Abstract
Aims
To evaluate the association of serum phosphorus with cardiac structure/function and incident heart failure.
Methods and results
We related serum phosphorus to echocardiographic left ventricular (LV) measurements cross-sectionally, and to incident heart failure prospectively in 3300 participants (mean age 44 years, 51% women) free of heart failure, myocardial infarction, and chronic kidney disease (estimated glomerular filtration rate [eGFR]<60 mL/min/1.73 m2). Cross-sectionally, serum phosphorus was related positively to LV mass, internal dimensions, and systolic dysfunction. On follow-up (mean 17.4 years), 157 individuals developed heart failure. In models adjusting for established risk factors as time-varying covariates, each mg/dL increment in serum phosphorus was associated with a 1.74-fold risk of heart failure [95% confidence intervals (CI) 1.17–2.59]. Individuals in the highest serum phosphorus quartile experienced a two-fold (95% CI 1.28–3.40) risk of heart failure compared with participants in the lowest quartile. These relations were maintained upon additional adjustment for LV mass/dimensions and systolic dysfunction. In analyses restricted to individuals with eGFR >90 mL/min/1.73 m2, no proteinuria and serum phosphorus <4.5 mg/dL, the association of serum phosphorus with heart failure remained robust.
Conclusion
In our community-based sample, higher serum phosphorus was associated with greater LV mass cross-sectionally, and with an increased risk of heart failure prospectively.
Keywords: Phosphorus, Congestive heart failure, Ventricle, Epidemiology, Risk factors
Introduction
Serum phosphorus concentration is tightly regulated, being determined by intestinal absorption, utilization, and renal excretion.1 The intestinal absorption of phosphorus correlates positively with dietary intake.2 Dietary phosphorus intake also regulates levels of the fibroblast growth factor-23 (FGF-23), which modulates renal excretion of phosphorus.3 In chronic kidney disease (CKD) patients, phosphorus excretion is impaired, which results in the elevation of serum phosphorus concentrations. High serum phosphorus levels in CKD patients are associated with increased cardiovascular disease (CVD) mortality.4 These clinical observations are consistent with experimental evidence that a greater dietary intake of phosphorus and higher resultant serum phosphorus levels are associated with cardiac fibrosis in uraemic rodents.5
Recent data suggest that higher serum phosphorus concentrations within the ‘normal range’ are associated with greater risk of developing CVD in individuals without CKD,6 and in the general population.7 One mechanism by which phosphorus may pose greater cardiovascular hazard is the association of higher serum concentrations with left ventricular (LV) hypertrophy8 and systolic dysfunction, a relationship well described in CKD.9 Investigators have also linked higher serum phosphorus to a greater prevalence10 and a higher incidence of heart failure in patients with prior myocardial infarction (MI).6
It is unclear, however, if serum phosphorus concentrations within the ‘normal’ range are associated with LV remodelling and heart failure in individuals without prior MI or CKD. We examined this hypothesis by relating serum phosphorus concentrations to LV mass, dimensions, and systolic function cross-sectionally, and to the incidence of heart failure longitudinally in a community-based sample.
Methods
Study sample
In 1972, the children of the original Framingham Study participants were enrolled along with their spouses into the Framingham Offspring Study (n = 5124).11 Attendees of the second examination of the Offspring cohort (1979–82) with available laboratory data for serum phosphorus (n = 3666) were eligible for the present investigation. We excluded 366 individuals for the following reasons: a previous MI, congestive heart failure, atrial fibrillation, or an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. After exclusions, 3300 participants (1684 women) remained eligible. For cross-sectional analyses, echocardiographic data were available on 3088 of the 3300 participants (93.6%). The present study complies with the Declaration of Helsinki. All participants provided written informed consent, and the study protocol was approved by Institutional Review Board of the Boston University Medical Center.
Measurement of serum phosphorus and risk factors
At Heart Study visits, attendees underwent medical history, physical examination, and laboratory assessment of vascular risk factors. Hypertension was defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg or the use of antihypertensive medications. Alcohol intake was assessed by averaging the self-reported weekly consumption of alcoholic drinks. Valvular disease was defined as the presence of any diastolic murmur, or of a systolic murmur ≥3/6 on physical examination by a Heart Study physician. Diabetes was defined as a fasting blood sugar level of ≥126 mg/dL or the use of any hypoglycaemic agent. Fasting concentrations of serum total cholesterol, high-density lipoprotein cholesterol, serum albumin, blood sugar, and haemoglobin were measured using standardized assays. Serum creatinine was measured using the modified Jaffe method, and values were calibrated to the Cleveland Clinic Laboratory standard using a correction factor of 0.23 mg/dL. The modification of diet in renal disease (MDRD) equation was used to calculate eGFR thus: 186.3 × (serum creatinine)−1.154 × age−0.203 × (0.742 for women). Proteinuria was measured by the urine dipstick test (Ames labstix, Elkhardt, IN, USA) and coded as none, trace, 1+, 2+, or more. For analytical purposes, we dichotomized proteinuria as ‘none’ vs. ≥‘trace.’ Serum phosphorus concentrations were measured on fasting blood samples using the standard colorimetric method (Roche Diagnostics, Alameda, CA, USA) with an intra-assay coefficient of variation of 5.6%.7
Echocardiographic measurements
All attendees at the second Offspring examination underwent routine transthoracic two-dimensionally guided M-mode echocardiography using a Hoffrel 201 ultrasound machine with an Aerotech 2.25-MHz transducer. The end-diastolic measurements of the left ventricle internal diameter (LVID) and the thicknesses of the posterior wall (PW) and interventricular septum (IVS) were obtained by averaging M-mode measurements in three cardiac cycles using a ‘leading edge’ technique, in accordance with the American Society of Echocardiography guidelines. Left ventricular wall thickness (LVWT) was derived by summing the diastolic thicknesses of the IVS and the PW. Left ventricular mass was calculated by using the formula:
LV mass = 0.8[1.04 (LVID + LVWT)3 − (LVID)3] + 0.6.
Left ventricular endocardial fractional shortening (FS) was calculated as (LVID − LV end-systolic diameter)/LVID.
Assessment of heart failure on follow-up
The follow-up period for the current investigation was from the second examination (1979–82) through December 2007. All Heart Study participants were under continuous surveillance for the occurrence of CVD. A team of three investigators reviewed all medical records for adjudicating possible CVD events. A diagnosis of heart failure was based on the presence of two major, or of one major and two minor criteria (Appendix 1).12
Statistical analyses
For cross-sectional analyses, we standardized the LV variables within each sex (mean = 0, SD = 1). We used sex-pooled multivariable linear regression to relate serum phosphorus (independent variable) to the following sex-standardized measurements (dependent variables): LV mass, LVWT, LVID, and FS. We used multivariable logistic regression models to assess the cross-sectional association of serum phosphorus with LV systolic dysfunction (FS <0.29, corresponding to an ejection fraction of 0.50).13 All multivariable models adjusted for age, sex, height, weight, diabetes mellitus, systolic blood pressure, treatment for hypertension, smoking, total cholesterol/HDL cholesterol ratio, valve disease, albumin, haemoglobin, eGFR, and proteinuria. We also assessed for interactions of serum phosphorus with age, sex, pulse pressure, and hypertension in multivariable models.
Our prospective analyses pooled sexes to maximize statistical power. We used Cox regression to relate serum phosphorus to the heart failure incidence, modelling serum phosphorus as a continuous variable, and as sex-specific quartiles. In primary analyses, models adjusted for baseline age, haemoglobin, and serum albumin, and for the following variables modelled as time-dependent covariates (updated every 4 years): body mass index, systolic and diastolic blood pressure, use of antihypertensive drugs, diabetes, smoking, total/high-density cholesterol ratio, presence of valve disease, eGFR, proteinuria, and occurrence of an MI on follow-up. We also performed several secondary analyses (Appendix 2). A two-sided P-value <0.05 was considered statistically significant.
Results
The baseline characteristics of the study sample according to sex are displayed in Table 1. Mean serum phosphorus levels were approximately 0.20 mg/dL lower in men compared with women in each corresponding quartile including in subgroups, as reported by others.14
Table 1.
Baseline characteristics of study participants
| Characteristic | Men (N = 1616) | Women (N = 1684) |
|---|---|---|
| Age (years) | 44.7 (10.3) | 44.0 (9.9) |
| Body mass index (kg/m2) | 26.9 (3.7) | 24.8 (4.8) |
| Systolic blood pressure (mmHg) | 126 (16) | 118 (16) |
| Diastolic blood pressure (mmHg) | 81 (9) | 75 (9) |
| Pulse pressure (mmHg) | 45 (11) | 43 (11) |
| Hypertension (%) | 28.0 | 17.2 |
| Treatment for hypertension (%) | 9.9 | 8.3 |
| Diabetes (%) | 6.5 | 3.0 |
| Smoking (%) | 35.7 | 37.0 |
| Alcohol (drinks/week) | 5.3 (6.5) | 2.3 (3.1) |
| Laboratory parameters | ||
| Total/HDL cholesterol | 5.1 (1.6) | 4.0 (1.3) |
| Triglycerides (mg/dL) | 125 (88) | 87 (70) |
| eGFR (mL/min/1.73 m2)a | 106 (43) | 114 (76) |
| Serum creatinine (mg/dL) | 1.2 (0.2) | 1.0 (0.2) |
| Albumin (gm/dL) | 4.5 (0.3) | 4.4 (0.3) |
| Haemoglobin (gm/dL) | 15.5 (1.0) | 13.7 (1.0) |
| Proteinuria (%) | 2.4 | 1.4 |
| Electrolytes | ||
| Calcium (mg/dL) | 9.64 (0.39) | 9.58 (0.38) |
| Corrected calcium (mg/dL)b | ||
| Quartile 1, mean (range) | 9.2 (6.1–9.4) | 9.1 (8.5–9.3) |
| Quartile 1, mean (range) | 9.6 (9.4–9.6) | 9.5 (9.3–9.6) |
| Quartile 1, mean (range) | 9.8 (9.7–9.9) | 9.7 (9.6–9.8) |
| Quartile 1, mean (range) | 10.1 (9.9–11.1) | 10.1 (9.8–11.2) |
| Phosphate (mg/dL)c | ||
| Quartile 1, mean (range) | 2.5 (1.6–2.7) | 2.7 (1.8–2.9) |
| Quartile 2, mean (range) | 2.9 (2.8–3.0) | 3.1 (3.0–3.2) |
| Quartile 3, mean (range) | 3.2 (3.1–3.3) | 3.4 (3.3–3.5) |
| Quartile 4, mean (range) | 3.6 (3.4–5.0) | 3.8 (3.6–6.2) |
| Echocardiographic variablesd | ||
| LV mass (g) | 186.3 (41.5) | 127.1 (28.8) |
| LV mass indexed to BSA | 94.2 (18.7) | 76.0 (14.0) |
| LV diastolic dimension (cm) | 5.12 (0.38) | 4.62 (0.34) |
| LV wall thickness (cm) | 1.93 (0.26) | 1.64 (0.22) |
| Fractional shortening | 0.36 (0.03) | 0.38 (0.04) |
All values are mean (standard deviation) unless otherwise indicated.
LV, left ventricle; BSA, body surface area.
aCorrected serum calcium = observed serum calcium + [0.8 × (4 − serum albumin)], if the serum albumin level is less than 4 g/dL.
bTo convert serum phosphorus levels from mg/dL to mmol/L multiply by 0.3229.
ceGFR denotes estimated glomerular filtration rate calculated by using the MDRD equation (see text).
dAll echocardiographic variables are standardized for each sex and represent the subgroup of individuals with available data (men = 1589, women = 1657).
Cross-sectional relations of serum phosphorus and echocardiographic variables
Higher serum phosphorus was positively associated with LV mass (P = 0.04) and LVID (P = 0.008) in multivariable-adjusted models (Table 2). The association of serum phosphorus with LV mass was maintained when LV mass was indexed to body surface area (P = 0.04) and when individuals with angina or coronary insufficiency (n = 38) were excluded from the analyses (P = 0.03). Left ventricular wall thickness was not related to serum phosphorus cross-sectionally. In logistic regression models, higher serum phosphorus was associated with a 2.2-fold higher odds of reduced FS (P = 0.052). We did not observe effect modification by age, sex, or hypertension status (P-value for interactions >0.05). The magnitude of the association with LV mass was quite modest (footnote, Table 2).
Table 2.
Cross-sectional relations of echocardiographic variables and serum phosphorus
| Echocardiographic variables | ||
|---|---|---|
| Linear regression | βa (standard error) | P-value |
| LV mass | 0.08 (0.04) | 0.04 |
| LV diastolic internal dimensions | 0.11 (0.04) | 0.008 |
| LV wall thickness | 0.03 (0.04) | 0.43 |
| Logistic regression | Odds ratio (95% CI)b | P-value |
| LV systolic dysfunction (fractional shortening <0.29) | 2.20 (0.99–4.85) | 0.052 |
aBeta coefficient is the increment in LV measure (dependent variable) in sex-specific standardized units for each 1 mg/dL increase in serum phosphorus (independent variable). Thus, a 1 mg/dL increment in serum phosphorus is associated with an increment in LV mass of: (0.08 × 41.5), or 3.32 gm in men; (0.08 × 28.8), or 2.30 gm in women.
bPer 1 mg/dL increment in serum phosphorus levels. There were 38 individuals with LV systolic dysfunction (total sample = 3088).
Relations of serum phosphorus to incidence of heart failure
On follow-up (mean 17.4 years), 157 participants (63 women) developed new-onset heart failure, 246 had an MI and 54 of them had both an MI and heart failure. Age- and sex-adjusted incidence rates of heart failure rose across quartiles of serum phosphorus in the entire sample and in the subsample with eGFR ≥90 mL/min/1.73 m2, those without proteinuria or with serum phosphorus <4.5 mg/dL (Table 3).
Table 3.
Age and sex-adjusted cumulative incidence of heart failure per 1000 person-years according to sex-specific quartiles of serum phosphorus
| Quartiles of serum phosphorusa | No. events/no. at risk (%) | Age- and sex-adjusted incidence of heart failure per 1000 person-years (95% CI) |
|---|---|---|
| All individuals (n = 3300) | ||
| Quartile 1 | 32/764 (4.19) | 2.36 (1.61–3.33) |
| Quartile 2 | 43/885 (4.86) | 2.70 (1.96–3.64) |
| Quartile 3 | 39/899 (4.34) | 2.58 (1.84–3.53) |
| Quartile 4 | 43/752 (5.72) | 3.45 (2.50–4.65) |
| Subgroupb (n = 1850) | ||
| Quartile 1 | 11/405 (2.72) | 1.59 (0.79–2.84) |
| Quartile 2 | 20/483 (4.14) | 2.30 (1.40–3.55) |
| Quartile 3 | 16/497 (3.22) | 1.99 (1.14–3.24) |
| Quartile 4 | 24/465 (5.16) | 3.17 (2.03–4.71) |
aAll quartile data are calculated by pooling sex-specific quartiles. Sex-specific quartile cut-points for serum phosphorus (in mg/dL) are displayed in Table 1.
bIndividuals with eGFR >90 mL/min/1.73 m2, without proteinuria, and serum phosphorus <4.5 mg/dL.
In multivariable models, a 1 mg/dL increase in baseline levels of serum phosphorus was associated with a 74% higher [95% confidence intervals (CI) 17–159%; P = 0.007] risk of new-onset heart failure (Table 4) even with adjustment for interim MI on follow-up. We also substituted pulse pressure in place of systolic blood pressure in multivariable models and found no significant difference in the results [hazard ratio (HR) per 1 mg/dL increase of serum phosphorus 1.90, 95% CI 1.27, 2.85; P = 0.002].
Table 4.
Cox proportional hazard models examining the relations of serum phosphorus levels to incidence of heart failure
| Modela | Entire sample |
Subsampleb |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Serum phosphorus, per mg/dL increment | 1.74 (1.17–2.59) | 0.007 | 2.40 (1.29–4.46) | 0.006 |
| Categorical modelc | ||||
| Quartile 1 | Referent | Referent | ||
| Quartile 2 | 1.62 (0.99–2.64) | 0.05 | 2.26 (1.00–5.11) | 0.05 |
| Quartile 3 | 1.18 (0.71–1.96) | 0.53 | 1.40 (0.59–3.32) | 0.45 |
| Quartile 4 | 2.09 (1.28–3.40) | 0.003 | 3.11 (1.40–6.94) | 0.006 |
| Trend across quartiles | 1.20 (1.03–1.40) | 0.02 | 1.33 (1.04–1.69) | 0.02 |
aAll multivariable models are adjusted for baseline age, sex, haemoglobin, serum albumin, and for the following time-dependent variables (updated every 4 years): BMI, diabetes mellitus, systolic blood pressure, treatment for hypertension, smoking, total/HDL cholesterol ratio, valve disease, eGFR, proteinuria, and interim MI on follow-up.
bSubsample included individuals with eGFR >90 mL/min/1.73 m2, without proteinuria and with serum phosphorus <4.5 mg/dL. There were 71 heart failure cases in this subgroup.
cAll quartiles for categorical models were analysed by pooling sex-specific quartiles (see Table 1 for quartile cut-points for the whole sample. Subsample quartiles for men were 1.6–2.7, 2.8–3.0, 3.1–3.3, and 3.4–4.4 mg/dL; and for women were 1.9–2.9, 3.0–3.2, 3.3–3.5, and 3.6–4.4 mg/dL).
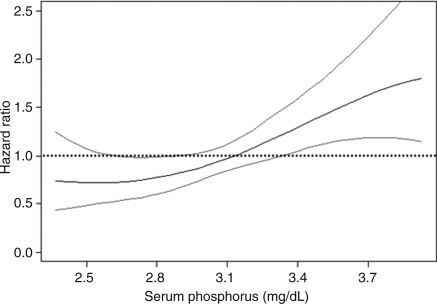
In quartile-based analyses, the highest quartile of serum phosphorus was associated with a two-fold risk (95% CI 1.28–3.40; P = 0.003) of heart failure relative to the lowest quartile (Table 4); the use of sex-specific quartiles complemented the analyses of serum phosphorus as a continuous variable, in which values for men and women were pooled and models adjusted for sex. There was a statistically significant trend of rising heart failure risk across quartiles of serum phosphorus (P = 0.02). In sensitivity analyses using pooled sex quartiles (instead of sex-specific quartiles), we obtained results consistent with the primary analysis [HR per quartile increment 1.29, 95% CI 1.10–1.52, P = 0.002; HR for the top quartile (relative to the lowest) 2.14, 95% CI 1.31–3.50, P = 0.003]. These results were corroborated with regression splines that demonstrated a linear trend for higher heart failure risk across serum phosphorus values (Figure 1). We did not observe any effect modification by age, sex, hypertension, pulse pressure, or eGFR (all P-values exceeded 0.05).
Figure 1.
Regression spline based on curve with multivariable models (adjusted for all baseline covariates—see text) examining relations of serum phosphorus to the incidence of heart failure on follow-up. Figure shows estimated multivariable hazard ratios for heart failure (Y axis) in relation to serum phosphate (X axis) as a function of penalized regression splines.
In adjusting for LV internal dimensions (or LV mass) and presence of LV systolic dysfunction, the results of primary analyses remained robust (Table 5). In analyses excluding the 38 individuals with prevalent LV systolic dysfunction, the association of serum phosphorus with heart failure was maintained (Table 5).
Table 5.
Cox proportional hazard models examining the relations of serum phosphorus levels to incidence of heart failure: additional adjustment for LV measurements
| Multivariable modelsa | Entire sample with echocardiographic data |
Subsampleb |
||
|---|---|---|---|---|
| Hazard ratio per mg/dL serum phosphorus incrementa (95% CI) | P-value | Hazard ratio per mg/dL serum phosphorus incrementa (95% CI) | P-value | |
| A. Samples including individuals with prevalent LV systolic dysfunctionc | ||||
| 1. Adjusting for LV mass and LV systolic dysfunction | 1.79 (1.14–2.81) | 0.01 | 3.06 (1.47–6.36) | 0.003 |
| 2. Adjusting for LV diastolic dimensions and LV systolic dysfunction | 1.71 (1.09–2.68) | 0.02 | 2.73 (1.33–5.62) | 0.007 |
| B. Samples excluding individuals with prevalent LV systolic dysfunctionc | ||||
| 1. Adjusting for LV mass | 1.86 (1.17–2.94) | 0.008 | 3.26 (1.56–6.81) | 0.002 |
| 2. Adjusting for LV diastolic dimensions | 1.79 (1.13–2.82) | 0.02 | 2.96 (1.43–6.15) | 0.004 |
aAll multivariable models are adjusted for baseline age, sex, haemoglobin, serum albumin, and for the following time-dependent variables (updated every 4 years): BMI, diabetes mellitus, systolic blood pressure, treatment for hypertension, smoking, total/HDL cholesterol ratio, valve disease, eGFR, proteinuria, and interim MI on follow-up.
bSubsample included individuals with eGFR >90 mL/min/1.73 m2, without proteinuria and with serum phosphorus <4.5 mg/dL, and with available echocardiographic measurements (n = 1739).
cA. There were 120 heart failure events in the entire sample with available echocardiographic data, and 56 heart failure events in the subsample (defined above). B. There were 115 heart failure events in the entire sample with available echocardiographic data, and 54 events in the subsample (defined above). Thirty-eight individuals with LV systolic dysfunction in the larger sample, and 15 individuals with LV systolic dysfunction in the smaller sample were excluded (compared with samples in A).
Subgroup analyses
Subgroup analyses were performed to exclude confounding by subclinical kidney disease. There were 71 incident heart failure events in the 1850 individuals with ‘normal’ renal function, (eGFR >90 mL/min/1.73 m2, no proteinuria) and with a serum phosphorus <4.5 mg/dL (Table 3). In time-dependent covariate models, a 1 mg/dL increase in serum phosphorus was associated with a 2.40-fold incidence of heart failure (Table 4). Individuals in the fourth quartile of serum phosphorus carried a 3.11-fold risk of new-onset heart failure compared with those in the lowest quartile (Table 4). In this subgroup of individuals with potentially normal kidney functions, the increasing trend of higher incidence of heart failure with greater serum phosphorus remained significant (P = 0.02).
In additional analyses, the association remained strong in models adjusting for LV systolic dysfunction and LV internal dimensions (or LV mass; Table 5), and in analyses excluding 15 individuals with LV systolic dysfunction in this subsample (Table 5).
Discussion
Principal findings
First, cross-sectionally higher serum phosphorus was associated with greater LV mass and larger LV internal dimensions in individuals without CKD or prior MI, and this association was independent of hypertension, favouring the possibility that serum phosphorus is associated with eccentric hypertrophy (as opposed to concentric hypertrophy). The magnitudes of these associations were modest, rendering their clinical significance unclear. Second, serum phosphorus within a ‘normal’ range was positively associated with the risk of heart failure. Individuals in the highest quartile of serum phosphorus experienced a more than two-fold risk of heart failure compared with the lowest quartile, a finding that remained robust in the subgroup of individuals with ‘normal’ kidney function (as defined by eGFR calculated with the MDRD equation). Third, the association of serum phosphorus with heart failure risk persisted in analyses adjusting for LV dimensions/mass, and in subsamples excluding individuals with LV systolic dysfunction, suggesting that the cross-sectional relations to subclinical LV remodelling could not entirely account for the observed association. This is not surprising, given the modest strength of the cross-sectional association. The strength of the association, its consistency across multiple analyses, the biological plausibility, and the temporal sequence are consistent with the possibility that the observed association may be causal. Of note, serum phosphorus levels may be modifiable with alterations of dietary intake2,15,16 and affirmed by the Institute of Medicine.17 However, this issue is controversial because some investigators disagree.18 Additionally, serum phosphorus may differ in individuals secondary to genetic polymorphisms influencing FGF-23 production,19 or to Klotho gene variants.20 Therefore, if confirmed, our observations raise the possibility that serum phosphorus may be a modifiable risk factor for heart failure.
Mechanisms
Several mechanisms may explain our observations. First, higher serum phosphorus may be a marker of low vitamin D levels.15 Lower serum vitamin D is cross-sectionally associated with greater plasma renin activity,21 higher blood pressure,22 prevalent heart failure,10 and with a greater risk of CVD in the community.23 Additionally, vitamin D supplementation lowers serum phosphorus and is associated with an improvement in LV systolic function24 and in the circulating cytokine profile in patients with overt heart failure.25 On a parallel note, vitamin D receptor knockout animals demonstrate hypertrophy of cardiomyocytes, perhaps secondary to over-stimulation of the renin-angiotensin system26 or as a result of the direct action of calcitriol.27 Thus, alterations in vitamin D levels in those with higher serum phosphorus may contribute to the observed link between the mineral and LV mass and heart failure.
Second, higher serum phosphorus levels (>2 mg/dL) are associated with greater vascular smooth muscle calcification in vitro, which may be partly regulated through increased osteopontin expression.28 Furthermore, higher serum phosphorus may indirectly stimulate smooth muscle cell to undergo phenotypic changes pre-disposing them to calcification through a sodium-dependent phosphate co-transporter identified as Pit-1.29 Such vascular calcification may increase vascular stiffness30 that can pre-dispose individuals to heart failure. Of note, in our analyses the association of serum phosphorus with heart failure risk was maintained in analyses adjusting for the occurrence of an MI during follow-up, suggesting that non-ischaemic mechanisms may contribute to the observed association.
Third, in animal models,26 in in vitro human parathyroid tissue cells31 and in humans,2 greater phosphorus levels have been linked to the development of secondary hyperparathyroidism. Higher parathyroid hormone (PTH) levels have been shown to induce cardiac hypertrophy32 and are related to higher CVD mortality in community dwelling individuals.33 Therefore, higher serum phosphorus may reflect subtle alterations in the PTH system, which may mediate the associations with LV mass and heart failure risk.
Strengths and limitations
The large community-based sample, the long follow-up time, the comprehensive adjustment for confounders as time-dependent covariates, and the consistency of findings in multiple analyses strengthen the present investigation. There are several limitations that must be acknowledged. We do not have data on serum PTH or vitamin D supplementation/levels or on dietary phosphorus intake at the baseline examination at which serum phosphorus was available to clarify mechanisms underlying the observed association. Also we did not have repeated phosphorus levels to assess intra-individual variability, however data from another cohort study suggests fairly good stability of serum phosphorus within individuals with a high (0.72) reliability coefficient.34 Participants in our study were mostly middle-aged and of European descent, which limits the generalizability of our results.
Conclusions
In our large community-based sample of individuals without prior MI or CKD, serum phosphorus within the ‘normal range’ was associated with greater LV mass cross-sectionally, and with increased risk of heart failure prospectively.
Funding
This work was supported by the NIH/NHLBI Contract No. N01-HC-25195; HL080124, and 2K24HL4334 (Dr R.S.V.).
Conflict of interest: none declared.
Appendix 1: heart failure criteria
Briefly, major criteria include the presence of paroxysmal nocturnal dyspnoea, jugular venous distension, orthopnoea, hepato-jugular reflex, pulmonary rales, acute pulmonary oedema, third heart sound, cardiomegaly on a radiograph, central venous pressure of >16 cm of water, and weight loss of >4.5 kg during first 5 days of treatment for suspected heart failure. Minor criteria consist of bilateral ankle oedema, dyspnoea on exertion, nocturnal cough, hepatomegaly, pleural effusion, and heart rate >120 b.p.m.22
Appendix 2: secondary analyses
In order to assess potential non-linearity of association between serum phosphorus and the risk of heart failure, we examined multivariable generalized additive models using penalized splines, adjusting for all the covariates in our multivariable models (same as in our primary analyses). We also evaluated effect modification of the association of serum phosphorus with heart failure risk by age, sex, hypertension, and eGFR by incorporating interaction terms in the multivariable models. We also performed additional analyses adjusting for baseline LV measurements (n = 3088, with available measurements were eligible) in addition to all covariates noted above, to evaluate if any potential association of serum phosphorus with heart failure was mediated by the association of the mineral with subclinical LV remodelling (as reflected by LV measurements).
To evaluate the impact of serum phosphorus within the ‘normal range’ and to avoid potential confounding by renal dysfunction, we repeated our analyses in a subsample that excluded all individuals with eGFR <90 mL/min/1.73 m2, those with proteinuria (trace or more) or with serum phosphorus ≥4.5 mg/dL (n = 1850). Paralleling the analyses of the larger sample, we performed additional analyses adjusting for LV measurements (n = 1739, with available measurements).
References
- 1.Weisinger JR, Bellorin-Font E. Magnesium and phosphorus. Lancet. 1998;352:391–396. doi: 10.1016/S0140-6736(97)10535-9. doi:10.1016/S0140-6736(97)10535-9. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg SJ, Shane E, de la CL, Segre GV, Clemens TL, Bilezikian JP. Abnormalities in parathyroid hormone secretion and 1,25-dihydroxyvitamin D3 formation in women with osteoporosis. N Engl J Med. 1989;320:277–281. doi: 10.1056/NEJM198902023200503. [DOI] [PubMed] [Google Scholar]
- 3.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. doi:10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 4.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 5.Amann K, Tornig J, Kugel B, Gross ML, Tyralla K, El Shakmak A, Szabo A, Ritz E. Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int. 2003;63:1296–1301. doi: 10.1046/j.1523-1755.2003.00864.x. doi:10.1046/j.1523-1755.2003.00864.x. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G for the Cholesterol, Recurrent Events (CARE) Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. doi:10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 7.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. doi:10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphate and left ventricular hypertrophy in young adults: the coronary artery risk development in young adults study. Kidney Blood Press Res. 2009;32:37–44. doi: 10.1159/000203348. doi:10.1159/000203348. [DOI] [PubMed] [Google Scholar]
- 9.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. doi:10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 10.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J. Am. Coll. Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. doi:10.1016/S0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 12.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 13.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. doi:10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo M, Ciacci C, De Santo NG. Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med. 2008;359:864–866. doi: 10.1056/NEJMc0800696. doi:10.1056/NEJMc0800696. [DOI] [PubMed] [Google Scholar]
- 15.Portale AA, Halloran BP, Murphy MM, Morris RC., Jr Oral intake of phosphorus can determine the serum concentration of 1,25–dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986;77:7–12. doi: 10.1172/JCI112304. doi:10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portale AA, Halloran BP, Morris RC., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987;80:1147–1154. doi: 10.1172/JCI113172. doi:10.1172/JCI113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine. Dietary Reference Intakes for Calcium Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1999. pp. 147–380. [PubMed] [Google Scholar]
- 18.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, APw-ppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. doi:10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. doi:10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 21.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 22.Kristal-Boneh E, Froom P, Harari G, Ribak J. Association of calcitriol and blood pressure in normotensive men. Hypertension. 1997;30:1289–1294. doi: 10.1161/01.hyp.30.5.1289. [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. doi:10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte KK, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, Clark AL, Cleland JG. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238–2244. doi: 10.1093/eurheartj/ehi442. doi:10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 25.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 26.Canalejo A, Hernandez A, Almaden Y, Concepcion MT, Felsenfeld A, Torres A, Rodriguez M. The effect of a high phosphorus diet on the parathyroid cell cycle. Nephrol Dial Transplant. 1998;13(Suppl. 3):19–22. doi: 10.1093/ndt/13.suppl_3.19. [DOI] [PubMed] [Google Scholar]
- 27.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–524. doi: 10.1016/j.jsbmb.2006.12.098. doi:10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giachelli CM, Speer MY, Li X, Rajachar RM, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–722. doi: 10.1161/01.RES.0000161997.24797.c0. doi:10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 29.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:10e–117. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 30.McCullough PA, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and Monckeberg's sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585–1598. doi: 10.2215/CJN.01930408. doi:10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- 31.Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez CL, Campistol JM, Torres A, Rodriguez M. High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol. 1998;9:1845–1852. doi: 10.1681/ASN.V9101845. [DOI] [PubMed] [Google Scholar]
- 32.Schluter KD, Piper HM. Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol. 1992;263:H1739–H1746. doi: 10.1152/ajpheart.1992.263.6.H1739. [DOI] [PubMed] [Google Scholar]
- 33.Hagstrom E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundstrom J, Melhus H, Held C, Lind L, Michaelsson K, Arnlov J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. doi:10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 34.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118:496–500. [PubMed] [Google Scholar]