Abstract
Background
Protein 4.2 deficiency caused by mutations in the EPB42 gene results in hereditary spherocytosis with characteristic alterations of CD47, CD44 and RhAG. We decided to investigate at which stage of erythropoiesis these hallmarks of protein 4.2 deficiency arise in a novel protein 4.2 patient and whether they cause disruption to the band 3 macrocomplex.
Design and Methods
We used immunoprecipitations and detergent extractability to assess the strength of protein associations within the band 3 macrocomplex and with the cytoskeleton in erythrocytes. Patient erythroblasts were cultured from peripheral blood mononuclear cells to study the effects of protein 4.2 deficiency during erythropoiesis.
Results
We report a patient with two novel mutations in EPB42 resulting in complete protein 4.2 deficiency. Immunoprecipitations revealed a weakened ankyrin-1-band 3 interaction in erythrocytes resulting in increased band 3 detergent extractability. CD44 abundance and its association with the cytoskeleton were increased. Erythroblast differentiation revealed that protein 4.2 and band 3 appear simultaneously and associate early in differentiation. Protein 4.2 deficiency results in lower CD47, higher CD44 expression and increased RhAG glycosylation starting from the basophilic stage. The normal downregulation of CD44 expression was not seen during protein 4.2(−) erythroblast differentiation. Knockdown of CD47 did not increase CD44 expression, arguing against a direct reciprocal relationship.
Conclusions
We have established that the characteristic changes caused by protein 4.2 deficiency occur early during erythropoiesis. We postulate that weakening of the ankyrin-1-band 3 association during protein 4.2 deficiency is compensated, in part, by increased CD44-cytoskeleton binding.
Keywords: erythropoiesis, erythrocyte, hereditary spherocytosis, protein 4.2, CD47, CD44
Introduction
Hereditary spherocytosis (HS) is the most common disorder of the erythrocyte membrane, affecting approximately 1 in 2,000 individuals of northern European ancestry. Clinical symptoms of hereditary spherocytosis include hemolysis, anemia, jaundice, gallstones and reticulocytosis.1–4 The disease results from mutations in the anion exchanger band 3 (SLC4A1), ankyrin-1 (ANK1), protein 4.2 (EPB42) or specific mutations in α/β-spectrin (SPTA1/SPTB) genes and affect the horizontal link between the membrane and the underlying skeleton resulting in spherocytic erythrocytes. Together with glycophorin A and the Rh complex [Rh associated glycoprotein (RhAG), the Rh polypeptides (RhCE, RhD), CD47, glycophorin B (GPB) and ICAM-4 (LW)],5 these proteins form the band 3 macrocomplex.6
The peripheral membrane protein, protein 4.2, shares significant homology to transglutaminases but lacks the catalytic triad residues required for transglutaminase activity.7–9 Stable expression of protein 4.2 in erythrocytes depends on its interaction with the band 3 and ankyrin-1.8,10–12 Protein 4.2 is thought to be expressed late during erythropoiesis13 and stabilizes the band 3-ankyrin-1 association.14–16 To date, nine mutations in EPB42 have been associated with hereditary spherocytosis,6, 17–22 some of these mutations influence protein 4.2 stability and band 3-ankyrin-1 binding.8,23 Protein 4.2 deficiency in humans causes a severe reduction in the marker of self,24 CD47 (by approximately 80%),6,25,26 suggesting an association between protein 4.2 and CD47. Furthermore, protein 4.2 deficiency causes elevated expression of the ankyrin binding protein CD4427 and increased RhAG glycosylation.6, 25 CD44 and CD47 may have a complementary role during cell migration from the bone marrow or in the circulation that explains their observed reciprocal expression during protein 4.2 deficiency. It is currently not known at what stage the characteristic alterations in protein 4.2 deficient erythrocytes occur, or whether the striking increase in CD44 is a compensatory mechanism of membrane stabilization25 or due to selective enrichment during erythrocyte membrane loss.28
To gain a better understanding of the effects of protein 4.2 deficiency, we examined the protein associations within the band 3 macrocomplex and with the cytoskeleton in peripheral erythrocytes from a new hereditary spherocytosis patient lacking protein 4.2. In addition, the patients’ peripheral blood mononuclear cells (PBMC) were used to culture progenitors and differentiated to enucleated cells, to establish when key membrane protein alterations occur during erythropoiesis.
Design and Methods
Case Study
Patient history, blood smears, erythrocyte indices and ektacyto-metric parameters are provided in the Online Supplementary Appendix.
Antibodies
Monoclonal antibodies (IBGRL, Bristol, UK) were BRIC235 and BRIC222 (CD44), BRIC172 (α-spectrin), BRIC274 (ankyrin-1), BRIC4 and BRIC10 (GPC), R1.3 (GPA/GPB), BRIC163 and BRIC256 (GPA), BRIC14 (band 3/GPA), BRIC126 and BRIC125 (CD47), LA1818 (RhAG), BRIC170, BRIC155, BRIC6 and BRAC66 (band 3;29), c-kit-PE (Pharmingen, San Diego, CA), CD71 (Pharmingen). Polyclonal antibodies to protein 4.2, protein 4.1, p55, aquaporin 1, Rh, RhAG, GPA and ankyrin-1 were described previously.26,28 Polyclonal actin and ERK-1 were purchased (Santa Cruz Biotechnology, CA, USA). CD47out1 was raised against a synthetic peptide to the first extra-cellular loop of CD47 (University of Bristol, Peptide Synthesis Facility, U.K). Secondary antibodies: rabbit anti-mouse PE conjugated, rabbit anti-mouse IgG2B and IgG1, HRP-conjugated rabbit anti-mouse and swine anti-rabbit (DAKO, Glostrup, Denmark).
cDNA and DNA analysis
mRNA isolation was carried out as described.30 Further details of the cDNA synthesis and DNA analysis are provided in Online Supplementary Figure S1.
Erythrocyte ghosts
Erythrocyte membranes were prepared as previously described.26,31
Immunoprecipitations and total cell lysates
Cells were lysed as described32 and quantified by Lowry assay (Biorad, Hercules, CA, USA). For immunoprecipitations 1.5×107 erythroblasts or 5×107 erythrocytes were pre-cleared (1h) with unbound protein-G-beads. Antibodies were pre-bound to beads and incubated with pre-cleared lysates for 1h at 4°C whilst rotating. Beads were washed 5 times in lysis buffer and proteins eluted from the beads twice using sample buffer at 95°C. Pooled eluates and total cell lysates were subjected to SDS-PAGE and stained directly with Coomassie G-25033 or electroblotted to PVDF membranes and visualized with specific antibodies stated in the legends.
C12E8 Detergent extractability of ghosts
C12E8 Detergent extractability was performed as previously described.34 Briefly, 1 volume of ghosts was added to 1 equal volume of 1% C12E8 and incubated for 10 min in hypotonic 5mM phosphate buffer (pH 7.2) at 4°C. Following ultracentrifugation at 100,000g (30 min), the supernatant contained the extractable fraction and the pellet contained the cytoskeleton fraction.
Flow cytometry
Cells (3×105) were washed in ice-cold PBS and ice-cold PB (PBS, 4% BSA). Cells were reconstituted in PB supplemented with normal rabbit serum and stained for 1h at 4°C with specific antibodies (see Figure legends). When appropriate, cells were washed once in ice-cold PB and incubated with PE conjugated anti-mouse secondary antibodies. Fluorescent signals were measured using Beckman Coulter (FC-500 MPL, Wycombe, UK) or FACS CantoII-F60 machine (BD Biosciences, Oxford, UK). To detect enucleation, aliquots (3×105) from cultures were washed once in PBS and loaded with 0.1 μg/mL Hoechst 33342 for 30 min at room temperature. Dead cells were measured with propidium Iodide (PI; 2μg/mL, Invitrogen). Fluorescent signals were measured using a BD Biosciences flow cytometer (LSR-II-F60). Data was analyzed using Flowjo 7.2.5 software (Flowjo, Ashland, OR).
Erythroblast expansion and differentiation
PBMC were isolated by density purification (Percoll; GE Healthcare, Little Chalfont, UK) (ρ1.077). Informed consent was given in accordance with the Declaration of Helsinki. Cells were cultured as described.35
Erythroblast lentiviral transduction
HEK293T cells were cultured in DMEM (Invitrogen) containing 10% fetal calf serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen). Cells were seeded in 10cm2 dishes and calcium-phosphate-transfected using lentiviral packaging vectors pVSV (5μg) and pPAX (15μg) and the shRNA containing lentiviral vector pTRIPempty or pTRIPshRNACD47 (20μg; sequences, cloning and vector information upon request). After 24h, DMEM was removed and cells were put on STEMSPAN expansion media as described above. Twenty-four hours later the media was filtered (0.45μm filters), 8μg/mL polybren (Sigma) was added and then incubated for 24h with erythroblasts to induce transduction.
Cytospins and hemoglobin assays
Cell volume was calculated by taking the diameter of cells (>20) during differentiation and using the formula 4/3π(d/2)3. Hemoglobin was measured as described.36 Hemoglobin concentration was normalized to cell volume.
Results
Genetic analysis
Genetic analysis showed that the patient was a compound heterozygote for two novel mutations within the EPB42 gene. One mutation referred to as Chartres-1, was C1305>G resulting in amino acid substitution Tyr435Stop (Online Supplementary Figure S1C). Stop mutations can influence gene transcription by giving rise to truncated transcripts or exon skipping.37 Here, C1305G resulted in mis-splicing and complete skipping of exon 9 from the mRNA. However, exon 8 remained in frame with exon 10 (Online Supplementary Figure S1D). The other mutation, referred to as Chartres-2, was a di-nucleotide deletion A1176-T1177 in exon 9 (Online Supplementary Figure S1E), taking the sequence out of frame and coding for a premature stop signal after five altered amino acids, Ser392, Cys393, Gly, Leu, Glu, Val, Leu, Stop. An alignment of mutant mRNAs with wild-type mRNA is shown in the Online Supplementary Figure S1F.
Erythrocyte membrane analysis confirmed protein 4.2 deficiency in the patient
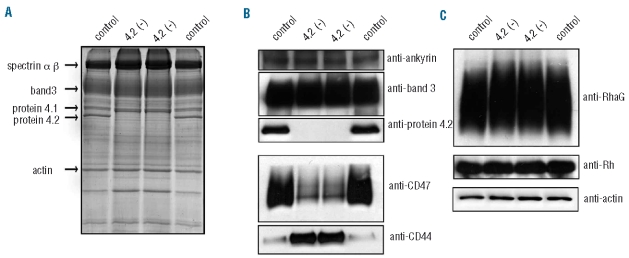
Analysis of membrane preparations (Figure 1) and lysates (data not shown) showed the absence of protein 4.2. It is currently unknown if this is due to instability of the mutant mRNAs or an instability of the proteins produced. No protein 4.2 peptides were detected in the patient’s lysates using mass spectrometry (data not shown). As reported previously for protein 4.2 deficient patients,6,25 CD47 expression was reduced to approximately 20%, CD44 expression increased 4-fold and RhAG migrated more slowly during SDS-PAGE (Figure 1B and C). Protein 4.1, p55, aquaporin-1, GPA, GPB and GPC levels were similar between control and mutant membranes (Online Supplementary Figure S2) and lysates (data not shown). Flow cytometry analysis confirmed these results (Online Supplementary Table S2).
Figure 1.
Erythrocyte membrane analysis reveals protein 4.2 deficiency. Control blood was matched to the patient’s serotype DCe/DCe. (A) 10μg control and 4.2(−) ghosts were subjected to SDS-PAGE and stained with Coomassie. (B and C), equal amounts of ghosts (5μg, right panels) were subjected to SDS-PAGE and immunoblotted using polyclonal antibodies against protein 4.2, CD47, RhAG, Rh, ankyrin-1 and actin and monoclonal antibodies against BRIC170 (band 3) and BRIC235 (CD44). Panels are representative of at least three experiments.
The band 3 association with ankyrin-1 is severely attenuated in protein 4.2(−) erythrocytes
Co-immunoprecipitations on erythrocyte lysates demonstrated unchanged interactions between band 3 and GPA, GPB and RhAG in protein 4.2(−) erythrocytes (Figure 2A and B). As expected, protein 4.2 was co-immunoprecipitated with multiple band 3 antibodies in control but not in protein 4.2(−) cells (Figure 2C). Although CD47 is reduced in protein 4.2(−) cells (Figure 1D) the remaining CD47 is still associated with band 3, and, when adjusted for CD47 levels, the amount of co-immunoprecipitated band 3 was similar to control (Figure 2D and E). Importantly, co-immunoprecipitation with ankyrin-1 antibody demonstrated that there is a severe reduction in the amount of band 3 associated with the cytoskeleton in protein 4.2(−) cells (Figure 2F).
Figure 2.
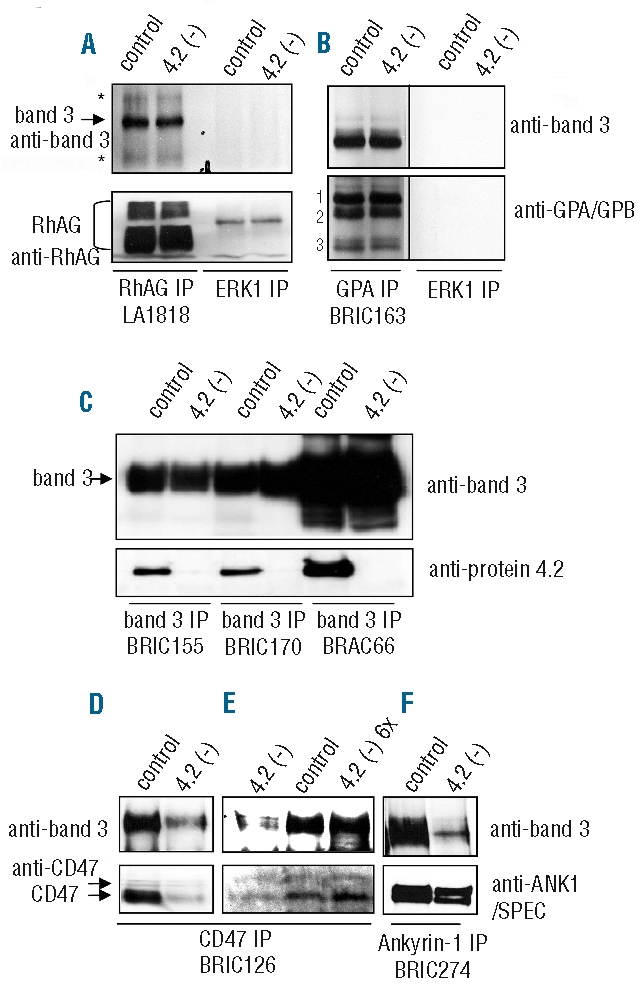
The band 3-ankyrin-1 association is attenuated in protein 4.2(−) erythrocytes. Erythrocytes were lysed in NP40 based lysis buffer and indicated proteins were immunoprecipitated, subjected to SDS-PAGE and Western blotted using the indicated antibodies. (A) RhAG (LA1818) immunoprecipitation. (B) GPA immunoprecipitations (BRIC163, GPA). Lower part: 1= GPB dimer; 2= GPA monomer and 3= GPB monomer. (C) BRIC155, BRIC170 and BRAC66 immunoprecipitates. BRAC66 consistently immunoprecipitated more band 3 and protein 4.2 than the other band 3 antibodies. (D and E) CD47 immunoprecipitates (BRIC126), 6x (E, last lane) indicates that 6 times more lysate was used to correct for lower CD47 levels in protein 4.2(−) cells. (F) Ankyrin-1 (BRIC274) immunoprecipitation showing that the band3-ankyrin-1 association is attenuated.
Protein 4.2 deficiency leads to increased CD44-cytoskeleton association and increased extractability of some band 3 macrocomplex proteins
CD44 interacts with ankyrin-1 in a multitude of cells.38 Despite the significant increase in CD44 protein levels in the protein 4.2(−) cells, we could not specifically co-immunoprecipitate ankyrin-1 with CD44 (results not shown). Furthermore we could not co-immunoprecipitate band 3 with CD44 or CD44 with band 3 (data not shown). Therefore, we used cytoskeleton extractability in the non-ionic detergent C12E814 to evaluate cytoskeleton linkage. Consistent with a reduced ankyrin-1-band 3 interaction, band 3 is more extractable from protein 4.2(−) ghosts (Figure 3A and B). The choice of detergent is important, as band 3 levels were unchanged in TritonX100 extractions.25 Protein 4.2 was not extractable from control ghosts indicating that the band 3-protein 4.2 complex was primarily associated with cytoskeleton. Rh and RhAG were more extractable from protein 4.2(−) ghosts but the majority of these proteins were non-extractable. A small amount of ankyrin-1, but not α/β-spectrin, was extractable from protein 4.2(−) ghosts confirming that specific interactions of the cytoskeleton with the plasma membrane were attenuated in strength but the spectrin cytoskeleton remains intact.
Figure 3.
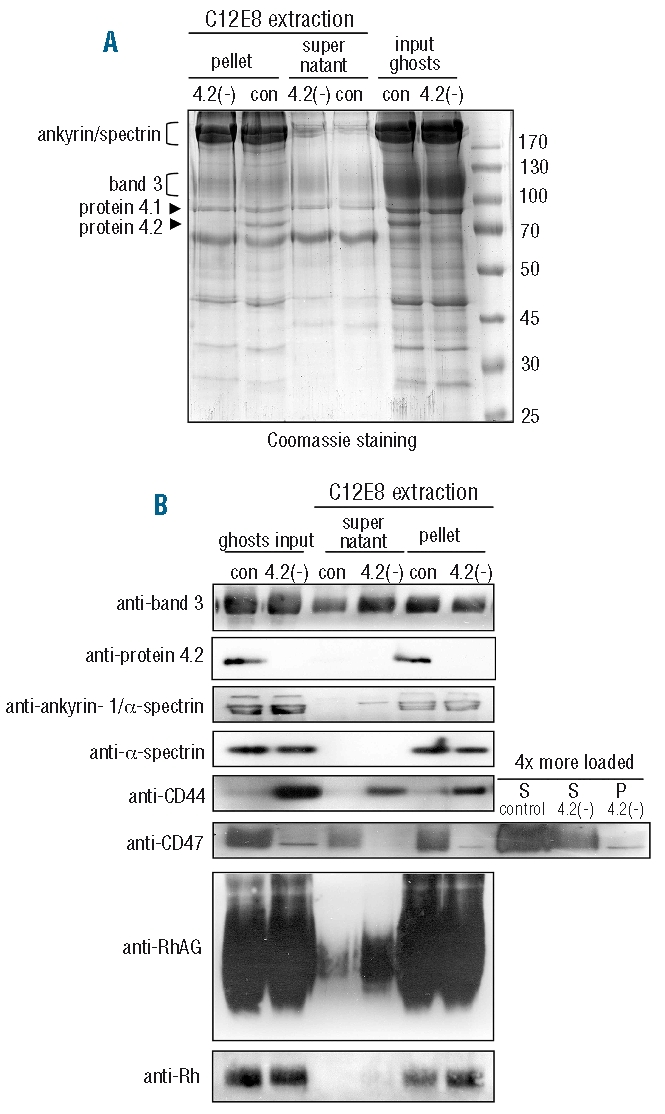
Protein 4.2 deficiency affects the extractability of band 3 macrocomplex proteins. (A and B), Protein 4.2 deficiency results in increased C12E8 extractability of band 3 macro-complex proteins. Control and protein 4.2(−) ghosts were subjected to the non-ionic detergent C12E8. (A) Coomassie staining showing 10μg of ghosts (input) extracted fraction (supernatant) and non-extractable (pellet) fraction. Arrows and brackets indicate the molecular height of specific proteins. (B) Western blot of C12E8 extracted ghosts. Antibodies used were: BRIC170: band 3; rabbit anti-protein 4.2; BRIC274: ankyrin-1 (and α/β-spectrin); BRIC172: α-spectrin; BRIC235: CD44; rabbit-CD47out1; rabbit anti-RhAG; rabbit anti-Rh30. For CD47, extended lanes, 4 times more extractable fractions were loaded to be able to detect CD47 in protein 4.2(−) ghosts.
Slightly more CD44 was present in cytoskeleton fractions in both control and protein 4.2(−) ghosts. However, the amount of CD44 residing in the cytoskeleton fraction was strikingly larger in protein 4.2(−) ghosts, suggesting that more CD44 associates with the cytoskeleton in protein 4.2 deficiency. In control cells, CD47 was mainly non-extractable but the remaining CD47 in protein 4.2(−) ghosts was completely extracted by C12E8 treatment, signifying that CD47 cytoskeleton attachment, primarily via band 3, is severely weakened.
Protein 4.2 deficiency does not interfere with erythroblast expansion and differentiation
To study how protein 4.2 deficiency affects erythropoiesis we used a modified version35 of a recently described in vitro culture system.39 A comparison between control and protein 4.2(−) cells revealed no obvious differences in cell proliferation rate and erythroblast yield (Figure 4A). Following differentiation induction, the initial proliferation followed by cell cycle arrest (Figure 4A), cell volume loss (Figure 4B) and enucleation rate (Figure 4D) were also similar. Cell death during differentiation remained typically below 10% (Figure 4C). A small increase in hemoglobinized cells in protein 4.2(−) cells was observed (Figure 4B) but overall, protein 4.2 deficiency did not grossly interfere with the general process of erythropoiesis.
Figure 4.
There are no gross defects in protein 4.2(−) in vitro erythropoiesis with respect to expansion, proliferation, cell volume, hemoglobinization, enucleation and cell morphology. Comparison of erythropoiesis between nomal (black squares) and 4.2(−) cells (white squares). (A) Outgrowth, expansion and differentiation of erythroblasts from 1×108 PBMC from control and protein 4.2(−)blood. The arrow indicates the time of differentiation induction and the dotted lines indicate the different culture phases as described in Design and Methods. (B) Hemoglobinization and cell volume of control (black squares; black circles) and protein 4.2(−) (white squares; white circles) during erythroblast differentiation (three independent measurements). (C) Cell death during differentiation as measured by propidium iodide (PI) positive population is represented as a percentage of the whole cell population. (D) Enucleation is approximately 95% after 7 days of differentiation. The three scatter plots show Hoechst33342 plotted against PI or undifferentiated cells (0 h) and fully differentiated cells (168 h, control and protein 4.2(−) cells). The Hoechst+/PI− population indicates nucleated cells (I), the Hoechst−/PI− population indicates enucleated cells (III) and the Hoechst+/Pi+ population indicates nuclei (II).
Protein 4.2 deficiency leads to changes in the band 3 macrocomplex during erythropoiesis
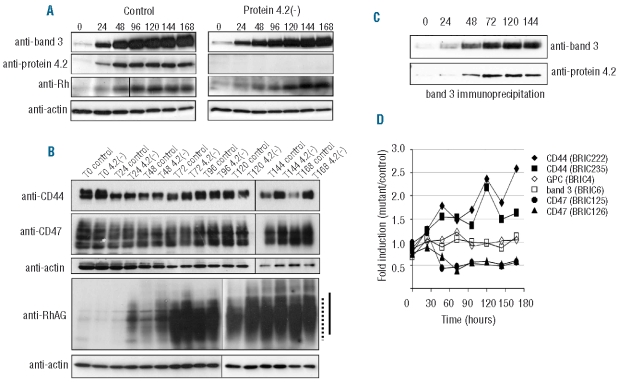
We explored the protein expression dynamics of band 3 macrocomplex proteins during differentiation of erythroblasts to reticulocytes by Western blotting. Figure 5A and B show that at the start of control cell differentiation (pro-erythroblast stage) most band 3 macrocomplex proteins are expressed at low levels, including band 3, protein 4.2, RhAG and Rh. However, expression of these proteins increased dramatically during erythroblast maturation. Protein 4.2 expression increased in tandem with band 3 and maximal expression was reached within 96h. Band 3 co-immunoprecipitated protein 4.2 from the moment these proteins appeared during differentiation (24h-144h; Figure 5C). CD47 was present at the earliest erythroblast stage and increased marginally during differentiation.
Figure 5.
Immunoblotting of proteins from control and 4.2(−) erythroblast differentiation courses reveal several hallmarks of protein 4.2 deficiency as observed in erythrocytes. (A) Equal amounts (20μg) of total cell lysates from control and protein 4.2(−) differentiating erythroblasts taken at 24 h intervals were subjected to SDS-PAGE. Immunoblotting was performed with polyclonal antibodies against protein 4.2, CD47, Rh, RhAG and actin and monoclonal antibody against band 3 (BRIC170). (B) Comparison by Western blotting, of CD44, CD47, RhAG, and actin levels during differentiation of normal and protein 4.2(−) erythroblasts. Equal cell numbers were loaded (1.0×106 cell/lane). (C) Protein 4.2 co-immunoprecipitates with band 3 during erythroblast differentiation. Lysates from differentiating erythroblasts were used to immunoprecipitate band 3 (BRAC66). Immunoprecipitates were subjected to SDS-PAGE and Western blotting and stained with BRIC170 (band 3, upper part) and rabbit anti-protein 4.2 (lower part). (D) Expanded erythroblasts were differentiated to reticulocytes over 7 days. The cell surface expression of the indicated proteins was followed daily by flow cytometry. The median intensity values for each antibody labeling of the protein 4.2(−) cells divided by the median intensity values of the control is defined as fold induction (a fold induction of 1 means no change). Intensity values were normalized against isotype controls. Time is measured in hours.
Protein 4.2 was not detected in protein 4.2(−) cells at any stage of differentiation (Figure 5A). The expression profiles of band 3, Rh, and CD47 between mutant and control were similar. Therefore, the gross reduction in CD47 observed in mature protein 4.2(−) erythrocytes is not simply explained by protein expression changes during differentiation. The gradual decrease of CD44 expression normally observed during erythropoiesis in control cells (Figure 5A) was absent in protein 4.2(−) cells, and thus CD44 expression was effectively increased. In addition, RhAG mobility was decreased during the later stages of differentiation (Figure 5B).
Cell surface expression of CD47 and CD44 but not other band 3 macrocomplex proteins are affected by protein 4.2 deficiency during differentiation
Consistent with previous reports40,41 and our Western blotting data, the majority of surface proteins (e.g. band 3, GPA, RhAG and Rh) investigated increased during differentiation albeit with different expression profiles (flow cytometry data, Online Supplementary Figure S3). Band 3 and GPA, and separately RhAG and Rh, follow parallel expression patterns. RhAG appeared slightly later in our culture system (at approximately two days) than previous reports40 coinciding closely with the appearance of the Rh proteins. CD47 and GPC cell surface expression only marginally increased during differentiation in control cells.
CD47 cell surface expression was 50% lower in protein 4.2(−) cells relative to control cells within 48h of differentiation (Figure 5D). This 50% decrease was maintained throughout differentiation. Simultaneously, we detected a 2-fold increase in CD44 cell surface levels relative to controls, which increased during the rest of the differentiation (Figure 5D). To test if increased CD44 was a direct result of decreased CD47 cell surface levels, CD47 was knocked-down using shRNA (Figure 6A and B; Online Supplementary Figure S4). A knockdown of CD47 below the level observed in protein 4.2(−) cells (60% reduction; Figure 5) showed no reciprocal rise in CD44 during differentiation (Figure 6A and B). Thus increased CD44 expression is not a direct response to decreased CD47 expression. In addition, CD44 levels were decreased in an ankyrin-1 deficient patient (E van den Akker, TJ Satchwell, and AM Toye, unpublished results, 2010) without affecting CD47 levels (Figure 6C).
Figure 6.
The relationship between CD47 and CD44 is not reciprocal. (A and B) Erythroblasts were induced to differentiate 5 days after transduction and the GFP positive population (expressing pTRIPshRNACD47) was compared to the GFP negative population in the same culture (culture 3, Online Supplementary Figure S4) by flow cytometry using anti-CD47 (BRIC125), anti-CD44 (BRIC235), anti-band 3 (BRIC6) and anti-IgG2b (isotypic control). (A) Dot plot and histogram data used to calculate the fold changes described in (B). The graphs display the cell surface binding of antibodies against band 3, CD47, CD44 and an IgG2b isotypic control on pTRIPCD47shRNA transduced cells as a function of GFP expression (top scatter plots) or alone as histograms (bottom panel; dark gray indicate GFP negative (GFP-) cells and light gray lines indicate GFP positive (GFP+) cells. Three independent experiments were performed. (B) Fold change was calculated on 0 h (gray bars) and 72 h in differentiation (white bars) as the median fluorescence intensity level of GFP- cells (non-transduced) divided by the GFP+ cells (transduced) in the same culture. No fold change is calculated for band 3 on time point 0 h in differentiation because at this stage the cells do not express band 3. (C) CD44 protein levels are changed in patient erythrocytes with reduced ankyrin-1 levels. (C) A representative Coomassie gel showing ankyrin-1/spectrin protein levels. (D) The cell surface expression fold change of CD44, CD47 and isotypic controls calculated as the median intensity of mutant divided by control (in percentages). *= <0.05 (Student’s t-test).
Discussion
We describe a patient with two novel protein 4.2 mutations (protein 4.2 Chartres 1 and 2) that result in complete absence of protein 4.2 during erythropoiesis and in erythrocytes. We confirm the characteristic membrane protein changes caused by protein 4.2 deficiency,6,25 and demonstrate that protein 4.2 (−) erythrocytes possess a dramatically weakened band 3/ankyrin-1 association with accompanying increased band 3 detergent extractability. This agrees with previous observations using reconstituted systems or in vitro binding assays14–16 that suggested that protein 4.2 influences the binding of ankyrin-1 to band 3. Only a small increase in Rh and RhAG extractability was observed in the patient’s erythrocytes, and the RhAG/band 3 association was unaffected. Importantly, the increased CD44 levels were accompanied by a marked increase in CD44 cytoskeleton attachment consistent with a compensatory change. Although describing the alterations and their consequences in patient’s erythrocytes is clearly important, these studies only provide information about the endpoint of erythropoiesis. So, complementary to the investigation of these protein complexes in erythrocytes, we extended the study to encompass erythropoiesis.
Protein 4.2 is expressed simultaneous to band 3 during erythropoiesis
We have shown that protein 4.2 and band 3 express simultaneously within 24h and associate at the basophilic erythroblast stage, which is in agreement with the expression profiles in mice.42 It contradicts an earlier report showing late expression of protein 4.2 during erythropoiesis (orthochromatic stage), with band 3 expressed earlier at the basophilic stage.40,41 However, an earlier simultaneous expression profile is in keeping with the known dependence of protein 4.2 on band 3 expression.8,10–12 The level and association of band 3 and protein 4.2 remains constant after 72h (normoblast stage) suggesting that the majority of protein 4.2 may already be associated with band 3 early in differentiation.
Since we observed that RhAG and Rh expression occurs later during erythropoiesis, this raises the exciting possibility that a basic band 3 tetrameric complex (consisting of band 3, ankyrin-1, protein 4.2, GPA) may form early during erythropoiesis and recruit CD47 ahead of the Rh proteins. This is consistent with observations in differentiating mice erythroblasts where the tetrameric band 3 complex is initially formed followed by the dimeric band 3 complexes.43
Protein 4.2 deficiency during erythropoiesis
We have shown that protein 4.2 deficiency does not affect pro-erythroblast differentiation, expansion or proliferation rate, and the expression of the majority of erythrocyte membrane proteins investigated was unaffected. The alterations to CD47 and CD44 in protein 4.2(−) cells occurred early in erythropoiesis (48h) and was maintained throughout the differentiation course. The changes were approximately 2-fold lower than those observed in peripheral erythrocytes. This may reflect the absence of in vivo context, i.e. erythroblastic islands, or that further changes in protein expression levels occur at the reticulocyte membrane remodeling stage or in circulation, which is not covered by our culture system. Erythrocyte aging by storage of erythrocytes for 12 weeks at 4°C in plasma revealed no significant difference in CD47 loss by exosomes between control and protein 4.2(−). This suggests no accelerated loss of CD47 occurs in mutant erythrocytes (data not shown).
The decrease in CD47 cell surface expression during erythropoiesis was not observed at the total protein level. Thus, during protein 4.2 deficiency, CD47 either has a reduced stability at the plasma membrane or has disturbed trafficking to the plasma membrane. Further work is necessary to explain why the absence of protein 4.2 causes a decrease in CD47 surface levels at this specific point in erythropoiesis. The reduction in CD47 leads to a concomitant increase in CD44 at the basophilic erythroblast stage and could indicate a correlative reciprocal connection. However, CD47 knockdown did not cause a simultaneous increase in CD44 during differentiation. Furthermore, CD44 was reduced in an ankyrin-1 deficient patient whose CD47 levels were normal. Taken together, these data strongly argue against a direct link between CD44 and CD47 expression.
Another characteristic of protein 4.2 deficiency in erythrocytes is increased glycosylation of RhAG and this was evident during the final stages of erythroid differentiation. RhAG appears to express at higher levels, earlier, in the protein 4.2(−) cells, so increased glycosylation may be a consequence of this earlier expression. In addition, although we did not detect an alteration in the RhAG association with band 3 in protein 4.2(−) erythrocytes, we cannot rule out the possibility that the interaction of band 3 with RhAG is compromised during erythropoiesis, causing enhanced recycling or slower trafficking through the Golgi complex.
CD44 may compensate for a weakened band 3-ankyrin-1 interaction in 4.2 deficiency and band 3 deficiency
The GPC complex of proteins represents another cytoskeleton attachment site but these proteins were unaffected in the mutant cells. We speculate that the augmented CD44-cytoskeleton association observed in protein 4.2 deficient cells may be a result of the weakened interaction of ankyrin-1 with band 3. This could potentially occur through competition for ankyrin-1 binding by CD44. Lokeshwar et al. demonstrated that CD44 competes with the cytoplasmic domain of band 3 for ankyrin-1 in in vitro binding studies.44 During erythropoiesis, CD44 may compete with the band 3-protein 4.2 complex for ankyrin-1 binding. In the absence of protein 4.2, the observed reduction in band 3 affinity for ankyrin-1 may enable a concomitant increase in CD44 association with ankyrin-1. This model predicts reduced CD44 protein levels upon ankyrin-1 deficiency in erythrocytes and indeed, lower levels of CD44 were observed in an ankyrin-1 deficient patient (unpublished mutant provided by Prof. J Delaunay, 20th May 2009). However, at this stage, we cannot rule out other compensatory cytoskeleton interactions for CD44. Future work will establish whether there is a direct link between ankyrin-1 availability and CD44 levels during erythropoiesis. The increased CD44-cytoskeleton association may explain the relatively mild phenotype observed in HS patients with protein 4.2 deficiency and also suggests a potential compensation mechanism for membrane stabilization during band 3 deficiency. Indeed, CD44 expression is increased in several hereditary spherocytosis patients with reduced band 3 protein levels despite unchanged or slightly decreased ankyrin-1 levels.26,28,45
In summary we have described, two novel protein 4.2 mutations that lead to complete protein 4.2-deficiency and hereditary spherocytosis. We have, for the first time, followed the consequences of protein 4.2 deficiency on erythrocyte membrane assembly during in vitro erythropoiesis and determined the stages at which key changes occur. This has identified a specific stage where protein 4.2 deficiency results in reduced CD47 cell surface expression and simultaneous CD44 stabilization. We are now entering an exciting stage where we can explore the consequences of specific protein deficiencies on membrane composition and function during erythropoiesis, and combine these studies with what is already known in erythrocytes in both health and disease.
Acknowledgments
the authors thank Dr. K. Heesom for mass spectrometry, Mr. P.G. Martin for DNA sequencing and Dr. W.J. Mawby for preparation of CD47out1 antibody. We thank Dr. Z. Benseddik for managing blood sampling, Dr. T. Cynober for ektocytometry and Dr. M. Fénéant-Thibault for initial SDS-PAGE analysis. We thank Dr. David Anstee and Dr. Rosey Mushens for monoclonal antibodies and Dr. Marieke von Lindern for providing cell culture reagents.
Footnotes
Funding: the work was supported by NHS Blood and Transplant (NHSBT) project grants for EvdA and SP (GD), a BBSRC DTA NHSBT Case Studentship for TJS (AMT), a NHSBT Wellcome Trust Career Development Fellowship (AMT), UK National Health Service R & D Directorate (LJB), INSERM Unit 779 and a grant “P2R Research Network Program in Human Genetics (2005–2006)” (JD).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
EvdA and TJS performed the experiments; EvdA, TJS and AMT conceived and designed experiments and wrote the manuscript; SP performed the flow cytometry on erythrocytes and helped with culturing of cells; LJB and JF identified the EPB42 gene mutations and wrote the manuscript; JD and MM provided the patient material and performed the diagnosis; EvdA, GD, JD, LJB and AMT edited the manuscript.
The authors declare no competing financial interests.
References
- 1.Eber S, Lux SE. Hereditary spherocytosis--defects in proteins that connect the membrane skeleton to the lipid bilayer. Semin Hematol. 2004;41(2):118–41. doi: 10.1053/j.seminhematol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141(3):367–75. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher PG. Red cell membrane disorders. Hematology Am Soc Hematol Educ Program. 2005:13–8. doi: 10.1182/asheducation-2005.1.13. [DOI] [PubMed] [Google Scholar]
- 4.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372(9647):1411–26. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- 5.Cartron JP. RH blood group system and molecular basis of Rh-deficiency. Baillieres Best Pract Res Clin Haematol. 1999;12(4):655–89. doi: 10.1053/beha.1999.0047. [DOI] [PubMed] [Google Scholar]
- 6.Bruce LJ, Ghosh S, King MJ, Layton DM, Mawby WJ, Stewart GW, et al. Absence of CD47 in protein 4.2-deficient hereditary spherocytosis in man: an interaction between the Rh complex and the band 3 complex. Blood. 2002;100(5):1878–85. doi: 10.1182/blood-2002-03-0706. [DOI] [PubMed] [Google Scholar]
- 7.Korsgren C, Lawler J, Lambert S, Speicher D, Cohen CM. Complete amino acid sequence and homologies of human erythrocyte membrane protein band 4.2. Proc Natl Acad Sci USA. 1990;87(2):613–7. doi: 10.1073/pnas.87.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toye AM, Ghosh S, Young MT, Jones GK, Sessions RB, Ramauge M, et al. Protein-4.2 association with band 3 (AE1, SLCA4) in Xenopus oocytes: effects of three natural protein-4.2 mutations associated with hemolytic anemia. Blood. 2005;105(10):4088–95. doi: 10.1182/blood-2004-05-1895. [DOI] [PubMed] [Google Scholar]
- 9.Satchwell TJ, Shoemark DK, Sessions RB, Toye AM. Protein 4.2: a complex linker. Blood Cells Mol Dis. 2009;42(3):201–10. doi: 10.1016/j.bcmd.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Korsgren C, Cohen CM. Purification and properties of human erythrocyte band 4.2. Association with the cytoplasmic domain of band 3. J Biol Chem. 1986;261(12):5536–43. [PubMed] [Google Scholar]
- 11.Korsgren C, Cohen CM. Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J Biol Chem. 1988;263(21):10212–8. [PubMed] [Google Scholar]
- 12.Rank G, Sutton R, Marshall V, Lundie RJ, Caddy J, Romeo T, et al. Novel roles for erythroid Ankyrin-1 revealed through an ENU-induced null mouse mutant. Blood. 2009;113(14):3352–62. doi: 10.1182/blood-2008-08-172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada H, Kanzaki A, Yawata A, Inoue T, Kaku M, Takezono M, et al. Late expression of red cell membrane protein 4.2 in normal human erythroid maturation with seven isoforms of the protein 4.2 gene. Exp Hematol. 1999;27(1):54–62. doi: 10.1016/s0301-472x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 14.Rybicki AC, Schwartz RS, Hustedt EJ, Cobb CE. Increased rotational mobility and extractability of band 3 from protein 4.2-deficient erythrocyte membranes: evidence of a role for protein 4.2 in strengthening the band 3-cytoskeleton linkage. Blood. 1996;88(7):2745–53. [PubMed] [Google Scholar]
- 15.Golan DE, Corbett JD, Korsgren C, Thatte HS, Hayette S, Yawata Y, et al. Control of band 3 lateral and rotational mobility by band 4.2 in intact erythrocytes: release of band 3 oligomers from low-affinity binding sites. Biophys J. 1996;70(3):1534–42. doi: 10.1016/S0006-3495(96)79717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybicki AC, Heath R, Wolf JL, Lubin B, Schwartz RS. Deficiency of protein 4.2 in erythrocytes from a patient with a Coombs negative hemolytic anemia. Evidence for a role of protein 4.2 in stabilizing ankyrin on the membrane. J Clin Invest. 1988;81(3):893–901. doi: 10.1172/JCI113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouhassira EE, Schwartz RS, Yawata Y, Ata K, Kanzaki A, Qiu JJ, et al. An alanine-to-threonine substitution in protein 4.2 cDNA is associated with a Japanese form of hereditary hemolytic anemia (protein 4.2NIPPON) Blood. 1992;79(7):1846–54. [PubMed] [Google Scholar]
- 18.Kanzaki A, Yasunaga M, Okamoto N, Inoue T, Yawata A, Wada H, et al. Band 4.2 Shiga: 317 CGC-->TGC in compound heterozygotes with 142 GCT-->ACT results in band 4.2 deficiency and microspherocytosis. Br J Haematol. 1995;91(2):333–40. doi: 10.1111/j.1365-2141.1995.tb05299.x. [DOI] [PubMed] [Google Scholar]
- 19.Takaoka Y, Ideguchi H, Matsuda M, Sakamoto N, Takeuchi T, Fukumaki Y. A novel mutation in the erythrocyte protein 4.2 gene of Japanese patients with hereditary spherocytosis (protein 4.2 Fukuoka) Br J Haematol. 1994;88(3):527–33. doi: 10.1111/j.1365-2141.1994.tb05069.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, Hatano N, Ideguchi H, Takahira H, Fukumaki Y. A novel mutation causing an aberrant splicing in the protein 4.2 gene associated with hereditary spherocytosis (protein 4.2Notame) Hum Mol Genet. 1995;4(7):1187–91. doi: 10.1093/hmg/4.7.1187. [DOI] [PubMed] [Google Scholar]
- 21.Hayette S, Morle L, Bozon M, Ghanem A, Risinger M, Korsgren C, et al. A point mutation in the protein 4.2 gene (allele 4.2 Tozeur) associated with hereditary haemolytic anaemia. Br J Haematol. 1995;89(4):762–70. doi: 10.1111/j.1365-2141.1995.tb08413.x. [DOI] [PubMed] [Google Scholar]
- 22.Beauchamp-Nicoud A, Morle L, Lutz HU, Stammler P, Agulles O, Petermann-Khder R, et al. Heavy transfusions and presence of an anti-protein 4.2 antibody in 4.2(−) hereditary spherocytosis (949delG) Haematologica. 2000;85(1):19–24. [PubMed] [Google Scholar]
- 23.Su Y, Ding Y, Jiang M, Hu X, Zhang Z. Protein 4.2 Komatsu (D175Y) associated with the lack of interaction with ankyrin in human red blood cells. Blood Cells Mol Dis. 2007;38(3):221–8. doi: 10.1016/j.bcmd.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 25.Mouro-Chanteloup I, Delaunay J, Gane P, Nicolas V, Johansen M, Brown EJ, et al. Evidence that the red cell skeleton protein 4.2 interacts with the Rh membrane complex member CD47. Blood. 2003;101(1):338–44. doi: 10.1182/blood-2002-04-1285. [DOI] [PubMed] [Google Scholar]
- 26.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, et al. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101(10):4180–8. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 27.Nunomura W, Takakuwa Y, Tokimitsu R, Krauss SW, Kawashima M, Mohandas N. Regulation of CD44-protein 4.1 interaction by Ca2+ and calmodulin. Implications for modulation of CD44-ankyrin interaction. J Biol Chem. 1997;272(48):30322–8. doi: 10.1074/jbc.272.48.30322. [DOI] [PubMed] [Google Scholar]
- 28.Toye AM, Williamson RC, Khanfar M, Bader-Meunier B, Cynober T, Thibault M, et al. Band 3 Courcouronnes (Ser667Phe): a trafficking mutant differentially rescued by wild-type band 3 and glycophorin A. Blood. 2008;111(11):5380–9. doi: 10.1182/blood-2007-07-099473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckmann R, Toye AM, Smythe JS, Anstee DJ, Tanner MJ. An N-terminal GFP tag does not alter the functional expression to the plasma membrane of red cell and kidney anion exchanger (AE1) in mammalian cells. Mol Membr Biol. 2002;19(3):187–200. doi: 10.1080/09687680210141043. [DOI] [PubMed] [Google Scholar]
- 30.Appleford NE, Wilson K, Houston F, Bruce LJ, Morrison A, Bishop M, et al. alpha-Hemoglobin stabilizing protein is not a suitable marker for a screening test for variant Creutzfeldt-Jakob disease. Transfusion. 2008;48(8):1616–26. doi: 10.1111/j.1537-2995.2008.01759.x. [DOI] [PubMed] [Google Scholar]
- 31.Wainwright SD, Tanner MJ, Martin GE, Yendle JE, Holmes C. Monoclonal antibodies to the membrane domain of the human erythrocyte anion transport protein. Localization of the C-terminus of the protein to the cytoplasmic side of the red cell membrane and distribution of the protein in some human tissues. Biochem J. 1989;258(1):211–20. doi: 10.1042/bj2580211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Lindern M, Parren-van Amelsvoort M, van Dijk T, Deiner E, van den Akker E, van Emst-de Vries S, et al. Protein kinase C alpha controls erythropoietin receptor signaling. J Biol Chem. 2000;275(44):34719–27. doi: 10.1074/jbc.M007042200. [DOI] [PubMed] [Google Scholar]
- 33.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9(6):255–62. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 34.Yi SJ, Liu SC, Derick LH, Murray J, Barker JE, Cho MR, et al. Red cell membranes of ankyrin-deficient nb/nb mice lack band 3 tetramers but contain normal membrane skeletons. Biochemistry. 1997;36(31):9596–604. doi: 10.1021/bi9704966. [DOI] [PubMed] [Google Scholar]
- 35.van den Akker E, Satchwell TJ, Pellegrin S, Daniels G, Toye AM. The majority of the in vitro erythroid expansion potential resides in CD34− cells, outweighing the contribution of CD34+ cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica. 2010 Apr 7; doi: 10.3324/haematol.2009.019828. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakker WJ, Blazquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, et al. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol. 2004;164(2):175–84. doi: 10.1083/jcb.200307056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmeyer S, Nurnberg P, Ritter H, Fahsold R, Leistner W, Kaufmann D, et al. Nearby stop codons in exons of the neurofibromatosis type 1 gene are disparate splice effectors. Am J Hum Genet. 1998;62(2):269–77. doi: 10.1086/301715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourguignon LY. CD44-mediated oncogenic signaling and cytoskeleton activation during mammary tumor progression. J Mammary Gland Biol Neoplasia. 2001;6(3):287–97. doi: 10.1023/a:1011371523994. [DOI] [PubMed] [Google Scholar]
- 39.Leberbauer C, Boulme F, Unfried G, Huber J, Beug H, Mullner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105(1):85–94. doi: 10.1182/blood-2004-03-1002. [DOI] [PubMed] [Google Scholar]
- 40.Southcott MJ, Tanner MJ, Anstee DJ. The expression of human blood group antigens during erythropoiesis in a cell culture system. Blood. 1999;93(12):4425–35. [PubMed] [Google Scholar]
- 41.Bony V, Gane P, Bailly P, Cartron JP. Time-course expression of polypeptides carrying blood group antigens during human erythroid differentiation. Br J Haematol. 1999;107(2):263–74. doi: 10.1046/j.1365-2141.1999.01721.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci USA. 2009;106(41):17413–8. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanspal M, Golan DE, Smockova Y, Yi SJ, Cho MR, Liu SC, et al. Temporal synthesis of band 3 oligomers during terminal maturation of mouse erythroblasts. Dimers and tetramers exist in the membrane as pre-formed stable species. Blood. 1998;92(1):329–38. [PubMed] [Google Scholar]
- 44.Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol. 1994;126(4):1099–109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro ML, Alloisio N, Almeida H, Gomes C, Texier P, Lemos C, et al. Severe hereditary spherocytosis and distal renal tubular acidosis associated with the total absence of band 3. Blood. 2000;96(4):1602–4. [PubMed] [Google Scholar]