Abstract
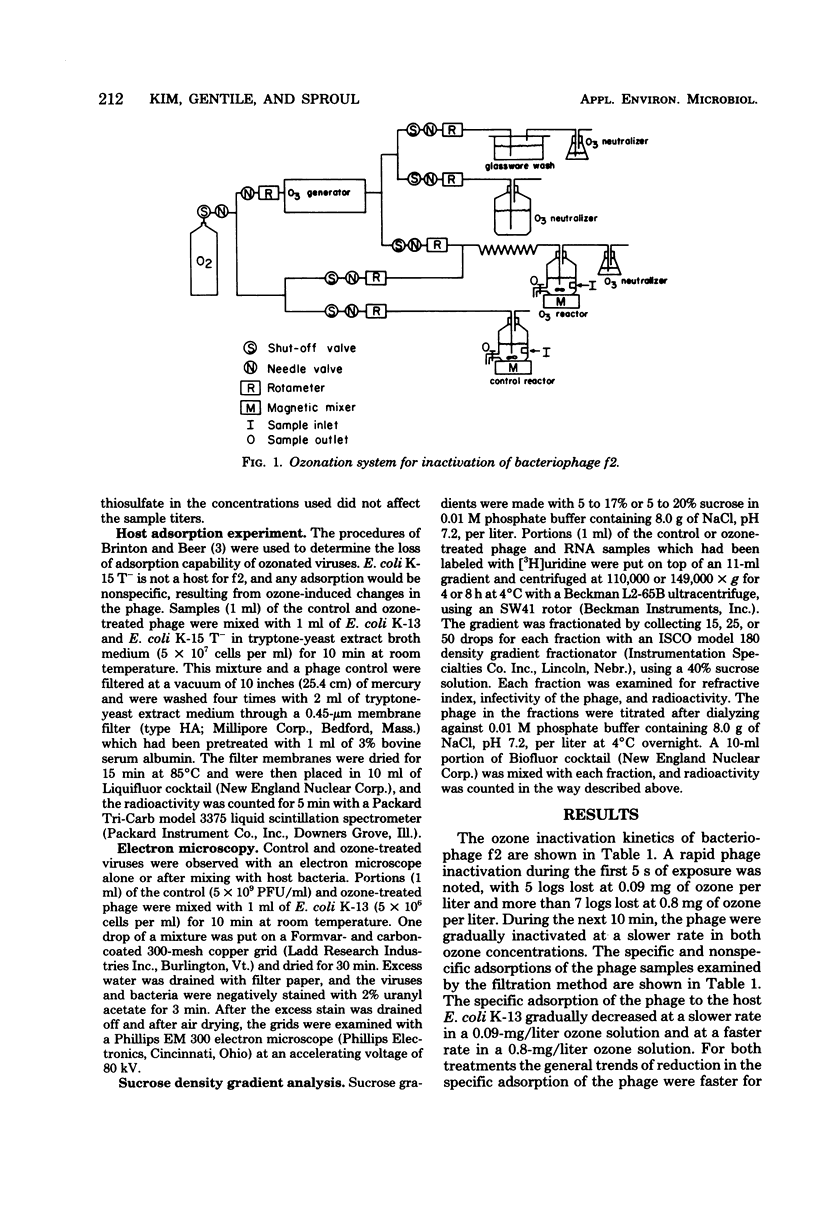
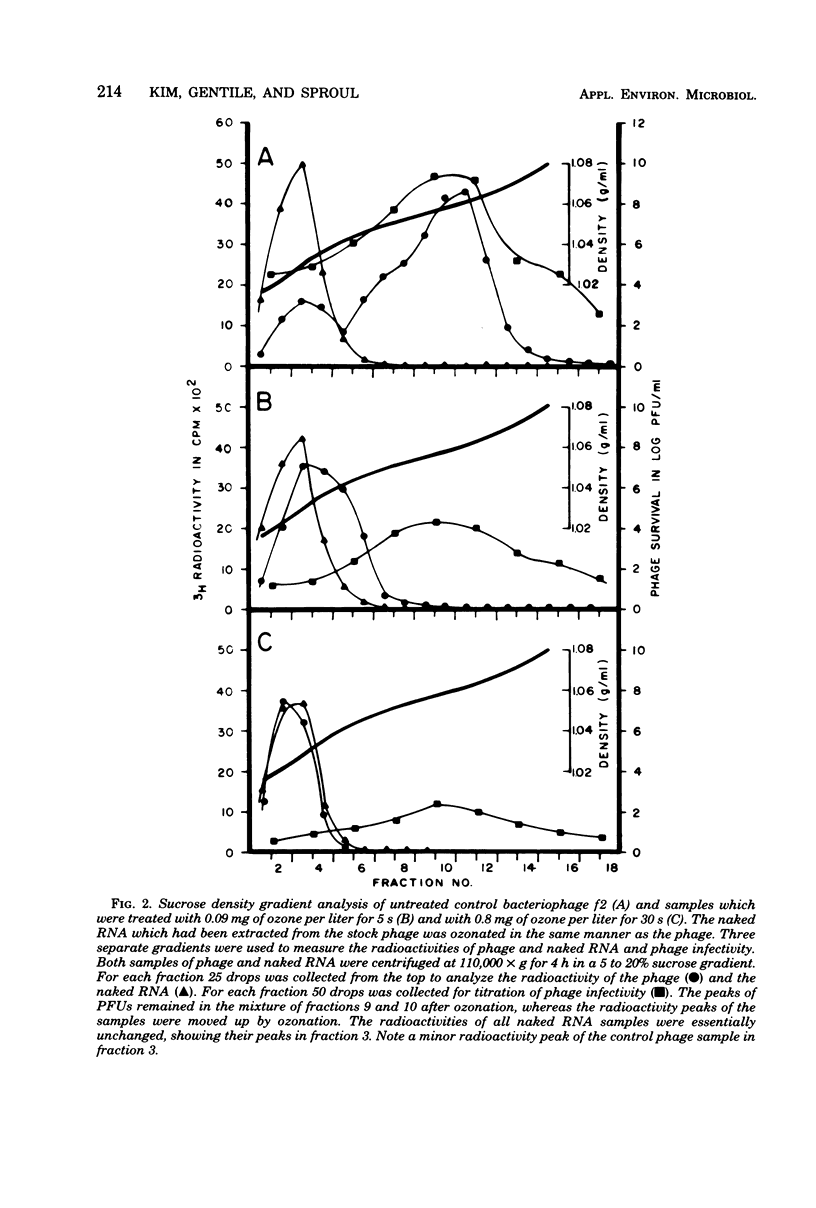
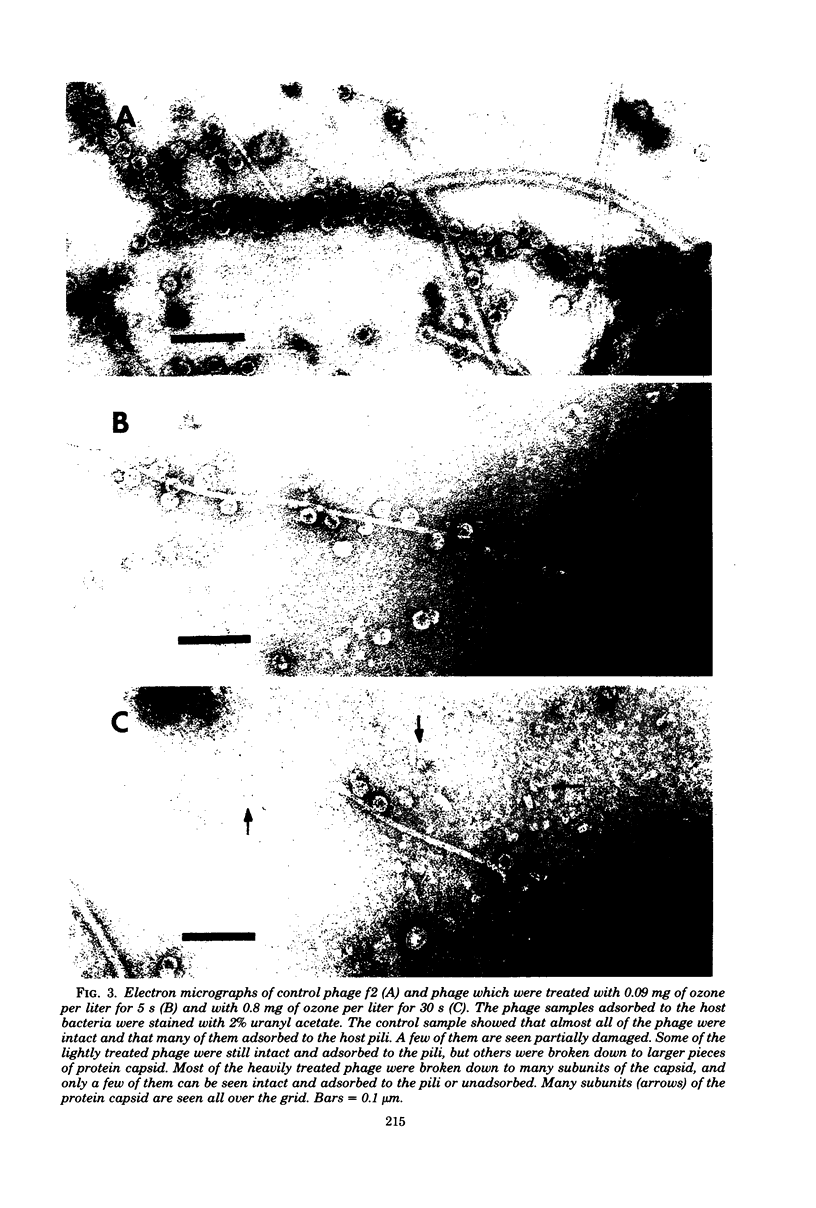
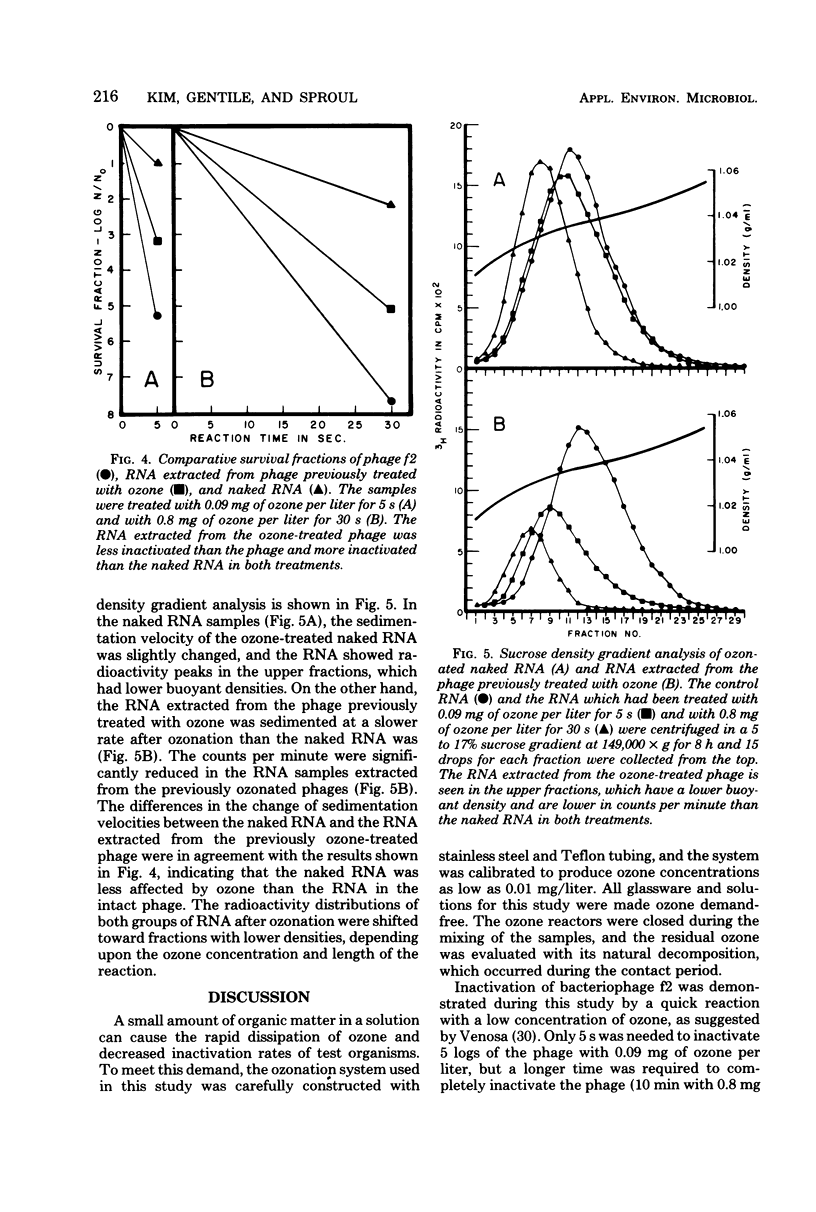
The inactivation kinetics of bacteriophage f2 were studied by using ozone under controlled laboratory conditions. The phage were rapidly inactivated during the first 5 s of the reaction by 5 and 7 logs at ozone concentrations of 0.09 and 0.8 mg/liter, respectively. During the next 10 min, the phage were further inactivated at a slower rate in both treatments. The [3H]uridine-labeled f2 phage and its ribonucleic acid (RNA) were examined to elucidate the mechanism of ozone inactivation, utilizing adsorption to host bacteria, sucrose density gradient analysis, and electron microscopy. The specific adsorption of the phage was reduced by ozonation in the same pattern as plaque-forming unit reduction. RNA was released from the phage particles during ozonation, although it had reduced infectivity for spheroplasts. Electron microscopic examination showed that the phage coat was broken by ozonation into many protein subunit pieces and that the specific adsorption of the phage to host pili was inversely related to the extent of phage breakage. The RNA enclosed in the phage coat was inactivated less by ozonation than were whole phage, but inactivated more than naked RNA. These findings suggest that ozone breaks the protein capsid into subunits, liberating RNA and disrupting adsorption to the host pili, and that the RNA may be secondarily sheared by a reduction with and/or without the coat protein molecules, which have been modified by ozonation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHRISTENSEN E., GIESE A. C. Changes in absorption spectra of nucleic acids and their derivatives following exposure to ozone and ultraviolet radiations. Arch Biochem Biophys. 1954 Jul;51(1):208–216. doi: 10.1016/0003-9861(54)90468-3. [DOI] [PubMed] [Google Scholar]
- Cramer W. N., Kawata K., Krusé C. W. Chlorination and iodination of poliovirus and f2. J Water Pollut Control Fed. 1976 Jan;48(1):61–76. [PubMed] [Google Scholar]
- Hsu Y. C., Nomura S., Krusé C. W. Some bactericidal and virucidal properties of iodine not affecting infectious RNA and DNA. Am J Epidemiol. 1965 Nov;82(3):317–328. doi: 10.1093/oxfordjournals.aje.a120552. [DOI] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S. B., Ceckler W. H., Sproul O. J. Inactivation of poliovirus in water by ozonation. J Water Pollut Control Fed. 1973 Dec;45(12):2433–2443. [PubMed] [Google Scholar]
- Mudd J. B., Leavitt R., Ongun A., McManus T. T. Reaction of ozone with amino acids and proteins. Atmos Environ. 1969 Nov;3(6):669–682. doi: 10.1016/0004-6981(69)90024-9. [DOI] [PubMed] [Google Scholar]
- Nebel C., Gottschling R. D., Hutchison R. L., McBride T. J., Taylor D. M., Pavoni J. L., Tittlebaum M. E., Spencer H. E., Fleischman M. Ozone disinfection of industrial-municipal secondary effluents. J Water Pollut Control Fed. 1973 Dec;45(12):2493–2507. [PubMed] [Google Scholar]
- O'Callaghan R., Bradley R., Paranchych W. Controlled alterations in the physical and biological properties of R17 bacteriophage induced by gunaidine hydrochloride. Virology. 1973 Aug;54(2):476–494. doi: 10.1016/0042-6822(73)90158-x. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Nathans D. Differential synthesis of bacteriophage-specific proteins in MS2-infected Escherichia coli treated with actinomycin. J Mol Biol. 1966 Dec 28;22(2):235–247. doi: 10.1016/0022-2836(66)90129-x. [DOI] [PubMed] [Google Scholar]
- Prat R., Nofre C., Cier A. Effets de l'hypochlorite de sodium, de l'ozone et des radiations ionisantes sur les constituants pyrimidiques d'Escherichia coli. Ann Inst Pasteur (Paris) 1968 May;114(5):595–607. [PubMed] [Google Scholar]
- Previero A., Coletti-Previero M. A., Jollès P. Localization of non-essential tryptophan residues for the biological activity of lysozyme. J Mol Biol. 1967 Mar 14;24(2):261–268. doi: 10.1016/0022-2836(67)90331-2. [DOI] [PubMed] [Google Scholar]
- Shah P. C., McCamish J. Relative chlorine resistance of poliovirus I and coliphages f2 and T 2 in water. Appl Microbiol. 1972 Oct;24(4):658–659. doi: 10.1128/am.24.4.658-659.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Young D. C., Sharp D. G. Poliovirus aggregates and their survival in water. Appl Environ Microbiol. 1977 Jan;33(1):168–177. doi: 10.1128/aem.33.1.168-177.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P. O., Haschemeyer R. H. Specific dissociation of bacteriophage f2 protein to an 11S component. Biochemistry. 1969 Sep;8(9):3587–3592. doi: 10.1021/bi00837a014. [DOI] [PubMed] [Google Scholar]
- de Mik G., de Groot I. Mechanisms of inactivation of bacteriophage phiX174 and its DNA in aerosols by ozone and ozonized cyclohexene. J Hyg (Lond) 1977 Apr;78(2):199–211. doi: 10.1017/s0022172400056096. [DOI] [PMC free article] [PubMed] [Google Scholar]