Abstract
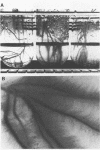
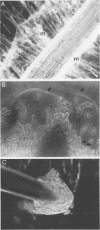
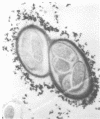
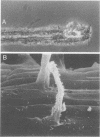
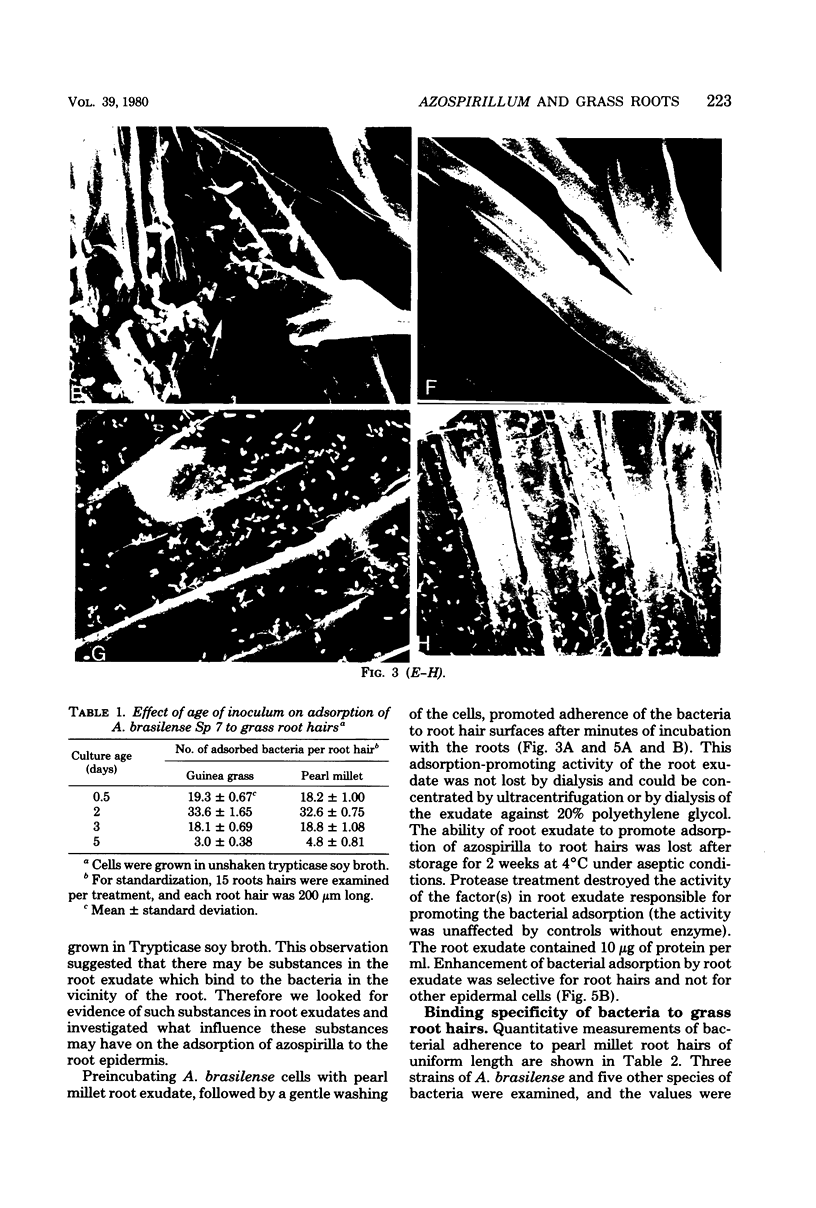
The association between grass roots and Azospirillum brasilense Sp 7 was investigated by the Fahraeus slide technique, using nitrogen-free medium. Young inoculated roots of pearl millet and guinea grass produced more mucilaginous sheath (mucigel), root hairs, and lateral roots than did uninoculated sterile controls. The bacteria were found within the mucigel that accumulated on the root cap and along the root axes. Adherent bacteria were associated with granular material on root hairs and fibrillar material on undifferentiated epidermal cells. Significantly fewer numbers of azospirilla attached to millet root hairs when the roots were grown in culture medium supplemented with 5 mM potassium nitrate. Under these growth conditions, bacterial attachment to undifferentiated epidermal cells was unaffected. Aseptically collected root exudate from pearl millet contained substances which bound to azospirilla and promoted their adsorption to the root hairs. This activity was associated with nondialyzable and proteasesensitive substances in root exudate. Millet root hairs adsorbed azospirilla in significantly higher numbers than cells of Rhizobium, Pseudomonas, Azotobacter, Klebsiella, or Escherichia. Pectolytic activities, including pectin transeliminase and endopolygalacturonase, were detected in pure cultures of A. brasilense when this species was grown in a medium containing pectin. These studies describe colonization of grass root surfaces by A. brasilense and provide a possible explanation for the limited colonization of intercellular spaces of the outer root cortex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber L. E., Tjepkema J. D., Russell S. A., Evans H. J. Acetylene reduction (nitrogen fixation) associated with corn inoculated with Spirillum. Appl Environ Microbiol. 1976 Jul;32(1):108–113. doi: 10.1128/aem.32.1.108-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Regulation by fixed nitrogen of host-symbiont recognition in the Rhizobium-clover symbiosis. Plant Physiol. 1978 Jul;62(1):18–21. doi: 10.1104/pp.62.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Northcote D. H., Pickett-Heaps J. D. A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem J. 1966 Jan;98(1):159–167. doi: 10.1042/bj0980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Methods for Growing Spirillum lipoferum and for Counting It in Pure Culture and in Association with Plants. Appl Environ Microbiol. 1977 Jan;33(1):85–88. doi: 10.1128/aem.33.1.85-88.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., Hebert R. R. Adsorption of rhizobia to cereal roots. Biochem Biophys Res Commun. 1978 Oct 16;84(3):736–742. doi: 10.1016/0006-291x(78)90766-0. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Bouton J. H., Schank S. C., Quesenberry K. H., Tyler M. E., Milam J. R., Gaskins M. H., Littell R. C. Nitrogen Fixation in Grasses Inoculated with Spirillum lipoferum. Science. 1976 Sep 10;193(4257):1003–1005. doi: 10.1126/science.193.4257.1003. [DOI] [PubMed] [Google Scholar]
- Tarrand J. J., Krieg N. R., Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978 Aug;24(8):967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]