Abstract
Background
Despite the favorable results of imatinib front line in chronic-phase chronic myeloid leukemia there is room for improvement.
Design and Methods
Early intervention during imatinib therapy was undertaken in 210 adults with chronic-phase chronic myeloid leukemia less than three months from diagnosis (Sokal high risk: 16%). Patients received imatinib 400 mg/day. At three months, dose was increased if complete hematologic response was not achieved. At six months, patients in complete cytogenetic response were kept on 400 mg and the remainder randomized to higher imatinib dose or 400 mg plus interferon-alfa. At 18 months, randomized patients were switched to a 2nd generation tyrosine kinase inhibitor if not in complete cytogenetic response and imatinib dose increased in non-randomized patients not in major molecular response.
Results
Seventy-two percent of patients started imatinib within one month from diagnosis. Median follow-up is 50.5 (range: 1.2–78) months. At three months 4 patients did not have complete hematologic response; at six months 73.8% were in complete cytogenetic response; among the remainder, 9 could not be randomized (toxicity or consent withdrawal), 17 were assigned to high imatinib dose, and 15 to 400 mg + interferon-alpha. The low number of randomized patients precluded comparison between the two arms. Cumulative response at three years was: complete hematologic response 98.6%, complete cytogenetic response 90% and major molecular response 82%. On an intention-to-treat basis, complete cytogenetic response was 78.8% at 18 months. At five years, survival was 97.5%, survival free from accelerated/blastic phase 94.3%, failure free survival 82.5%, and event free survival (including permanent imatinib discontinuation) 71.5%.
Conclusions
These results indicate the benefit of early intervention during imatinib therapy (ClinicalTrials.gov Identifier: NCT00390897).
Keywords: chronic myeloid leukemia, treatment, imatinib
Introduction
Chronic myeloid leukemia (CML) is a clonal disorder of a pluripotent hemopoietic stem cell that harbors the BCR/ABL rearrangement, a molecular abnormality that results in the activation of an oncogenic protein, the Bcr/Abl tyrosine kinase, which confers the neoplastic cells a proliferative advantage over the benign hemopoietic progenitors.1 Imatinib, a selective inhibitor of the Bcr/Abl protein, has dramatically changed the treatment of CML.2 Following the publication of the early results of the IRIS trial,3 a phase III study that compared imatinib 400 mg daily versus interferon-alfa (IFN) plus low-dose Ara-C in patients newly diagnosed with chronic-phase CML (CP-CML), imatinib became the front-line therapy for CML patients. Further updates of the IRIS trial have shown that the responses to imatinib are mostly durable and that treatment failures tend to occur at the beginning of therapy, with a steady decline being observed after two years.4, 5 These results, which have been recently confirmed in an individual series,6 were taken as a basis for the design of studies aimed at further improving the results of front-line imatinib therapy.7–10
In this context, the Spanish PETHEMA group undertook a collaborative study of early intervention during imatinib therapy in patients with de novo CP-CML. The study was designed before publication of the first European LeukemiaNet recommendations for CML treatment.11 Following the availability of the 2nd generation tyrosine kinase inhibitors (TKIs) dasatinib12 and nilotinib,13 first in clinical trials and then in clinical practice, the study was amended to allow administration of these newer drugs in patients with failure or intolerance to imatinib, as well as imatinib dose increase in patients not in major molecular response at 18 months of treatment. This paper reports the results of the CML/PETHEMA study.
Design and Methods
Patients and inclusion criteria
Between July 2003 and August 2006, 215 patients consecutively diagnosed with Ph-positive CP-CML in 47 institutions in Spain were registered for inclusion in the study, after approval by the local ethics committees. Criteria of inclusion were: age from 18 to 72 years, less than three months from diagnosis, performance status 2 or less of the ECOG scale, negative HIV test, no pregnancy or breast feeding in fertile women, no neoplasia for the preceding five years, renal and liver function tests lower than 1.5 times the upper normal range, and written informed consent in accordance with the Declaration of Helsinki. The reason to exclude patients over the age of 72 years was the possibility that they could be assigned to the interferon arm, considering the well-known poor tolerability to this drug in the elderly. Five screening failures were registered, due to criteria of accelerated phase (n=2), and recent neoplasia, a positive pregnancy test and more than three months from diagnosis (one case each). Therefore, 210 patients were finally included in the study. Their main characteristics are detailed in the Results section.
Treatment and monitoring
Following the diagnosis of Philadelphia chromosome-positive CP-CML, the use of hydroxyurea was allowed for a maximum of three months. Imatinib was administered orally at a dose of 400 mg/day. At three months, in patients failing to achieve a complete hematologic response (CHR), the imatinib dose was increased to 800 mg daily while it was maintained at 400 mg in the remaining patients. Then, depending on the cytogenetic response obtained at six months from imatinib start, patients were kept on imatinib 400 mg daily if they had achieved a complete cytogenetic response (CCyR) or randomized to receive either higher imatinib doses (600 mg and then 800 mg) or imatinib 400 mg/day plus subcutaneous low-dose IFN 2b (3 million units 3 times a week) if a CCyR had not been obtained at that time. The rationale to add IFN in one of the experimental arms was the fact that, before imatinib availability, it was the only drug able to produce consistent cytogenetic responses in CML. Since the International Scale (IS) had not been described at the time when the study was designed, a 3-log reduction in the BCR/ABL transcripts was initially employed to define a MMolR, following the example of the IRIS study.14 However, once the IS was created15 and the national standardization process carried out in Spain, the values at 18 months and thereafter were reassessed and expressed using the IS by means of a laboratory-specific conversion factor provided via the EUTOS for CML programme (http://www.eutos.org). Imatinib levels were not available during most of the study period and, therefore, they were not used to optimize dosage. Due to the availability of the 2nd generation TKIs during the study, an amendment was introduced in January 2006 allowing the switch to dasatinib or nilotinib in those patients randomized at six months who had not achieved a CCyR at one year of randomization, as well as in patients with intolerance to imatinib. The study amendment also included imatinib dose increase in those patients in CCyR at six months who were not in MMolR at 18 months of treatment. In order to maintain imatinib dose intensity as much as possible, the use of G-CSF was permitted in patients experiencing prolonged or recurrent grade 3–4 neutropenia during treatment.
A complete medical history, physical examination, blood counts, and comprehensive biochemistry tests were obtained before the start of therapy and at appropriate intervals, which varied depending on the time from the start of treatment, the patients’ clinical situation and the level of response (either hematologic, cytogenetic or molecular) already obtained. Cytogenetic studies were performed in bone marrow by chromosome banding analysis after short-term culture with standard G or Q banding techniques. At least 20 metaphases were required for evaluation. If 20 metaphases could not be obtained, fluorescence in situ hybridization (FISH) of interphase cells in peripheral blood was recommended. Once CCyR was achieved, the BCR-ABL transcript levels were assessed in peripheral blood every three months by real-time quantitative polymerase chain reaction (RT-Q-PCR) according to standard methods, with GUS being used as control. As mentioned before, MMolR was initially defined as a 3-log reduction with regard to the baseline BCR/ABL transcript level14 and later expressed as a ratio BCR-ABL/control gene of 0.1% or less of the IS.15 Complete molecular response was defined as two consecutive samples with no detectable transcripts, corresponding to a greater than 4.5 log-reduction in the BCR/ABL copies. The molecular studies were performed in three reference laboratories, located in Barcelona, Córdoba and Salamanca, Spain, which used the same technique and exchanged representative samples for cross-checking.
Criteria of response and loss of response
Complete hematologic response (CHR) was defined as normalization of the peripheral blood counts and leukocyte differential with disappearance of the palpable spleen. Cytogenetic response was graded according to standard criteria.11 If 20 metaphases could not be obtained in the bone marrow study (which occurred in one or more occasions in a total of 29 patients), a CCyR was considered if less than 1% of the nuclei were positive for the BCR/ABL at FISH study of 200 or more cells from peripheral blood.16,17
For the purpose of the present analysis, treatment failure was defined according to the updated criteria of the European LeukemiaNet.18 In brief, it included the lack of CHR at three months of treatment, no cytogenetic response (bone marrow Ph+ cells > 95%) at six months, less than a partial cytogenetic response (i.e. Ph+ cells > 35%) at 12 months, lack of CCyR at 18 months, the loss of response at anytime (defined as the loss of CHR, also including progression to the accelerated or blastic phases, and the loss of CCyR), and death. The accelerated phase (AP) and blastic phase (BP) were defined according to standard criteria.11 Overall survival was calculated as the time from imatinib start until death, whatever the cause. Progression free survival (PFS) was defined as the time from imatinib start to the appearance of AP/BP or death, whatever the cause. Failure free survival (FFS) was defined as the time from imatinib start to failure, including death, progression to AP/BP and the lack of achievement or the loss of a previously achieved CHR or CCyR. Event free survival (EFS) included the above parameters plus the permanent discontinuation of imatinib for any reason (e.g. intolerability, allogeneic stem cell transplantation, treatment of a second neoplasia or others).
Statistical analysis
Survival and time to event curves were calculated from the start of imatinib by the Kaplan-Meier method19 and compared by the log rank test. Patients lost to follow up were censored at the time of the last visit, according to standard actuarial procedures. Patients who underwent allogeneic stem cell transplantation (allo-SCT) were considered until last follow up for the calculation of overall survival, progression free survival and failure free survival. For the calculation of cytogenetic and molecular response, patients were censored at the time of imatinib discontinuation. Potential predictors of the achievement of CCyR at 12 and 18 months were analyzed by binary logistic regression. All the statistical analyses were performed with Stata software, version 10 (www.stata.com).
Results
Table 1 shows the main characteristics at diagnosis of the 210 patients. As can be seen, there was a predominance of patients in Sokal and Hasford low-risk groups and a low proportion of patients in the high-risk group.20,21 One hundred and forty-one patients received hydroxyurea before imatinib. Median time from diagnosis to imatinib start was 0.5 (range: 0–2.8) months and 72% of patients started imatinib therapy within one month from diagnosis. At the time of the analysis, median follow up from diagnosis was 50.5 (range: 1.2–78) months and median follow up from imatinib start 50.3 (range: 0.7–77) months.
Table 1.
Main initial characteristics of 210 patients newly diagnosed with chronic phase chronic myeloid leukemia treated with imatinib upfront.
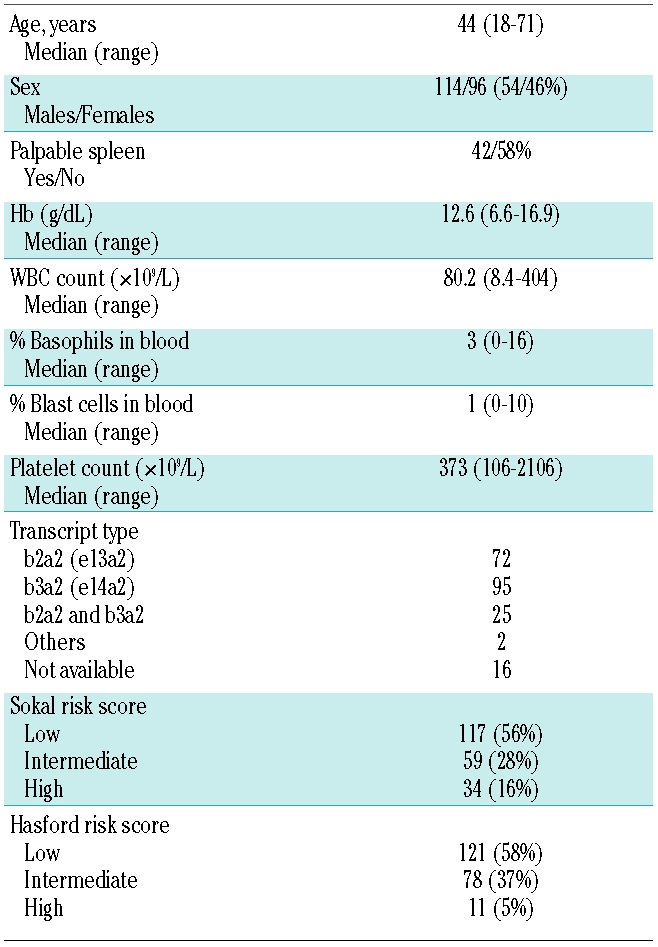
Figure 1 shows the flow diagram of the study since imatinib start until 18 months of treatment. At three months, 4 patients had not achieved CHR. Two of these patients failed to obtain a favorable response following an increase in the imatinib dose; one of them rapidly evolved into BP and subsequently died, while the other finally responded to a second generation TKI. On an intention-to-treat (ITT) basis, at six months, CCyR had been obtained in 73.8% of the patients. Of the 41 patients not in CCyR at that time point, 9 could not be randomized because of toxicity (n=3) or withdrawal of consent (n=6) and were followed-up on an observational basis, 17 were assigned to a higher imatinib dose, and 15 to imatinib 400 mg daily plus IFN. However, 6 of the latter patients withdrew consent and, therefore, only 9 patients actually started IFN, of whom one received the drug only for one month due to poor tolerability. Because of the low number of patients randomized, no comparison could be established between the two arms.
Figure 1.
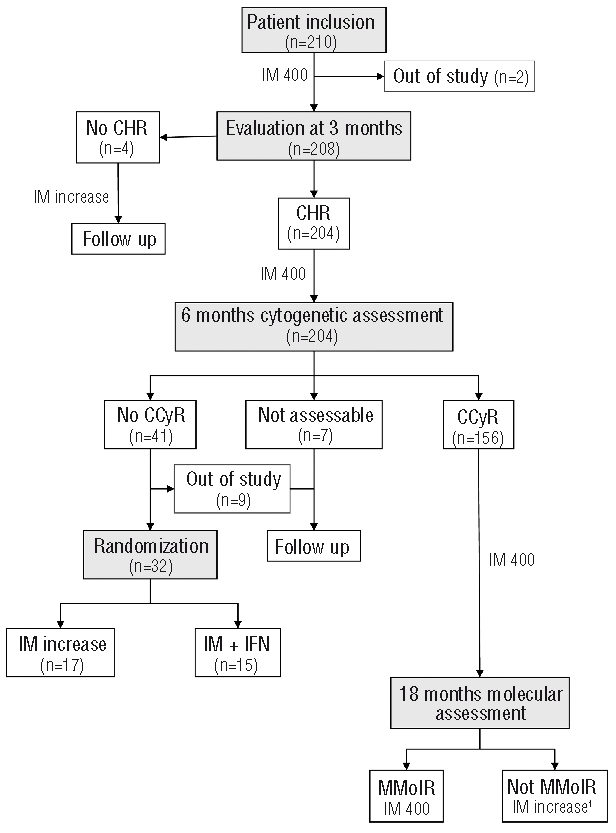
Flow diagram of the study design from imatinib start until 18 months of treatment. IM: imatinib; CHR: complete hematologic response; CCyR: complete cytogenetic response; IFN: interferon-α; MMolR: major molecular response; 1: after study amendment.
Of the 17 patients who underwent imatinib dose increase at six months, 16 achieved a CCyR at 18 months (without a MMolR in 4 cases) while the remaining one achieved the CCyR and also a MMolR at 24 months while on high-dose imatinib. Two of the patients eventually lost the response: one of them, in whom the cause was lack of adherence to therapy, regained the response after resuming the high imatinib dose; the other was administered a second generation TKI and subsequently responded. Due to either poor tolerability to the 800 mg imatinib dose or persistence of the good response in the long term, imatinib dose was eventually reduced in most patients to 600 (n=6) or 400 mg (n=8). All 17 patients were alive and responding at last follow up.
Among the 15 patients assigned to the imatinib plus IFN arm, 7 of the 9 who actually started IFN were in CCyR at 18 months (with an MMolR in 6 cases) and 2 failed to achieve this response. One of the latter 2 patients subsequently responded to a higher imatinib dose while the other one evolved into an AP that eventually responded to a second generation TKI. Of the 6 patients who withdrew consent to receive IFN, one was submitted to an allo-SCT shortly afterwards while in the chronic phase of CML, one patient evolved into AP and was also transplanted, another responded to imatinib dose escalation, and the remaining 3 continued on imatinib 400 mg daily. All patients were alive at last follow up.
In the group of 156 patients in CCyR at six months, during the period from month 6 to month 18 the following events were registered: evolution to AP/BP (n=2, one of them successfully rescued with an allo-SCT), imatinib discontinuation due to toxicity (n=2) or appearance of a second neoplasia (n=1), allo-SCT while in response to imatinib (n=1), loss to follow up (n=2), and loss of the cytogenetic response (n=6). Of the remaining patients, 16 were not in MMolR at 18 months and 9 of them were dose escalated. In the remaining 7, the reasons for not increasing the imatinib dose were that the amendment recommending escalation in this situation was implemented in January 2006 (n=6) and there was one death from an unrelated cause (n=1). Of the 9 patients who were dose escalated at 18 months, 8 achieved an MMolR following escalation while the remaining one developed cytogenetic resistance a few months later and was switched to a 2nd generation TKI. In the overall group of patients in CCyR at six months, 3 additional cases of AP/BP were registered after the initial 18 months of therapy.
Finally, among the 7 patients not assessable for cytogenetic response at six months, one developed sudden BP shortly afterwards and died. Of the 9 patients not in CCyR at six months and not being randomized because of toxicity or withdrawal of consent, 6 were kept on imatinib at lower or higher dosages (depending on whether the reason for the lack of randomization was toxicity or withdrawal of consent) and 3 were switched to a second generation TKI. No instance of evolution to AP/BP has been registered in the latter group of patients.
In the overall group of patients, cumulative response rates at three years were: CHR 98.6%, CCyR 90%, and MMolR 82% (Figure 2). On an intention-to-treat basis, CCyR rate was 78.8% at 18 months; at last follow up, MMolR and complete molecular response rates were 63% and 38%, respectively. No association was found between any of the initial clinical and laboratory features evaluated and the lack of CCyR at 12 and 18 months of imatinib. Moreover, no differences were noted in CCyR rate according to Sokal or Hasford risk groups, although it must be noted that the proportion of patients in the high-risk group was low.
Figure 2.
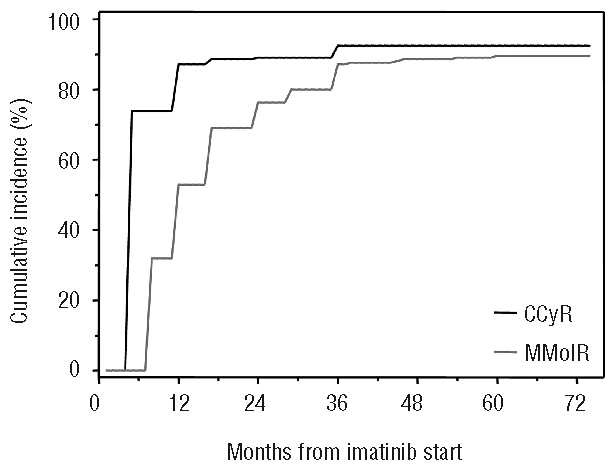
Cumulative incidence of first time achievement of complete cytogenetic response (CCyR, black line) and major molecular response (MMolR, gray line).
With current follow up, only 5 of the 210 patients have died, one of them from a CML-unrelated cause (poisoning) while in CCyR to standard-dose imatinib. Evolution into AP/BP was seen in 9 patients: 3 in the first year of treatment, 3 in the second year, 2 in the third year, and one in the fourth year, whereas no instance of such complication has been observed so far in patients having completed their fifth year of treatment. With regard to the characteristics of the 9 patients developing AP/BP, only 2 belonged to Sokal’s low-risk group while the remaining 7 had intermediate (n=5) or high-risk (n=2) CML. Eight patients were lost to follow up, 9 discontinued imatinib because of toxicity, 3 due to the appearance of a second neoplasia, and 2 female patients because of pregnancy. Five patients underwent allo-SCT between 13 and 27 months from diagnosis: 3 were in CP-CML at time of transplantation (2 in response to imatinib and one after losing a previously achieved CCyR) and 2 had evolved to AP/BP. At last follow up, 131 patients were receiving daily imatinib 400 mg, 23 patients 600 mg, 7 patients 800 mg and 4 patients 300; 16 patients were on dasatinib and 6 on nilotinib.
Figure 3 shows the curves of overall and progression free survival. At five years, overall survival was 97.5% and PFS 94.3%. Figure 4 shows the actuarial curves of failure free and event free survival, the 5-year projected percent-ages being 82.5% and 71.5%, respectively.
Figure 3.
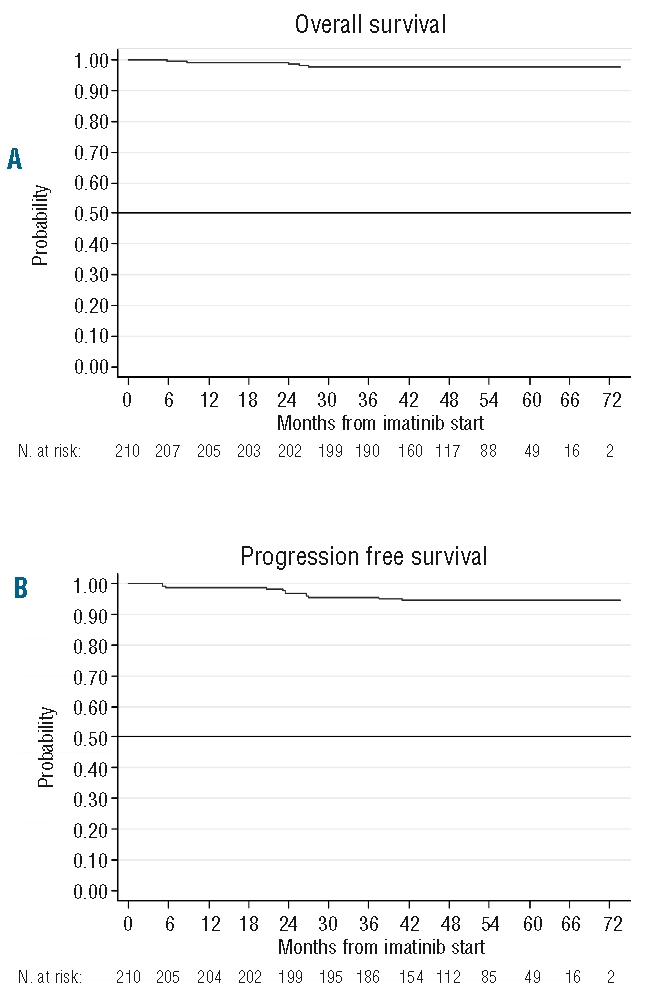
(A) Overall survival in the 210 patients. (B) Progression free survival, defined as the time from imatinib start to the appearance of AP/BP or death, whatever the cause.
Figure 4.
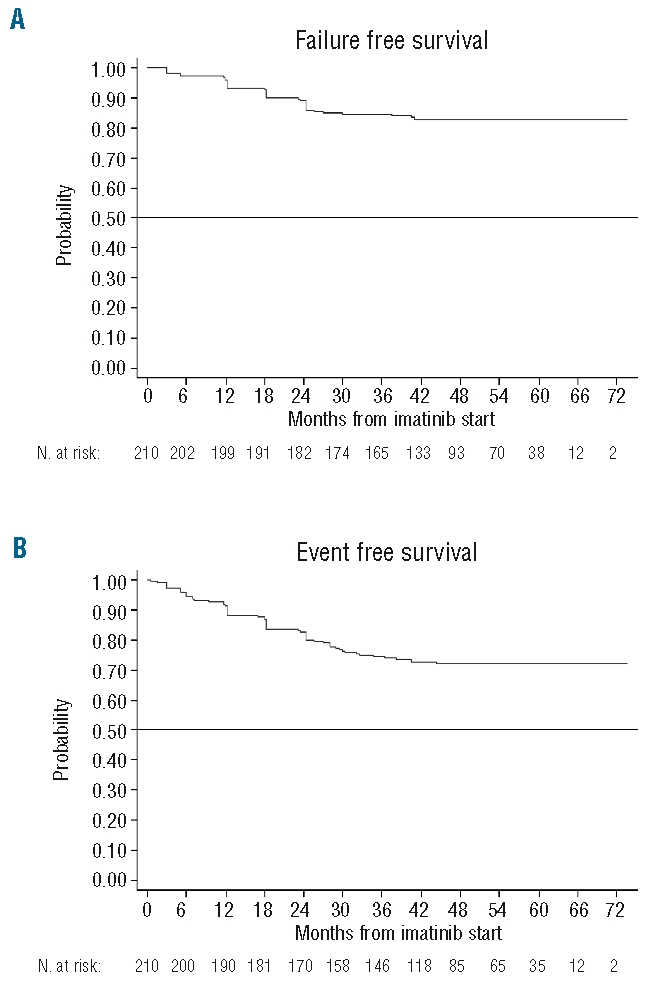
(A) Actuarial curves of failure free survival and (B) event free survival, including failure plus permanent imatinib discontinuation, whatever the cause.
During imatinib treatment, a total of 27 patients (12.8% of the overall group) experienced WHO grade 3–4 hematologic toxicity, including neutropenia (9.5% of patients), thrombocytopenia (2.8%), and anemia (1.4%). Grade 3–4 hematologic toxicity was observed during the first six months of therapy in 20 patients. After this time period, it was observed in 3 non-randomized patients, in 3 patients of the imatinib plus IFN arm and in one of those assigned to high-dose imatinib. In only 5 patients did the hematologic toxicity appear beyond one year of therapy. Seven patients required temporary administration of G-CSF for recurrent neutropenia. Grade 3–4 non-hematologic side effects occurred in 32 patients (15.4% of the series), the most frequent being liver toxicity (5.7%). These grade 3–4 non-hematologic side effects were registered in 13 patients during the first six months of therapy; after this they were observed in 17 of the non-randomized patients and in one receiving high-dose imatinib. Seventy of the 210 patients required transient imatinib interruption due to toxicity in a total of 91 occasions (one episode, n=42; two episodes, n=21; three or more episodes, n=7). Median duration of the imatinib discontinuation periods was one week (range 0.6–13). Three patients developed a second neoplasia (uterus, lung, and pancreas) while on imatinib treatment.
Discussion
The introduction of imatinib has dramatically changed the outcome of CML patients. Following the favorable results of imatinib in patients resistant or intolerant to IFN,22 the IRIS study showed that front-line imatinib therapy of CP-CML results in high rates of complete cytogenetic and major molecular responses in the short- and mid-term,3,4 that these responses are mostly durable in the long-term,4,5 and that a progressive deepening of the molecular response is observed over time with continuous treatment.5 All of these achievements have translated into historically high rates of overall and progression free survival in this disease.5 The results of the IRIS study have been confirmed in the single center series of the Hammersmith Hospital.6 Following the publication of the early results of the IRIS, collaborative efforts were initiated to further improve the results of imatinib therapy.7–10 However, to date, only the early results of two such studies have been published7,8 whereas the preliminary results of other studies have been communicated as abstracts.9,10
In the above context, the Spanish PETHEMA group undertook a collaborative study of early intervention during imatinib therapy in 210 patients newly diagnosed with CP-CML. Since the study was designed prior to the publication of the first recommendations of the European LeukemiaNet for the management of CML,11 the criteria triggering early intervention were different but actually more stringent. Thus, the design was aimed at early intervention in cases in whom a very good response had not been obtained in the early period of therapy. In addition, during the course of the study, an increase in the imatinib dose was also recommended in cases in whom a major molecular response had not been achieved at 18 months of treatment. This policy was subsequently adopted by the European LeukemiaNet which considered as suboptimal response the lack of MMolR by that time.11 Additionally, advantage was also taken of the availability of the second generation TKIs dasatinib and nilotinib for patients who failed or were intolerant to imatinib.12,13
An explanation must be found for the improved results of the present study as compared with those of the IRIS. It must be noted that the two populations were not totally comparable, since our patients were younger and had a better risk profile than those in the IRIS. Some of the differences between the two series can be ascribed to the inferior upper age limit (72 years) established for inclusion in our study. However, this does not seem to be the only reason for the differences. Indeed, it is well known that in Mediterranean countries, the median age at CML diagnosis and the proportion of high-risk patients are lower than in the USA and other countries from Central and Northern Europe. Thus, in a CML series from Spain, including all patients consecutively diagnosed in a single institution over a long period of time, median age at diagnosis was 46 years.23 Moreover, the younger age and lower risk profile of the Italian as compared with the German patients was also pointed out.24 This having been said, it also seems logical to ascribe part of the better results obtained in the present study to the earlier use of imatinib in our patients, who started the TKI at a median of only two weeks from diagnosis, with almost three-quarters of them already on imatinib within one month from diagnosis. This is in contrast to the IRIS study in which the median interval from diagnosis to imatinib start was 2.1 months and some of the patients started imatinib as late as 10.4 months from diagnosis.3 Two other facts would also likely contribute to our better results. First, earlier intervention, as soon as at three and at six months, in cases in whom there was an inappropriate response; this was not allowed in the IRIS, as it was a registration study. Second, a greater experience in the management of the toxicity to imatinib, and especially of the cytopenias, allowing the early use of G-CSF to maintain dose intensity as much as possible; this was not permitted during the early phase of the IRIS study.
In the present study, a high rate of CHR was achieved at three months of imatinib therapy, with only 4 of the 210 patients failing to achieve this response by that time. Of note, 2 of the 4 patients who were not in CHR at three months of treatment subsequently failed after imatinib dose increase; this confirms the poor prognosis of the lack of CHR at three months previously reported by the Hammersmith group25 and lends additional support to the consideration of this situation as a treatment failure in the updated recommendations of the European LeukemiaNet.18 The higher than expected proportion of patients already in CCyR at six months in the present study (73.8% vs. 52% in the IRIS) precluded comparison between the two randomization arms. It must also be noted that almost one quarter of the patients eligible for randomization at six months could not be randomized due to severe toxicity at the time of randomization or refusal of randomization to the IFN arm. In fact, of the 15 patients assigned to the IFN arm, 6 withdrew consent once they were informed of the result of randomization, whereas an additional patient stopped IFN after one month because of poor tolerability. This refusal or low compliance of IFN should not be surprising, given the convenient administration form and usually good tolerability of imatinib, as opposed to the subcutaneous administration and frequent side effects of IFN. As a result, only 8 of the 210 patients of the study finally received low doses of IFN for one year. This means that, in practice, the study consisted of imatinib optimization. On an ITT basis, the CCyR rate increased from 73.8% at six months to 78.8% at 18 months. Such a modest increase is explained by the already good results at six months and also by the fact that, although a majority of patients randomized to higher imatinib dose or to imatinib plus IFN actually achieved a CCyR at one year of randomization (i.e. at 18 months from imatinib start), this was partially counterbalanced by some CCyR losses, occasional evolution to AP/BP, allo-SCT (in some cases while in response to imatinib), imatinib discontinuation due to intolerance or appearance of a second neoplasia, and loss to follow up. Cumulative rate of MMolR at three years was 82%. On an ITT basis, it was 63% at last follow up, with 38% of patients being in complete molecular response.
The fact that no prognostic factors for the achievement of CCyR could be identified in our patients might be attributed to the size of the series but, especially, to the high rate of favorable responses obtained.
Survival of patients in the present study was highly favorable, with an overall survival of 97.5% at five years and a survival free from evolution to AP/BP of 94.3%. In keeping with the results of the IRIS and Hammersmith studies,5, 6 the unfavorable events tended to accumulate during the first years of treatment and progressively declined after the third year. This observation would reinforce the relevance of achieving an early favorable response to imatinib to prevent disease progression26 providing the basis for the use of more stringent criteria for the early assessment of response in the new recommendations by the European LeukemiaNet.18 These aim to identify as soon as possible those patients without an adequate response to imatinib and promote early therapeutic interventions. On the other hand, event free survival (EFS) at five years was 71% in our patients. It must be noted that the term EFS has a different meaning in the present study and in the IRIS, in which it was formerly known as progression free survival (PFS)3,4 and renamed EFS in the last update report.5 Thus, in our study, EFS encompassed primary/secondary resistance, evolution to AP/BP, death from any cause, and the definitive discontinuation of imatinib for any reason, using the concept coined by the Hammersmith group,6 as it represents a more realistic approach than considering the events of progression only. By contrast, in the IRIS study, EFS refers to death from any cause, evolution to AP/BP, and primary/secondary resistance, but it does not include the definitive discontinuation of imatinib for any reason. This would explain the apparent discrepancy between the two studies, but also the concordance of our results with those reported by the Hammersmith group.6
In our patients, toxicity to imatinib was generally moderate. Thus, although grade 1 and 2 non-hematologic side effects were frequent during the early period of treatment (data not shown), grade 3–4 non-hematologic toxicity was relatively low and it occurred mostly at the beginning of therapy. With regard to hematologic side effects, a low rate of grade 3–4 hematologic toxicity was observed, mainly consisting of neutropenia, which usually appeared during the first year of treatment and could be managed with temporary imatinib discontinuation or dose reduction or with the addition of G-CSF in the few patients with recurrent episodes of such complication. This policy allowed adequate dose intensity and the continuous administration of the drug in the majority of patients, a fact likely contributing to the favorable therapeutic results obtained in the present study.
In conclusion, prompt use of imatinib, early intervention after inadequate response and improved management of toxicity, together with rescue of resistant patients with 2nd generation TKIs, could improve the outcome of patients newly diagnosed with CP-CML. Ongoing studies comparing imatinib upfront with the second generation TKIs will determine whether these outstanding results can be improved further.
Participating institutions
In addition to the investigators included in the list of authors, the following participated in the study: Concha Boqué, Institut Català d’Oncologia, Hospitalet de Llobregat; Blanca Xicoy, Hospital Germans Trias i Pujol, Badalona; Isabel Massagué, Hospital Vall d’Hebron, Barcelona; Alberto Álvarez-Larrán, Hospital del Mar, Barcelona; Lourdes Escoda, Hospital Joan XXIII, Tarragona; Alba Bosch, Hospital de Mataró; Elena Ràmila, Corporació Sanitària Parc Taulí, Sabadell; Llorenç Font, Hospital Verge de la Cinta, Tortosa; Andrés Novo, Hospital Son Dureta, Palma de Mallorca; Joan Bargay, Hospital Son Llàtzer, Palma de Mallorca; Magdalena Sánchez, Hospital Universitario de Valencia; Eulogio Conde; Hospital Marqués de Valdecilla, Santander; José Luis López, Fundación Jiménez Díaz, Madrid; Santiago Larregla, Hospital Doce de Octubre, Madrid; Gracia Bravo, Hospital Puerta de Hierro, Madrid; Consuelo Cañizo, Hospital Clínico Universitario de Salamanca; Fernando Ortega, Complejo Hospitalario de Palencia; Pilar Fisac, Hospital General de Segovia; Antonio Alcalá, Complejo Hospitalario de Jaén; Concepción Ruiz, Hospital Carlos Haya, Málaga; Santiago Castillo, Hospital de la Victoria, Málaga; Antonio Fernández, Hospital Juan Ramón Jiménez, Huelva; Felipe Prósper, Clínica Universitaria de Navarra, Pamplona; María-Cruz Viguria, Hospital de Navarra, Pamplona; Esmeralda Benítez, Hospital Xeral de Vigo; Manuel Lite, Hospital Meixoeiro, Vigo; María-José Ramírez, Hospital de Jerez; Carmen Burgaleta, Hospital Príncipe de Asturias, Alcalá de Henares; Secundino Ferrer, Hospital Doctor Peset, Valencia; Lucía Villalón, Hospital Fundación de Alcorcón; Guillermo Debén, Hospital Juan Canalejo, La Coruña; María-Luisa Martín-Mateos, Hospital San Pedro de Alcántara, Cáceres; Abelardo Pérez, Hospital Nuestra Señora de Sonsoles, Avila; Montserrat Pérez, Hospital Virgen de la Concha, Zamora; Juan-Manuel Sánchez, Hospital Arnau de Vilanova, Lérida.
Footnotes
Funding: we thank Novartis for providing financial support for the collection of the data and the centralized molecular studies. This work has been supported in part by the grants FIS 060038 from the Instituto de Salud Carlos III and RD06/0020/0004 from the RETICS, Spanish Ministry of Health.
Authorship and Disclosures
FC designed the study, collected the data and wrote the manuscript; DC, JR and MG performed the molecular studies; AP carried out the statistical analysis; PLG, MIM, FJ, JM, JCHB, MC, AS, GFR, JBN, and CPL collected data and contributed to the study.
FC has received speaker fees from Novartis and Bristol-Myers Squibb.
References
- 1.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341(3):164–72. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Talpaz M, Rasta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. The IRIS International study: Imatinib versus interferon and low-dose Ara-C in patients with newly-diagnosed chronic phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of imatinib therapy for newly diagnosed chronic myelogenous leukemia in chronic-phase. N Engl J Med. 2006;355(23):2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–61. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 6.De Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly-diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–63. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 7.Deenik W, van der Holt B, Verhoef GE, Smit WM, Kersten MJ, Kluin-Nelemans HC, et al. Dose-finding study of imatinib in combination with intravenous cytarabine: feasibility in newly diagnosed patients with chronic myeloid leukemia. Blood. 2008;111(5):2581–8. doi: 10.1182/blood-2007-08-107482. [DOI] [PubMed] [Google Scholar]
- 8.Palandri F, Iacobucci I, Castagnetti F, Testoni N, Poerio A, Amabile M, et al. Front-line treatment of Philadelphia positive chronic myeloid leukemia with imatinib and interferon-alpha: 5-year outcome. Haematologica. 2008;93(5):770–4. doi: 10.3324/haematol.12265. [DOI] [PubMed] [Google Scholar]
- 9.Hehlmann H, Saussele S, Lauseker M, Proeter U, Kovalevskaya E, Leitner A, et al. Randomized comparison of Imatinib 400 mg Vs. Imatinib + IFN Vs. Imatinib + Ara-C Vs. Imatinib after IFN Vs. Imatinib 800 mg: optimized treatment and survival: designed first interim analysis of the German CML Study IV (abstract) Blood. 2008;112 Abstract 184. [Google Scholar]
- 10.Guilhot F, Mahon F-X, Guilhot J, Rigual-Huguet F, Maloisel F, Rousselot P, et al. Randomized comparison of Imatinib versus Imatinib combination therapies in newly diagnosed chronic myeloid leukemia (CML) patients in chronic phase (CP): first results of the phase II (SPIRIT) trial from the French CML Group (FI LMC) (abstract) Blood. 2008;112 Abstract 183. [Google Scholar]
- 11.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia. Recommendations from a panel expert on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 12.Hochhaus A, Kantarjian H, Baccarani M, Lipton J, Apperley J, Druker B, et al. Dasatinib induces notable, durable hematologic and cytogenetic responses in chronic phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109(6):2303–9. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–6. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 14.Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon α plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349(9):1423–32. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 15.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-Abl transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raanani P, Ben-Bassat I, Gan S, Trakhtenbrot L, Mark Z, Ashur-Fabian O, et al. Assessment of the response to imatinib in chronic myeloid leukemia patients: comparison between the FISH, multiplex and RT-PCR methods. Eur J Haematol. 2004;73(4):243–50. doi: 10.1111/j.1600-0609.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 17.Testoni N, Marzocchi G, Luatti S, Amabile M, Baldazzi C, Stacchini M, et al. Chronic myeloid leukemia: a prospective comparison of interphase fluoresecence in situ hybridization and chromosome banding analysis for the definition of complete cytogenetic response: a study of the GIMEMA CML WP. Blood. 2009;114(24):4939–43. doi: 10.1182/blood-2009-07-229864. [DOI] [PubMed] [Google Scholar]
- 18.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia. Management recommendations of the European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 20.Sokal JE, Cox EB, Baccarani M, Gomez JE, Robertson JE, Tso CY, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–99. [PubMed] [Google Scholar]
- 21.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon α. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90(11):850–8. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Paserini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 23.Cervantes F, Rozman C. A multivariate analysis of prognostic factors in chronic myeloid leukemia. Blood. 1982;60(6):1298–304. [PubMed] [Google Scholar]
- 24.Hasford J, Baccarani M, Hehlmann R, Anseri H, Tura S, Zuffa E. Interferon-alpha and hydroxyurea in early chronic myeloid leukemia: a comparative analysis of the Italian and German chronic myeloid leukemia trials with interferon-alpha. Blood. 1996;87(12):5384–91. [PubMed] [Google Scholar]
- 25.Marin D, Milojkovic D, Olavarria E, Khorashad JF, de Lavallade H, Reid AG, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437–44. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintás-Cardama A, Kantarjian H, Jones D, Shan J, Borthakur G, Thomas D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113(25):6315–21. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]


