way to overcome this problem is the inclusion of a random intercept.18
To determine the optimal number of trajectories, the Aiken Information Criterion 3 (AIC3) was used, with a higher AIC3 indicating a better fit. However, a difference of less than three will favour the least complex model. Recent studies have shown that AIC3 is a better criterion than BIC (Bayesian Information Criterion) and AIC in determining the number of LCs in LC models.19,20
For comparison between classes, we used the χ2 test for discrete variables. Adjusted standardized residuals (ASRs) were used to identify groups responsible for significant differences. A residual greater than 2.0 was taken to indicate a significantly higher frequency, and a residual less than −2.0 was considered to indicate a significantly lower frequency, than expected if the independence hypothesis was true.21 Variables that were significant in the univariate χ2 analyses were entered into a multivariate multinomial logistic regression model to assess whether demographic, medical, and psychological variables were predictors of trajectories of symptoms of fatigue.
Cox's proportional hazards regression was used to assess whether fatigue trajectories predicted mortality beyond the 12-month follow-up. In multivariate Cox's regression, we included age, gender, BMI, physical inactivity, diabetes mellitus, and a measure of disease severity (LVEF) because of their relation with cardiovascular prognosis and fatigue. The LC cluster analysis was performed with the LCA program Latent GOLD.17 All other data were analysed using SPSS 15.0.1 for Windows. A similar approach has previously been used in MI,22 PCI,23 and PAD patients.14
Results
Trajectories of exertion fatigue
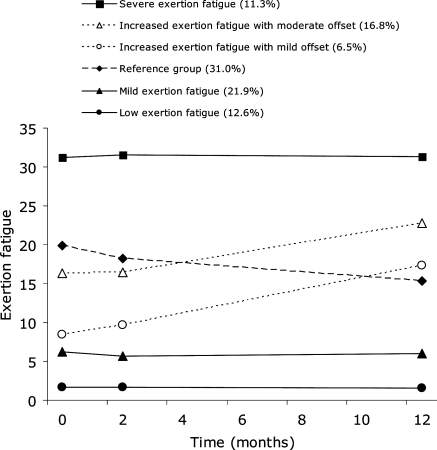
Figure 1 displays the six distinct developmental trajectories for exertion fatigue. The AIC3 improved from one class of exertion fatigue (AIC3 = −6353) to six classes of exertion fatigue (AIC3 = −5899), whereas a relative decline was observed in the seven class model (AIC3 = −5903). Compared with the five-class model (AIC3 = −5915), the six-class model achieved a significant improvement in fit. The six-class model was therefore adopted for further analysis.
Figure 1.
Observed trajectories of exertion fatigue.
The exertion fatigue first class (12.6% of the sample) was classified as the low exertion fatigue group and was stable over time (intercept = 1.69, P < 0.001; slope = −0.0054, P = 0.76). The level of exertion fatigue in the second class (21.9%) was slightly higher than in the first class and stable over time (intercept = 5.91, P < 0.001; slope = −0.011, P = 0.72), and was therefore classified as the mild exertion fatigue group. Class three (31.0%) had a moderate offset with an observed mean DEFS score at baseline of 19.94 [95% confidence interval (CI): 18.61–21.27] but showed a significant decrease in exertion fatigue over time (intercept = 18.87, P < 0.001; slope = −0.28, P = 0.004). Since the third class comprised the largest group of patients, it was conceptualized as the reference group in the present study. The fourth class (6.5%) was described as increasingly fatigued with a mild offset (observed mean baseline DEFS score = 8.40, 95% CI: 6.50–10.30; model intercept = 8.07, P < 0.001; slope = 0.74, P < 0.001). Class five (16.8%) had an increase in exertion fatigue and a moderate offset (observed mean baseline DEFS score = 16.33, 95% CI: 14.06–18.60; model intercept = 17.03, P < 0.001; slope = 0.31, P = 0.05). Finally, the sixth class (11.3%) was classified as severely fatigued across all assessment points (intercept = 31.45, P < 0.001; slope = −0.006, P = 0.92).
Trajectories of general fatigue
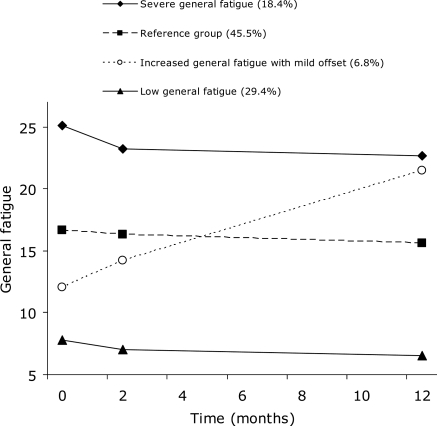
The levels of general fatigue were relatively stable over time for most patients (Figure 2). The AIC3 improved from one class of general fatigue (AIC3 = −5863) to four classes of general fatigue (AIC3 = −5741), whereas a relative decline was observed in the five-class model (AIC3 = −5749). Compared with the three-class model (AIC3 = −5745), the four-class model achieved a significant improvement in fit. A four-class model for general fatigue was therefore adopted for further analysis.
Figure 2.
Observed trajectories of general fatigue.
The first class (29.4% of the sample) was classified as the low general fatigue group and was stable over time (intercept = 8.55, P < 0.001; slope = −0.095, P = 0.06). The second class (45.5%) had fairly stable moderate levels of fatigue and was conceptualized as the reference group since this class comprised the largest group of patients (intercept = 15.10, P < 0.001; slope = −0.090, P = 0.04). The third class (6.8%) was described as increasingly fatigued with a mild offset (observed mean baseline FAS score = 12.05, 95% CI: 9.84–14.26; model intercept = 13.13, P < 0.001; slope = 0.71, P < 0.001). Finally, the fourth class (18.4%) was classified as severely fatigued across all assessment points (intercept = 22.50, P < 0.001; slope = −0.10, P = 0.06).
Baseline characteristics stratified by fatigue class
There were a number of differences in demographic, clinical, and psychological characteristics at baseline as a function of exertion fatigue class (Table 1) and general fatigue class (Table 2). Departure from independence was most pronounced in the extreme exertion fatigue groups (Table 1, numbers in bold). Patients in the low exertion fatigue group were younger (ASR = −3.6) and were less likely to be obese (ASR = −2.2), diabetic (ASR = −2.9), have a low exercise capacity (ASR = −4.2), a higher NYHA class (ASR = −3.3), or to be on diuretics (ASR = −2.5). Patients in the severe exertion fatigue group were more likely to be female (ASR = 2.9), obese (ASR = 2.0), physically inactive (ASR = 5.0), to have co-morbidities (ASR = 2.1), low exercise capacity (ASR = 6.3), to be on diuretics (ASR = 2.6), or psychotropic medication (ASR = 5.7), and to be in a higher NYHA class (ASR = 3.8).
Table 1.
Baseline characteristics stratified by exertion fatigue classa
| Variable, % (n) | Total | Low exertion fatigue (n = 39) | Mild exertion fatigue (n = 68) | Reference group (n = 96) | Increased exertion fatigue with mild offset (n = 20) | Increased exertion fatigue with moderate offset (n = 52) | Severe exertion fatigue (n = 35) | P-value |
|---|---|---|---|---|---|---|---|---|
| Male sex | 70.0 (217) | 82.1 (32) | 85.3 (58) | 64.6 (62) | 65.0 (13) | 67.3 (35) | 48.6 (17) | <0.001 |
| Age ≥ 60 | 72.6 (225) | 48.7 (19) | 72.1 (49) | 76.0 (73) | 85.0 (17) | 75.0 (39) | 80.0 (28) | 0.01 |
| Having no partner | 71.3 (221) | 79.5 (31) | 80.9 (55) | 66.7 (64) | 60.0 (12) | 69.2 (36) | 65.7 (23) | 0.20 |
| Low educational level | 15.2 (47) | 17.9 (7) | 22.1 (15) | 12.5 (12) | 25.0 (5) | 9.6 (5) | 8.6 (3) | 0.21 |
| Smoking | 22.6 (70) | 17.9 (7) | 22.1 (15) | 19.8 (19) | 15.0 (3) | 32.7 (17) | 25.7 (9) | 0.44 |
| Obesity (BMI > 30) | 27.7 (86) | 12.8 (5) | 20.6 (14) | 35.4 (34) | 20.0 (4) | 28.8 (15) | 40.0 (14) | 0.03 |
| Ischaemic aetiology | 53.9 (167) | 46.2 (18) | 54.4 (37) | 60.4 (58) | 35.0 (7) | 51.9 (27) | 57.1 (20) | 0.34 |
| Cardiac historyb | 57.1 (177) | 48.7 (19) | 54.4(37) | 60.4 (58) | 45.0 (9) | 55.8 (29) | 71.4 (25) | 0.31 |
| Physical inactivity | 43.2 (134) | 35.9 (14) | 23.5 (16) | 45.8 (44) | 25.0 (5) | 50.0 (26) | 82.9 (29) | <0.001 |
| Hypertension | 37.4 (116) | 15.4 (6) | 41.2 (28) | 37.5 (36) | 40.0 (8) | 40.4 (21) | 48.6 (17) | 0.06 |
| Hypercholesterolaemia | 47.7 (148) | 35.9 (14) | 45.6 (31) | 53.1 (51) | 30.0 (6) | 55.8 (29) | 48.6 (17) | 0.20 |
| Diabetes | 23.9 (74) | 5.1 (2) | 23.5 (16) | 27.1 (26) | 15.0 (3) | 34.6 (18) | 25.7 (9) | 0.03 |
| Co-morbiditiesc | 35.2 (109) | 23.1 (9) | 26.5 (18) | 43.8 (42) | 20.0 (4) | 34.6 (18) | 51.4 (18) | 0.02 |
| LVEF < 30% | 40.0 (124) | 41.0 (16) | 39.7 (27) | 41.7 (40) | 45.0 (9) | 36.5 (19) | 37.1 (13) | 0.98 |
| NYHA class III/IV | 39.4 (122) | 15.4 (6) | 25.0 (17) | 46.9 (45) | 40.0 (8) | 42.3 (22) | 68.6 (24) | <0.001 |
| ACE-inhibitors | 71.9 (223) | 76.9 (30) | 66.2 (45) | 76.0 (73) | 65.0 (13) | 71.2 (37) | 71.4 (25) | 0.72 |
| Diuretics | 73.2 (227) | 56.4 (22) | 73.5 (50) | 76.0 (73) | 80.0 (16) | 65.4 (34) | 91.4 (32) | 0.01 |
| β-Blocker | 68.7 (213) | 79.5 (31) | 69.1 (47) | 69.8 (67) | 80.0 (16) | 55.8 (29) | 65.7 (23) | 0.18 |
| Statins | 51.0 (158) | 12.8 (5) | 20.6 (14) | 35.4 (34) | 20.0 (4) | 28.8 (15) | 40.0 (14) | 0.45 |
| Aspirin | 43.5 (135) | 48.7 (19) | 50.0 (34) | 43.8 (42) | 30.0 (6) | 34.6 (18) | 45.7 (16) | 0.44 |
| Psychotropic medication | 12.6 (39) | 5.1 (2) | 2.9 (2) | 12.5 (12) | 5 (1) | 13.5 (7) | 42.9 (15) | <0.001 |
| 6MWT < 300 m | 41.6 (129) | 10.3 (4) | 19.1 (13) | 49.0 (47) | 30.0 (6) | 51.9 (27) | 91.4 (32) | <0.001 |
6MWT, 6 min walk test.
aNumbers in bold represent an absolute adjusted standardized residual >2.0.
bHistory of MI, CABG, and PCI.
cStroke, chronic obstructive pulmonary disease, peripheral arterial disease, and renal insufficiency.
Table 2.
Baseline characteristics stratified by general fatigue classa
| Variable | Total | Low general fatigue (n = 91) | Reference group (n = 141) | Increased general fatigue with mild offset (n = 21) | Severe general fatigue (n = 57) | P-value |
|---|---|---|---|---|---|---|
| Male sex | 70.0 (217) | 75.8 (69) | 70.2 (99) | 66.7 (14) | 61.4 (35) | 0.31 |
| Age ≥ 60 | 72.6 (225) | 70.3 (64) | 74.5 (105) | 76.2 (16) | 70.2 (40) | 0.85 |
| Having no partner | 71.3 (221) | 74.7 (68) | 72.3 (102) | 57.1 (12) | 68.4 (39) | 0.41 |
| Low educational level | 15.2 (47) | 20.9 (19) | 14.2 (20) | 19.0 (4) | 7.0 (4) | 0.13 |
| Smoking | 22.6 (70) | 18.7 (17) | 19.1 (27) | 19.0 (4) | 38.6 (22) | 0.02 |
| Obesity (BMI > 30) | 27.7 (86) | 24.2 (22) | 27.7 (39) | 28.6 (6) | 33.3 (19) | 0.69 |
| Ischaemic aetiology | 53.9 (167) | 47.3 (43) | 57.4 (81) | 47.6 (10) | 57.9 (33) | 0.39 |
| Cardiac historyb | 57.1 (177) | 50.5 (46) | 60.3 (85) | 42.9 (6) | 64.9 (37) | 0.15 |
| Physical inactivity | 43.2 (134) | 31.9 (29) | 40.4 (57) | 42.9 (9) | 68.4 (39) | <0.001 |
| Hypertension | 37.4 (116) | 35.2 (32) | 37.6 (53) | 38.1 (8) | 40.4 (23) | 0.94 |
| Hypercholesterolaemia | 47.7 (148) | 42.9 (39) | 49.6 (70) | 57.1 (12) | 47.4 (27) | 0.61 |
| Diabetes | 23.9 (74) | 19.8 (18) | 22.0 (31) | 33.3 (7) | 31.6 (18) | 0.26 |
| Co-morbiditiesc | 35.2 (109) | 35.2 (32) | 37.6 (53) | 19 (4) | 35.1 (20) | 0.43 |
| LVEF < 30% | 40.0 (124) | 37.4 (34) | 42.6 (60) | 38.1 (8) | 38.6 (22) | 0.87 |
| NYHA class III/IV | 39.4 (122) | 29.7 (27) | 40.4 (57) | 42.9 (9) | 50.9 (29) | 0.05 |
| ACE-inhibitors | 71.9 (223) | 68.1 (62) | 73.0 (103) | 81.0 (17) | 71.9 (41) | 0.66 |
| Diuretics | 73.2 (227) | 67.0 (61) | 75.9 (107) | 71.4 (15) | 77.2 (44) | 0.43 |
| β-Blocker | 68.7 (213) | 73.6 (67) | 68.1 (96) | 61.9 (13) | 64.9 (37) | 0.60 |
| Statins | 51.0 (158) | 50.5 (46) | 54.6 (77) | 57.1 (12) | 40.4 (23) | 0.30 |
| Aspirin | 43.5 (135) | 38.5 (35) | 46.8 (66) | 57.1 (12) | 38.6 (22) | 0.29 |
| Psychotropic medication | 12.6 (39) | 4.4 (4) | 15.6 (22) | 9.5 (2) | 19.3 (11) | 0.03 |
| 6MWT < 300 m | 41.6 (129) | 24.2 (22) | 46.1 (65) | 38.1 (8) | 59.6 (34) | <0.001 |
6MWT, 6 min walk test.
aNumbers in bold represent an absolute adjusted standardized residual >2.0.
bHistory of MI, CABG, and PCI.
cStroke, chronic obstructive pulmonary disease, peripheral arterial disease, and renal insufficiency.
In the general fatigue groups, departure from independence was only observed in the low and the severe fatigue groups (Table 2, numbers in bold). Patients in the low general fatigue group were less likely to be physically inactive (ASR = −2.6), to be in a higher NYHA class (ASR = −2.2), to be on psychotropic medication (ASR = −2.8), and to have a low exercise capacity (ASR = −4.0). Patients in the severe general fatigue group were more likely to be smokers (ASR = 3.2), to be physically inactive (ASR = 4.3), to be in a higher NYHA class (ASR = 2.0), and to have low exercise capacity (ASR = 3.1).
Predictors of fatigue trajectories
Multivariate predictors of exertion fatigue trajectories are shown in Tables 3 and 4. Multinomial logistic regression analysis revealed that younger patients, patients without diabetes mellitus or other co-morbidities, and patients with a preserved exercise capacity were more likely to be in the low exertion fatigue class when compared with the reference group, whereas patients in NHYA class III/IV were less likely to be in the low exertion fatigue class (Table 3). Furthermore, male, physically active patients without co-morbidities or impaired exercise capacity were more likely to be in the mild exertion fatigue class when compared with the reference group. Finally, female, physically inactive patients on psychotropic medication and with impaired exercise capacity were more likely to be in the severe exertion fatigue group (Table 3).
Table 3.
Multivariate predictors of fatigue class: exertion fatigue classa
| Variable | Low exertion fatigue |
Mild exertion fatigue |
Increased exertion fatigue with mild offset |
Increased exertion fatigue with moderate offset |
Severe exertion fatigue |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | |
| Male sex | 2.56 | 0.08 | 3.32 | 0.006 | NS | NS | NS | NS | 0.40 | 0.08 |
| Age ≥ 60 | 0.35 | 0.02 | NS | NS | NS | NS | NS | NS | NS | NS |
| Obesity (BMI > 30) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Physical inactivity | NS | NS | 0.41 | 0.02 | NS | NS | NS | NS | 6.71 | 0.001 |
| Diabetes | 0.21 | 0.05 | NS | NS | NS | NS | NS | NS | NS | NS |
| Co-morbiditiesb | 0.31 | 0.02 | 0.44 | 0.03 | 0.33 | 0.07 | NS | NS | NS | NS |
| NYHA class III/IV | 0.32 | 0.04 | 0.48 | 0.06 | NS | NS | NS | NS | NS | NS |
| Diuretics | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Psychotropic medication | NS | NS | 0.22 | 0.07 | NS | NS | NS | NS | 5.43 | 0.003 |
| 6MWT < 300 m | 0.22 | 0.02 | 0.39 | 0.02 | NS | NS | NS | NS | 9.62 | 0.001 |
6MWT, 6 min walk test.
aSignificant levels >0.1 are displayed as NS, i.e. not significant.
bStroke, chronic obstructive pulmonary disease, peripheral arterial disease, and renal insufficiency.
Bold entries indicate statistical significance at the P < 0.05 level.
Table 4.
Multivariate predictors of fatigue class: general fatigue classa
| Variable | Low general fatigue |
Increased general fatigue with mild offset |
Severe general fatigue |
|||
|---|---|---|---|---|---|---|
| OR | P-value | OR | P-value | OR | P-value | |
| Smoking | NS | NS | NS | NS | 3.03 | 0.002 |
| Physical inactivity | NS | NS | NS | NS | 2.96 | 0.002 |
| NYHA class III/IV | NS | NS | NS | NS | NS | NS |
| Psychotropic medication | 0.28 | 0.03 | NS | NS | NS | NS |
| 6MWT < 300 m | 0.42 | 0.007 | NS | NS | NS | NS |
6MWT, 6 min walk test.
aSignificant levels >0.1 are displayed as NS, i.e. not significant.
Bold entries indicate statistical significance at the P < 0.05 level.
Patients with a preserved exercise capacity and not on psychotropic medication were more likely to be in the low general fatigue class when compared with the reference group (Table 4). In contrast, patients who were physically inactive smokers were more likely to be in the severe general fatigue class. An additional analysis controlling for disease severity (LVEF) did not alter the results reported in Tables 3 and 4.
Trajectories of fatigue and chronic heart failure prognosis
The mean follow-up period was 28.3 months (SD = 11.8). During this period, 50 patients (16.6%) died. The exertion fatigue classes had the following event rates: reference group (18.8%, n = 18), low exertion fatigue (2.6%, n = 1), mild exertion fatigue (14.7%, n = 10), increased exertion fatigue with mild offset (15.0%, n = 3), increased exertion fatigue with moderate offset (15.4%, n = 8), and severe exertion fatigue (28.6%, n = 10). For the general fatigue classes, the event rates were: reference group (13.5%, n = 19), low general fatigue (12.1%, n = 11), increased general fatigue with mild offset (19.0%, n = 4), and severe general fatigue (28.1%, n = 16). Older age, being physically active, and a lower LVEF were associated with a higher incidence of mortality (Table 5). Obesity showed a trend towards significance. Of note, being physically active was only significantly associated with mortality in the multivariate analysis and may therefore represent a suppressor effect.
Table 5.
Trajectories of fatigue and chronic heart failure prognosis (multivariate)
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Model including exertion fatigue | |||
| Male sex | 1.67 | 0.85–3.31 | 0.14 |
| Age ≥ 60 | 3.23 | 1.26–8.26 | 0.01 |
| Obesity (BMI > 30) | 0.48 | 0.23–1.02 | 0.06 |
| Physical inactivity | 0.43 | 0.21–0.85 | 0.02 |
| Diabetes | 1.23 | 0.63–2.37 | 0.55 |
| LVEF < 30% | 2.17 | 1.22–3.87 | 0.009 |
| Low exertion fatigue | 0.12 | 0.02–0.93 | 0.04 |
| Mild exertion fatigue | 0.59 | 0.27–1.32 | 0.20 |
| Increased exertion fatigue with mild offset | 0.65 | 0.19–2.21 | 0.49 |
| Increased exertion fatigue with moderate offset | 1.03 | 0.44–2.41 | 0.95 |
| Severe exertion fatigue | 2.59 | 1.09–6.16 | 0.03 |
| Model including general fatigue | |||
| Male sex | 1.22 | 0.63–2.36 | 0.55 |
| Age ≥ 60 | 3.70 | 1.45–9.47 | 0.006 |
| Obesity (BMI > 30) | 0.53 | 0.25–1.15 | 0.11 |
| Physical inactivity | 0.56 | 0.31–1.02 | 0.06 |
| Diabetes | 1.26 | 0.64–2.46 | 0.51 |
| LVEF < 30% | 2.20 | 1.24–3.90 | 0.007 |
| Low general fatigue | 0.89 | 0.42–1.89 | 0.75 |
| Increased general fatigue with mild offset | 1.71 | 0.58–5.07 | 0.33 |
| Severe general fatigue | 3.20 | 1.62–6.31 | 0.001 |
Bold entries indicate statistical significance at the P < 0.05 level.
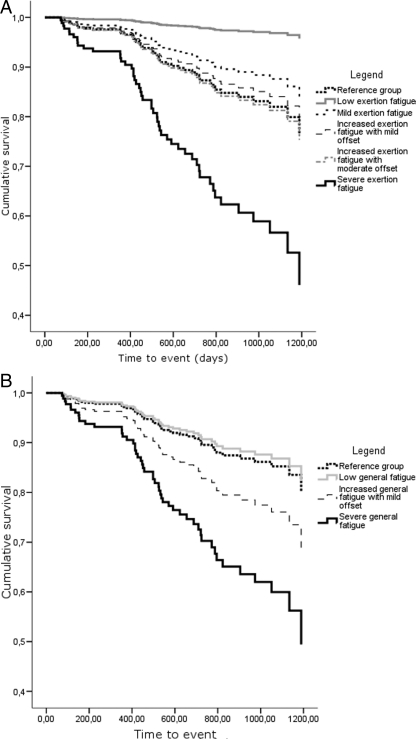
A first Cox's regression model showed that both low exertion fatigue and severe exertion fatigue predicted mortality beyond the 12-month follow-up (Table 5 and Figure 3A). Compared with the reference group (mortality rate = 19%), patients in the low exertion fatigue class had a decreased mortality rate [3%, hazards ratio (HR) = 0.12, 95% CI: 0.02–0.93, P = 0.04) and those in the severe exertion fatigue class an increased mortality rate (29%, HR = 2.59, 95% CI: 1.09–6.16, P = 0.03). A second Cox's regression model showed that severe general fatigue predicted mortality beyond the 12-month follow-up (Table 5 and Figure 3B). Patients in the severe general fatigue class had an increased risk for mortality when compared with the reference group (mortality rate = 28 vs. 14%, HR = 3.20, 95% CI: 1.62–6.31, P = 0.001).
Figure 3.
Event-free survival stratified by exertion fatigue class (A) and general fatigue class (B).
An additional analysis controlling for the use of psychotropic medication (P > 0.5 in both models) did not significantly alter the model results displayed in Table 5. A final analysis showed that when exertion fatigue and general fatigue were entered in the multivariate model simultaneously, both severe exertion fatigue (HR = 2.90, 95% CI: 1.14–7.36, P = 0.03) and severe general fatigue (HR = 3.00, 95% CI: 1.47–6.14, P = 0.003) remained significant predictors of mortality. Similarly, the protective effect of low exertion fatigue (HR = 0.12, 95% CI: 0.02–0.99, P = 0.05) remained significant as well. This result suggests that independently from each other, both types of fatigue add to the risk of mortality in CHF.
Discussion
To our knowledge, this is the first study to examine the course of symptoms of fatigue in patients with CHF. Similar to previous studies,5,12 we differentiated between exertion and general fatigue and found six distinct trajectories for exertion fatigue and four distinct trajectories for general fatigue. Multinomial logistic regression analysis revealed that sex, age, physical inactivity, diabetes mellitus, co-morbidities, NYHA class, exercise capacity, and psychotropic medication were predictors of exertion fatigue, varying according to fatigue classes. Predictors of general fatigue, again varying according to fatigue classes, comprised smoking, physical inactivity, and exercise capacity. Cox's regression showed that patients in the severe exertion fatigue class and severe general fatigue class independently had an almost three-fold increased risk for mortality when compared with the reference group. Patients in the low exertion fatigue group had a decreased risk for mortality.
Fatigue is considered as one of the most important symptoms affecting patients' quality of life. However, fatigue remains under-recognized in clinical practice,24,25 as well as an unresolved issue in CHF.12 In the current study, we were able to further refine our understanding of the course of fatigue in CHF and to characterize most of the trajectories by means of baseline variables. Overall, patients with low/mild levels of exertion/general fatigue were characterized by relatively good physical health (no diabetes mellitus, no co-morbidities, no physical inactivity, and good exercise capacity) and psychological health (no psychotropic medication), whereas the opposite was true for patients with high levels of fatigue. Age and gender effects were found for exertion fatigue. However, we were unable to describe the trajectories that showed an increase in fatigue over time (increased exertion fatigue with mild offset, increased exertion fatigue with moderate offset, and increased general fatigue with mild offset). An explanation for this could be that (i) the groups were relatively small and therefore lacked statistical power to reveal significant differences, (ii) the overall response levels of fatigue in these trajectories were more similar to the reference group when compared with the more extreme groups, resulting in smaller differences with respect to baseline variables, and (iii) persistence of fatigue is more important with regard to prognosis when compared with changes in fatigue over time.
From a clinical point of view, knowledge about factors characterizing trajectories that display changes in fatigue over time is important as they identify targets for intervention. Importantly, the findings of the present study indicate that fatigue trajectories are differently related to an increase or decrease in mortality rate. This finding provides further fuel to the ‘debate’ about whether the experience of fatigue is an important factor in itself or merely an unwelcome by-product of disease progression.26 In previous studies, we have shown that fatigue was associated with rehospitalization2 and with the combined endpoint of rehospitalization and death.5 The problem with rehospitalization as an outcome measure is its semi-objective nature, since the decision to rehospitalize is not solely based on objective diagnostic measures but also on symptom presentation and symptom interpretation. Therefore, it is important to note that in the present study, both exertion and general fatigue independently predicted mortality in CHF, above and beyond disease severity and other clinical risk factors. Large-scale studies should give a more in-depth insight into the differential effect of the fatigue trajectories on mortality.
The mechanisms through which fatigue may exert its effect on mortality are unclear. Future studies should assess physiological measures that are known to be abnormal in CHF patients and that are relevant with respect to fatigue, e.g. measures of abnormal muscle metabolism and an enhanced ergo reflex response;6,7 inflammatory markers may also be an interesting avenue for research in this regard.27,28 Increased fatigue may also have impact on the patient's ability for self-care, which has been associated with poor prognosis in CHF.29
The results of the present study advocate the use of a latent growth mixture modelling approach in clinical cardiology research, because it allows for the identification of subgroups with distinct developmental patterns. As such, this approach provides additional information beyond that obtained from studying prevalence rates or change scores. An LC approach has been used in previous studies,14,22,23,30,31 however, without the inclusion of a random intercept. Similar to our studies, these studies also found support for multiple rather than single trajectories, although they examined depressive symptoms,14,22,30 anxiety,23 and quality of life,31 but not fatigue.
This study has a number of limitations. First, the cardiologist or heart failure nurses asked patients to participate in the study, and this interaction pattern might have influenced patient selection. Secondly, the examined predictors of the fatigue trajectories were only assessed once. Thirdly, we did not include a control group of patients suffering from fatigue without CHF. Fourthly, the number of events was relatively small when compared with the number of LCs. This might have resulted in less reliable risk estimates. Nevertheless, the strengths of the current study were the repeated assessment of fatigue over time, the prospective design examining the course of fatigue over time using a state-of-the-art modelling approach, and the use of an objective medical outcome. Within the sampling frame as defined in the method section, we were able to collect a representative sample of systolic CHF patients. Finally, we used reliable and valid measures of both exertion and general fatigue.
In summary, we found six distinct trajectories for exertion fatigue and four distinct trajectories for general fatigue in patients with CHF. Several predictors, varying according to fatigue classes, were identified, with age, sex, physical inactivity, co-morbidities, exercise capacity, and psychotropic medication use being the most prominent ones. Severe exertion fatigue and severe general fatigue independently predicted an increased mortality risk beyond the 12-month follow-up, whereas low exertion fatigue was associated with a decreased mortality risk. Given that this was the first study to examine the course of fatigue in CHF patients, future studies are warranted to confirm these findings in larger samples with more frequent measures of fatigue and a longer follow-up. The results of the present study may help identify distinct groups of patients with potentially differential risks of adverse health outcomes, guide future interventions, and therefore be valuable for both research and clinical practice.
Funding
The present research was supported by a VICI grant (#453-04-004) from the Netherlands Organisation for Scientific Research, The Hague, The Netherlands, and by a grant from the Dutch Heart Foundation (#2003B038) to J.D. Funding to pay the Open Access publication charges for this article was provided by the Department of Medical Psychology and Neuropsychology, Tilburg University, The Netherlands.
Conflict of interest: none declared.
References
- 1.Drexler H, Coats AJ. Explaining fatigue in congestive heart failure. Annu Rev Med. 1996;47:241–256. doi: 10.1146/annurev.med.47.1.241. [DOI] [PubMed] [Google Scholar]
- 2.Smith OR, Gidron Y, Kupper N, Winter JB, Denollet J. Vital exhaustion in chronic heart failure: symptom profiles and clinical outcome. J Psychosom Res. 2009;66:195–201. doi: 10.1016/j.jpsychores.2008.10.021. doi:10.1016/j.jpsychores.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Ekman I, Cleland JG, Swedberg K, Charlesworth A, Metra M, Poole-Wilson PA. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005;11:288–292. doi: 10.1016/j.cardfail.2005.03.007. doi:10.1016/j.cardfail.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Ingle L, Rigby AS, Carroll S, Butterly R, King RF, Cooke CB, Cleland JG, Clark AL. Prognostic value of the 6 min walk test and self-perceived symptom severity in older patients with chronic heart failure. Eur Heart J. 2007;28:560–568. doi: 10.1093/eurheartj/ehl527. doi:10.1093/eurheartj/ehl527. [DOI] [PubMed] [Google Scholar]
- 5.Smith OR, Denollet J, Schiffer AA, Kupper N, Gidron Y. Patient-rated changes in fatigue over a 12-month period predict poor outcome in chronic heart failure. Eur J Heart Fail. 2009;11:400–405. doi: 10.1093/eurjhf/hfp002. doi:10.1093/eurjhf/hfp002. [DOI] [PubMed] [Google Scholar]
- 6.Clark AL. Origin of symptoms in chronic heart failure. Heart. 2006;92:12–16. doi: 10.1136/hrt.2005.066886. doi:10.1136/hrt.2005.066886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witte KK, Clark AL. Why does chronic heart failure cause breathlessness and fatigue? Prog Cardiovasc Dis. 2007;49:366–384. doi: 10.1016/j.pcad.2006.10.003. doi:10.1016/j.pcad.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Ekman I, Ehrenberg A. Fatigue in chronic heart failure—does gender make a difference? Eur J Cardiovasc Nurs. 2002;1:77–82. doi: 10.1016/S1474-5151(01)00016-0. doi:10.1016/S1474-5151(01)00016-0. [DOI] [PubMed] [Google Scholar]
- 9.Ekman I, Cleland JG, Andersson B, Swedberg K. Exploring symptoms in chronic heart failure. Eur J Heart Fail. 2005;7:699–703. doi: 10.1016/j.ejheart.2005.07.003. doi:10.1016/j.ejheart.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Falk K, Swedberg K, Gaston-Johansson F, Ekman I. Fatigue and anaemia in patients with chronic heart failure. Eur J Heart Fail. 2006;8:744–749. doi: 10.1016/j.ejheart.2006.01.016. doi:10.1016/j.ejheart.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Falk K, Swedberg K, Gaston-Johansson F, Ekman I. Fatigue is a prevalent and severe symptom associated with uncertainty and sense of coherence in patients with chronic heart failure. Eur J Cardiovasc Nurs. 2007;6:99–104. doi: 10.1016/j.ejcnurse.2006.05.004. doi:10.1016/j.ejcnurse.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Smith OR, Michielsen HJ, Pelle AJ, Schiffer AA, Winter JB, Denollet J. Symptoms of fatigue in chronic heart failure patients: clinical and psychological predictors. Eur J Heart Fail. 2007;9:922–927. doi: 10.1016/j.ejheart.2007.05.016. doi:10.1016/j.ejheart.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Friedman MM, King KB. Correlates of fatigue in older women with heart failure. Heart Lung. 1995;24:512–518. doi: 10.1016/s0147-9563(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 14.Smolderen KG, Aquarius AE, de Vries J, Smith OR, Hamming JF, Denollet J. Depressive symptoms in peripheral arterial disease: a follow-up study on prevalence, stability, and risk factors. J Affect Disord. 2008;110:27–35. doi: 10.1016/j.jad.2007.12.238. doi:10.1016/j.jad.2007.12.238. [DOI] [PubMed] [Google Scholar]
- 15.Tiesinga LJ, Dassen TW, Halfens RJ. DUFS and DEFS: development, reliability and validity of the Dutch Fatigue Scale and the Dutch Exertion Fatigue Scale. Int J Nurs Stud. 1998;35:115–123. doi: 10.1016/s0020-7489(98)00005-4. doi:10.1016/S0020-7489(98)00005-4. [DOI] [PubMed] [Google Scholar]
- 16.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J Psychosom Res. 2003;54:345–352. doi: 10.1016/s0022-3999(02)00392-6. doi:10.1016/S0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 17.Vermunt JK, Magidson J. Latent GOLD's User's Guide. Boston: Statistical Innovations Inc.; 2000. [Google Scholar]
- 18.Magidson J, Vermunt JK. Use of latent class regression models with a random intercept to remove overall response level effects in ratings data. In: Rizzi A, Vichi M, editors. Proceedings in Computational Statistics. Heidelberg: Springer; 2006. pp. 351–360. [Google Scholar]
- 19.Andrews RL, Currim IS. A Comparison of segment retention criteria for finite mixture logit models. J Marketing Res. 2003;40:235–243. doi:10.1509/jmkr.40.2.235.19225. [Google Scholar]
- 20.Dias JG. The Netherlands: University of Groningen; 2004. Finite mixture models: review, applications, and computer intensive methods. PhD Dissertation. [Google Scholar]
- 21.Everitt B. The Analysis of Contigency Tables. London: Chapman and Hall; 1977. [Google Scholar]
- 22.Martens EJ, Smith OR, Winter J, Denollet J, Pedersen SS. Cardiac history, prior depression and personality predict course of depressive symptoms after myocardial infarction. Psychol Med. 2008;38:257–264. doi: 10.1017/S0033291707001377. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen SS, Smith OR, De Vries J, Appels A, Denollet J. Course of anxiety symptoms over an 18-month period in exhausted patients post percutaneous coronary intervention. Psychosom Med. 2008;70:349–355. doi: 10.1097/PSY.0b013e3181656540. doi:10.1097/PSY.0b013e3181656540. [DOI] [PubMed] [Google Scholar]
- 24.Bennet SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Deer M, Murray MD. Discriminant properties of commonly used quality of life measures in heart failure. Qual Life Res. 2002;11:349–359. doi: 10.1023/a:1015547713061. doi:10.1023/A:1015547713061. [DOI] [PubMed] [Google Scholar]
- 25.Yennurajalingam S, Bruera E. Palliative management of fatigue at the close of life: ‘it feels like my body is just worn out. JAMA. 2007;297:295–304. doi: 10.1001/jama.297.3.295. doi:10.1001/jama.297.3.295. [DOI] [PubMed] [Google Scholar]
- 26.Swain MG. Fatigue in chronic disease. Clin Sci (Lond) 2000;99:1–8. doi:10.1042/CS19990372. [PubMed] [Google Scholar]
- 27.Appels A, Bar FW, Bar J, Bruggeman C, de Baets M. Inflammation, depressive symptomatology, and coronary artery disease. Psychosom Med. 2000;62:601–605. doi: 10.1097/00006842-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behav Immun. 2005;19:555–563. doi: 10.1016/j.bbi.2005.01.001. doi:10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Krumholz HM, Amatruda J, Smith GL, Mattera JA, Roumanis SA, Radford MJ, Crombie P, Vaccarino V. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83–89. doi: 10.1016/s0735-1097(01)01699-0. doi:10.1016/S0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- 30.Kaptein KI, de Jonge P, van den Brink RH, Korf J. Course of depressive symptoms after myocardial infarction and cardiac prognosis: a latent class analysis. Psychosom Med. 2006;68:662–668. doi: 10.1097/01.psy.0000233237.79085.57. doi:10.1097/01.psy.0000233237.79085.57. [DOI] [PubMed] [Google Scholar]
- 31.Le Grande MR, Elliott PC, Murphy BM, Worcester MU, Higgins RO, Ernest CS, Goble AJ. Health related quality of life trajectories and predictors following coronary artery bypass surgery. Health Qual Life Outcomes. 2006;4:49. doi: 10.1186/1477-7525-4-49. doi:10.1186/1477-7525-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]