Abstract
Background
There is currently no international consensus for first-line treatment (prior to autologous stem cell transplantation) in mantle cell lymphoma patients. Here, we investigated the efficacy and tolerance of VAD associated with chlorambucil (VAD+C) and rituximab or not before autologous stem cell transplantation.
Design and Methods
Between 1996 and 2005, 113 previously untreated mantle cell lymphoma patients were enrolled in two consecutive prospective phase II studies. Responses and response factors to the (R)VAD+C regimen were evaluated. The survival prognostic value of the MIPI score and Ki67 were also analyzed.
Results
The induction phase of 4 courses of (R)VAD+C showed very low hematologic and extra-hematologic toxicity (grade 3–4 thrombopenia and neutropenia, 9% and 2.7%, respectively and grade 3–4 extra-hematologic toxicities, 1.6%). Overall and complete response rates were 73% and 46%, respectively, and rose to 83% and 51% for the 70% of patients with less than two independent response factors (LDH, B symptoms and lymphocytosis). At the end of treatment, 65% of patients were in complete remission. Progression free and overall survival were significantly better in the transplanted population. The MIPI score was confirmed as a predictor of survival. Ki67, serum LDH, Performance Status (PS) and B symptoms were identified as independent prognostic factors of survival. A prognostic scoring system could stratify patients into three risk groups with markedly different median overall survival of 112, 44 and 11 months, respectively.
Conclusions
The (R)VAD+C is an effective regimen with very low toxicity. In addition to the MIPI score, Ki67 expression provides additional independent prognostic information for the prediction of overall survival (ClinicalTrials.gov Identifier: NCT00285389).
Keywords: autologous stem cell transplantation, mantle cell lymphoma, chlorambucil
Introduction
Mantle cell lymphoma (MCL) is an aggressive form of lymphoma described in the international WHO classification.1 Over the last decade the median overall survival (OS) for MCL patients has risen from 3–4 years to five years.2 A much shorter survival is, however, associated with some histological subtypes, such as blastic variants where median OS is 14 months compared to 53 months for the common forms.3
There is currently no consensual front-line therapy for MCL. Prior to the introduction of rituximab, and despite the non-superiority of regimens containing anthracyclines,4,5 the CHOP regimen was initially widely used as the reference treatment in view of its efficacy in aggressive large B-cell lymphoma. Reported overall response (OR) and complete remission rates (CR) range from 57 to 92%, and 7 to 30%, respectively.6–8 High-dose cytarabine-based regimens, such as DHAP8 or Hyper-CVAD,9 have been shown to achieve better responses; around 95% OR and between 38% and 84% CR, respectively.9,10 However, higher toxicities make these regimens difficult to complete in elderly patients.
Several reports have demonstrated that high-dose consolidation therapy including autologous stem cell transplantation (auto-SCT) improves PFS8,9,11–15 This was confirmed by the European MCL network in a randomized prospective phase III study.16 Auto-SCT associated to rituximab treatment of molecular relapse has been shown to extend median OS to five years.17
Here we report the long-term follow-up results of two French GOELAMS studies which evaluated the tolerance and efficacy of the VAD+C regimen with or without rituximab in a total of 113 newly diagnosed MCL patients.18 The prognostic value of the recently-devised MIPI score19 and Ki67 expression status were also evaluated.
Design and Methods
Study design
The designs of the LM 1996 and LM2001 trials are summarized in Figure 1. The goal of LM 1996 was to evaluate the efficacy and tolerance of the VAD+C regimen.18 This trial was opened for patients aged between 18 and 75 years old. The primary objective of LM2001 was to evaluate the association of rituximab to the VAD+C, in terms of efficacy and toxicity in patients under 65 years of age.
Figure 1.
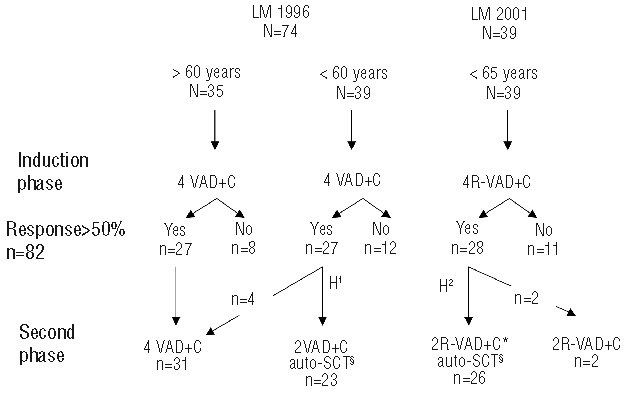
Study design of the LM 1996 and LM 2001 trials and number of responders at the intermediate and final step of evaluation. N= number of patients recruited in each arm. VAD+C: vincristine 0.4 mg/D, D1 to D4; doxorubicin 9 mg/m2/D, D1 to D4; dexamethasone 40 mg/D, D1 to D4; chlorambucil 12 mg/D, D20 to D29 ; 2 cycle delay 35 days. RVAD+C: rituximab 375 mg/m2, D1 of each VAD cycle. H1 and H2: stem cell harvest; H1, steady state manner; H2, cyclophosphamide mobilization 4 g/m2 and in vitro purge with rituximab 375 mg/m2. 2(R)VAD+C*, one RVAD+C followed by one VAD+C. §Melphalan 140 mg/m2 and TBI 8 gy/4 fractions.
In LM1996, all patients underwent an induction phase of treatment including 4 cycles of the VAD+C regimen. The VAD regimen (vincristine 0.4 mg/d, doxorubicine 9 mg/m2/d infused intravenously over four days associated with dexamethasone 20 mg/12h intravenous (IV) or oral (PO) administration on days 1 to 4) was applied every five weeks (d1 to d1) and associated with ten days of chlorambucil 12 mg/d PO from day 20 to day 29 of each cycle.18 Responses were evaluated after four cycles. All patients showing more than a partial response (over 50%) were eligible for the second phase. During this second phase, patients under 61 years of age received 2 additional cycles of the VAD+C regimen followed four weeks later by high-dose melphalan (140 mg/m2) and a fractioned total body irradiation (TBI) (8 grays, 4 fractions) with peripheral blood stem cell (PBSC) support. The fourth VAD cycle did not contain chlorambucil to limit the risk of harvest failure. Patients over 61 years not eligible for high-dose therapy received 4 additional cycles of VAD+C.
In the LM2001 trial, all 39 patients received the VAD+C induction associated to rituximab, (375 mg/m2, at day 1 of each cycle). PBSC collection was performed as for LM1996 with, in addition, high-dose cyclophosphamide (4 g/m2), mobilization (one course) and in vivo purging with one injection of rituximab (375 mg/m2). Prior to autologous transplantation, patients received one cycle of RVAD+C and one cycle of VAD+C. The preparative regimen for transplant was identical to that used in the LM1996 trial.
Patients’ selection
From 1996 to 2005, 113 newly diagnosed, previously untreated mantle cell lymphoma patients (according to the WHO classification1) were enrolled in the two consecutive phase II trials by the French GOELAMS group described above. Ninety patients were included in LM1996 (inclusions proceeded from September 1996 through December 2000) and 39 patients in the LM2001 trial (from September 2003 through December 2005). Additional inclusion criteria were an Ann Arbor (AA) stage II–IV and a performance status (PS) between 0 and 2 according to the ECOG scale. Ann Arbor staging was based on clinical examination, CT scan, bone marrow biopsy and gastric endoscopy. Peripheral blood infiltration was assessed by lymphocyte count. Patients were required to have normal renal (creatinine clearance > 50 mL/mn), cardiac (ventricular ejection fraction > 50%) and hepatic (ASAT/ALAT < 3 times the upper limit) functions. Patients with positivity for HIV, HCV or HBV, or reporting a previous malignancy, were not included. These phase II studies were approved by the ethics committee of Grenoble University Hospital and by the GOELAMS institutional review board (IRB). All recruited patients provided written informed consent.
Tumor analysis
The initial pathological examination prior to inclusion was performed locally and included morphological analysis and immunohistochemical detection of at least CD20 expression. All diagnoses were reviewed centrally by 3 pathologists from the GOELAMS pathology panel. MCL were classified, according to the criteria of the WHO 2001 classification of Lymphoma, in two groups: the common group with two variants (small cells and marginal zone-like cells) and the blastoid group with the lymphoblastic-like and the pleomorphic variants.
A total of 127 tumors were reviewed (83 lymph nodes and 44 extranodal tissues as follows: bone marrow n=18, spleen n=11, gastrointestinal (GI) tract n=8, tonsils n=3, skin n=1, orbital tumor n=2, salivary gland n=1).
Immunohistochemistry was performed using a labeled streptavidin-biotin-peroxydase system with diamino-benzidin as chromogen (Ultra-tech, Beckman Coulter, Miami, FL, USA). The following monoclonal antibodies were used: anti-CD5 (Novocastra, Newcastle upon Tyne, UK), anti-CD23 (Novocastra), anti-IgD (Dako, Glostrup, Denmark) anti-cyclin D1 CCND1 (Novocastra) and anti-Ki67 (Novocastra). In the LM1996 study, Ki67 immunostaining and quantification were performed by counting a total of one thousand cells in two areas of high Ki67 expression (2x500). The mean Ki67 count (26%) was used as the cut-off that could distinguish two different prognostic groups. In the 2001 trial, Ki67 was quantified by counting 2x250 cells also showing high CCND1 expression. If this first count yielded 20–30% Ki67 positivity (approaching the cut off of 26%), an additional observation of 2x250 cells was performed to confirm the initial count.
Evaluation and response criteria
Physical examination and complete blood cell count (CBC) preceded each course of (R)VAD. A CT scan was performed after the induction phase and after completion of the treatment plan. A bone marrow aspiration was also performed at these checkpoints. Subsequently, follow up was performed every three months during the first year and then every six months, including physical examination and CBC counts each time. CT scans were repeated every six months and bone marrow biopsies once yearly. Response was defined according to the International Working Group criteria.20
Statistical and prognostic factor analyses
Sixteen parameters were analyzed as potential adverse prognostic factors for response rate: age at diagnosis (<61 years vs. ≥61 years), sex, pathology subtype (common form vs. blastoid variants), lymphocytosis at diagnosis (<5×109/L vs. ≥5×109/L), PS (ECOG 0–1 vs. 2), B symptoms, LDH serum level (Normal vs. > N), bulky tumor (maximal diameter <10 cm vs. ≥10cm), number of extranodal sites (<2 vs. ≥2), bone marrow infiltration, GI tract localization, spleen localization, rituximab administration or not, and Ki67 status. Response rate after the induction phase (<50% vs. ≥50%) and auto-SCT (yes vs. no) were also studied as prognostic factors of survival.
A logistic regression was applied with the SPSS (Chicago, IL, USA) software to identify which factors impacted on the response rate. According to the international response criteria,21 the probability of overall survival (OS) was calculated for all patients from day one of the first cycle, until death, and the probability of progression free survival (PFS) until death or progression. OS and PFS were plotted with the StatView software (1998 SAS institute Inc.). Cox’s regression analysis with the forward stepwise method was applied with all factors, except the response after induction phase, to establish which clinical or biological factors at diagnosis impacted independently on OS and/or PFS. The log rank test was used to validate an index, determined by independent factors, which was compared with the MIPI score. P values lower than 0.05 were considered significant.
Results
Patients’ characteristics
Patients’ characteristics are summarized in Table 1. After pathology review and control of the inclusion/exclusion criteria, 74 patients were included in the LM1996 and all 39 patients of the LM2001 trial were considered eligible for the study. Fourteen patients of the 129 initially selected (15%) were excluded because of initial misdiagnosis (7 patients diagnosed as B chronic lymphocytic leukemia, 4 follicular and 3 marginal zone lymphoma). Two other patients presented exclusion criteria.
Table 1.
Patients’ and tumor characteristics.
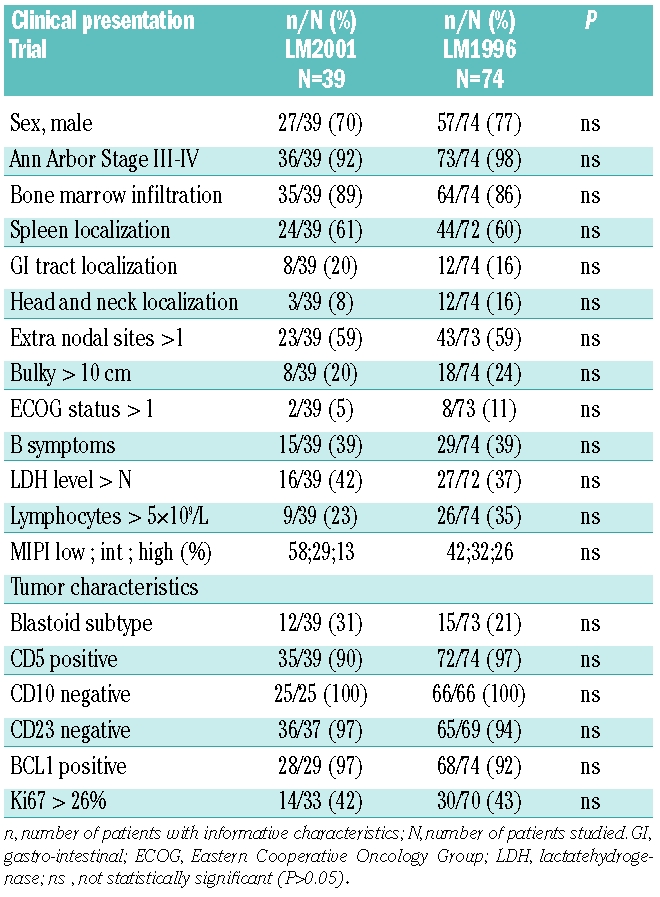
Eighty-five patients (75%) were diagnosed as common MCL and 27 (24%) with a blastoid variant. One patient could not be classified because only a bone marrow biopsy was available.
There were 84 males and 29 females (ratio 2.9). All but 4 patients had stage III–IV disease (n=109, 96%). There was bone marrow involvement in 88% of the patients (99/113) and 60% (68/113) showed spleen involvement, 16% (18/113) head/neck or orbit involvement and 18% (20/113) a GI localization (3 with polyposis). Fifty-nine percent of the patients had more than one extranodal site localization. Bulky tumor, defined by a diameter larger than 10 cm, was present in 23% of the cases and 39% of the cases displayed B symptoms. A PS over 1 was observed for 9% of the patients; 38% had increased LDH serum levels. Lymphocytosis over 5×109/L was observed in 31% of the patients and Ki67 over the defined threshold in 43%. According to the MIPI score, 47% (n=52/110) of the patients were in the low-risk group, 31% (n=34/110) in the intermediate group and 22% (n=24/110) in the high-risk group. There was no statistically significant difference in clinical and biological characteristics between the patients receiving the VAD+C regimen without rituximab and those who received rituximab (Table 1). Seventy-eight patients (70%, 78/113) were considered, at diagnosis, to be eligible for intensive therapy, i.e. 39 patients in each trial.
(R)VAD+C toxicity
Of the 414 VAD+C cycles performed during the induction phase (including rituximab in 150 cycles), 18 (4%) were delayed. WHO grade 3–4 neutropenia and thrombopenia were seen after 35 cycles (8%) and 11 cycles (3%), respectively. Fifteen patients required red blood cell (n=13) or platelet (n=9) transfusion during this phase. All presented with anemia or thrombopenia at diagnosis. The most serious extra-hematologic side effects consisted of WHO grade 3–4 infections (n=8), grade 3 cardiac side-effects (n=2) and grade 3 transaminase alterations (n=1). Fifty-eight patients had at least one WHO Grade 1–2 gastrointestinal, infectious, neurological, or cardiac side-effects. No renal complications were reported.
Response rates after 4 (R)VAD+C (induction phase)
Overall (ORR) and complete (CR/CRu) response rates after 4 cycles of (R)VAD+C were 73% (n=82) and 46% (n=52), respectively. There were no differences in ORR or CR/CRu between the VAD+C and R-VAD+C regimen (73% vs. 72% for ORR and 49% vs. 44% for CR/Cru, respectively). ORR and CR/CRu did not differ between young (n=78) and elderly patients (n=35): ORR was 70.5% (55/78) and 77% (27/35) (P=0.46) and CR/CRu was 44% (34/78) and 48% (17/35) (P=0.62). Thirty-one patients (27%) were not eligible for the second phase: one patient died, 12 did not achieve partial response (PR), and 18 progressed while on therapy. The median OS of these patients was ten months.
Stem cell collection was performed in 52 young patients. A median of 2 cytaphereses were necessary to collect an average 3.46×106 CD34+ cells/kg (0.9–11.5): 3.34×106 CD34+ cells/kg (0.9–10.7) in LM1996 and 5.94×106 CD34+ cells/kg (2.02–11.5) in LM2001. Harvest failed in 3 patients.
Response rate at the end of the treatment plan
Out of the 82 responding patients, 33 were not transplanted. Among them, 31 received a total of 8 cycles of VAD+C without rituximab (27 elderly and 4 young patients of LM 1996) and 2 young patients of LM 2001 received a total of 6 cycles of R-VAD+C.
Forty-nine young patients were transplanted: 23 of LM1996 patients received 6 cycles of VAD+C and 26 of LM2001 patients received 6 cycles of R-VAD+C before auto-SCT. Six patients did not receive the scheduled auto-SCT because of stem cell mobilization failure (n=3), protocol violation (n=1), coronary thrombosis (n=1) or metastatic carcinoma (n=1).
For the 33 patients treated with (R)VAD+C alone, disease status at the end of treatment was CR/CRu in 29 cases (88%), PR in one case and three progressions. For the 49 transplanted patients, the disease status at the end of the treatment was CR/CRu in 44 cases (89%), PR in 3 cases, one progression and one death. In summary, 73 of the 113 patients (66%) were in CR/CRu after the end of treatment.
Overall survival and progression free survival
The final analyses were performed in December 2007 and September 2009 for LM 1996 and LM 2001, respectively. The median follow up (FU) for the 39 living patients was 62 months (104 and 53 months, respectively, for LM1996 and LM2001 patients). The 3-year OS rate of the 113 patients was 62% (IC 95%, 46–68%) and median OS was 52 months. For the 78 young patients, the 3-year OS rate was 66.5% with a median OS of 63 months while for the 35 elderly patients treated with 8 VAD+C without rituximab these values were 51% and 36 months, respectively (Figure 2A). OS was significantly better for the 49 transplanted patients compared to 64 non-transplanted patients: 3-year OS=81% (95%CI, 50–90%) vs. 47% (95%CI, 37 to 56%) P<0.0001 (Figure 2B). The PFS was also significantly better for patients who received auto-SCT: 3-year FS = 62% (95%IC, 28–68%) vs. 6% (95% IC, 2–12%) P<0.0001 (Figure 2C).
Figure 2.
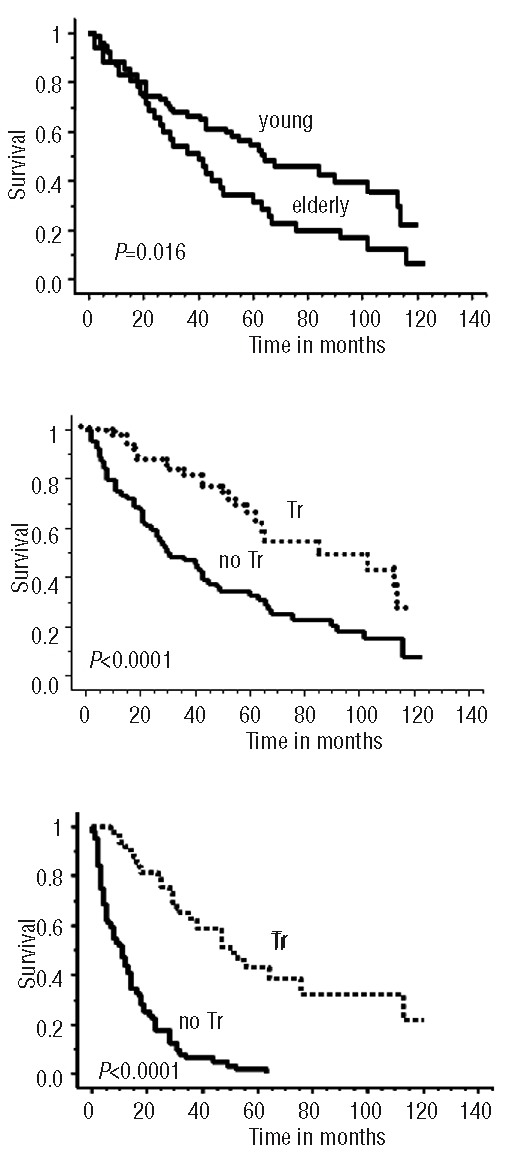
(A) Overall Survival (OS) as intent to treat of 78 young and 35 elderly patients receiving first-line (R)VAD+C regimen followed or not by auto-SCT. (B) Overall survival. (C) Progression free survival, of the 49 transplanted patients (Tr) versus 64 non-transplanted patients (no Tr).
For the 35 elderly patients, 3-year PFS and median PFS were 11% and 16 months, respectively. For the 49 transplanted patients there was a trend to a better PFS for those receiving rituximab prior to auto-SCT (P=0.054) (Figure 3A) which did not translate into a better OS (P=0.49) (Figure 3B).
Figure 3.
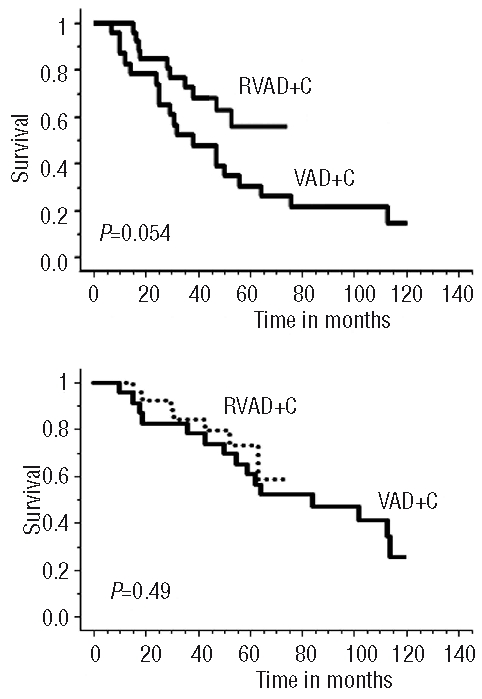
(A) Progression free survival and (B) overall survival, of the transplanted patients after RVAD+C or VAD+C regimen.
Response factors to the induction phase and prognostic factors for survival
Predictive factors of response to the 4 cycles of induction were analyzed. Logistic regression analyses identified three independent factors: LDH (P=0.009), B symptoms (P=0.007) and lymphocytosis (P=0.05). The ORR and CR/CRu between 78 patients (70%) with few adverse response factors (RF) (0 or 1) and 35 patients (30%) with high RF (2 or 3) were statistically different: ORR 83% with few RF vs. 47% with high RF (P=0.001) and CR/CRu 51% for few RF vs. 31% (P=0.047).
We next analyzed prognostic factors (PF) influencing OS. In a monoparametric analysis, eight PF had a significant impact: response to the induction phase, auto-SCT, PS, lymphocytosis more than 5×109/L, presence of B symptoms, elevated LDH, spleen involvement and Ki67 expression. In a multiparametric Cox’s regression model, four independent PF influenced OS: LDH level, Ki67 proliferation index, PS and B symptoms. It is, therefore, possible to propose a “GOELAMS index” of MCL prognostic factors related to the number of pejorative PF observed at diagnosis within these four criteria: Ki67>26%, ECOG>1, B symptoms and LDH>N. This allowed an OS prognostic index to be defined that stratified patients into three statistically significant distinct risk groups (Figure 4A, P<0.0001): group 1 (16 patients with 3–4 PF) with a median OS of 11 months, group 2 (64 patients with 1–2 PF) with a median OS of 44 months and group 3 (33 patients without any PF) with a median OS of 112 months.
Figure 4.
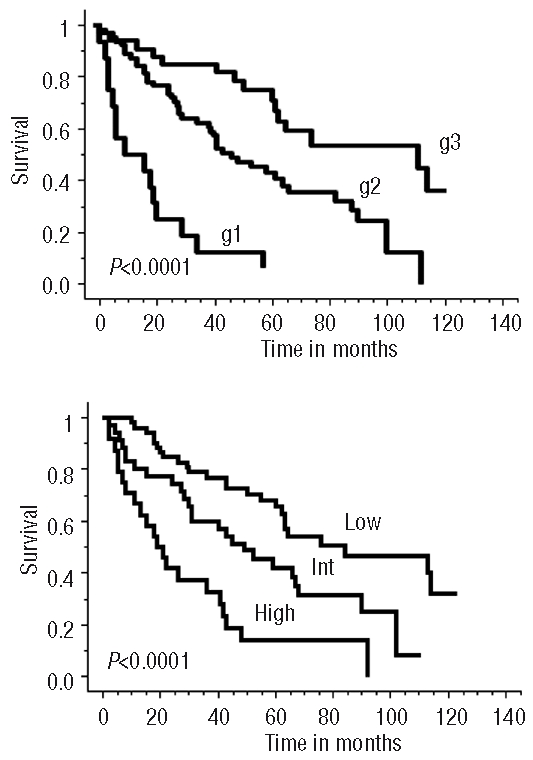
(A) Overall survival according to the GOELAMS index related to the number of criteria observed at diagnosis among 4 pejorative prognostic factors (PF): Ki67>26%, ECOG>1, B symptoms and LDH>N. g1 = group with 3 or 4 PF, g2 = group with 1 or 2 PF and g3 = group without PF. (B) Overall survival according to the MIPI, Low=low-risk, Int = intermediate-risk and High = high-risk.
The MIPI score, applied to 110 patients of our cohort, was efficient in distinguishing three independent prognostic groups: the high-risk group (n=24) had a median OS of 20 months, the intermediate group (n=34) had a median OS of 42 months and the low-risk group (n=52) had a median OS of 75 months (Figure 4B).
Discussion
In this study, 113 previously untreated mantle cell lymphoma patients were enrolled into two consecutive phase II prospective studies in order to explore the efficacy and tolerance of the VAD+C regimen, previously tested in relapsed patients,18 with or without addition of rituximab.
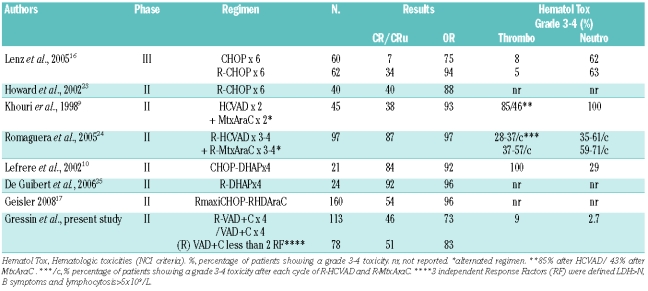
With 73% and 46% ORR and CR rates, respectively, 4 cycles of the (R)VAD+C compared favorably with 6 cycles of CHOP.8,16 However 70% of our patients (n=78) with less than 2 independent response factors integrating LDH level, B symptoms and lymphocyte count had response rates close to 6 cycles of RCHOP22,23, with a more favorable toxicity profile (Table 2). Moreover, the median OS (36 months) and PFS (16 months) of our 35 elderly patients treated with 8 cycles of VAD+C without rituximab was similar to that reported in the literature with 6 CHOP or 6 RCHOP cycles.22,23 Rituximab did not improve the response rate of the VAD+C regimen in this study. One reason could be a trend to a higher MIPI score for patients treated with rituximab (Table 1). Indeed, rituximab has been reported to improve all other regimens in first line (CHOP, DHAP or HyperCVAD) (Table 2).22,24,26 As expected, the efficacy was lower than that observed for high-dose cytarabine-containing regimens R-Hyper-CVAD or R-DHAP.8,24,25 However, the low toxicity profile observed means the (R)VAD+C regimen offers a good efficacy/tolerance ratio particularly recommended for elderly patients. RVAD+C has to be compared with other combination regimens which have shown a good efficacy in first line for elderly patients. The RFC regimen is currently compared to RCHOP in a phase III study of the EU MCL network. The British national cancer research network is completing a phase II trial that compares FC with or without rituximab in order to evaluate the impact of rituximab in non-transplanted MCL patients. Of note, the bendamustin-rituximab (BR) regimen has shown a good efficacy/toxicity profile on indolent lymphoma including MCL in relapse and was recently reported as effective as RCHOP in first line.27,28
Table 2.
Selected trials of the main different (R)-Chemotherapy protocols used in first line for mantle cell lymphoma comparing efficacy and hematologic toxicity.
This study confirms that intensive strategies including auto-SCT prolong PFS, as previously demonstrated in a randomized study conducted by the European MCL network.16 We also demonstrated a better OS in transplanted patients; but these were non-randomized trials and the populations of transplanted and non-transplanted patients differed by age. The 3-year OS and PFS rates (81% and 62%, respectively) of the 49 transplanted patients were comparable to those previously published.8,16,17,26 Unlike the recent study previously reported by the Nordic group, no survival plateau was observed in our study.17 The use of rituximab before auto-SCT seemed to give a PFS advantage as recently reported.29,30 We also observed, as published by Murali et al., long-term relapses with up to ten years of follow up in patients who did not benefit from auto-SCT.31 In this setting, maintenance therapy strategies or treatment of molecular relapses as recently published are of particular interest.32
In addition to confirming the prognostic value of the MIPI score, this work shows that additional important prognostic information can be obtained from quantifying Ki67 expression, in agreement with recent studies from the EU MCL network and German low-grade lymphoma study group (GLSG).19 Recent recommendations to assess the Ki67 index have been published by the MCL network.33 We used very similar screening procedures for Ki67 evaluation. Ki67 expression, together with three additional parameters available at diagnosis (PS > 1, presence of B symptoms, elevated serum LDH), was combined into a new scoring system that proved particularly powerful for the identification of very high- and low-risk patients, respectively. Of note, the 26% threshold for Ki67 expression is very close to that (30%) reported by the EU MCL network and the GLSG.34 The very high-risk group could benefit from more intensive treatment strategies such as allogeneic stem cell transplantation which has shown promise in relapsed MCL.35–37 Conversely, low-risk patients could benefit from less intensive (continuous) treatment regimens, and the (R)VAD+C could be a good option in this setting.38
In conclusion, this study demonstrates the low toxicity and efficacy of the (R)VAD+C regimen as first-line therapy in mantle cell lymphoma patients. This regimen can be considered an efficient therapeutic option especially for elderly patients and for low-risk younger patients scheduled to undergo auto-SCT. However, it is reasonable to propose that younger patients would benefit from more intensive treatment programs such as R-DHAP or RmaxiCHOP-RHDAraC followed by auto-SCT.17,25 Refinement of the current prognostic scoring systems for MCL could be very useful to accurately assign MCL patients to appropriate treatment plans as early as possible, particularly in the younger patient group.
Acknowledgments
The GOELAMS group acknowledges the following clinicians for their significant contributions to this study: Dr. Dutel JL; Dr. de Jaureguiberry JP; Dr. Maloisel F; Dr. Rodon P; Dr. Thyss A; Pr. Feugier P; Dr. Le Maignan C; Dr. Caillères S; Dr. Lucas V; Dr. Grulois I; Dr. Bellange A and Dr. Ghandour. C; The GOELAMS acknowledges the following pathologists for their participation sending slides or blocks for the histological review and study: Angonin R, Peoc’h M, Déchelotte P, Kemeny JL, Rousselet MC, Lefrancq T, De Muret A, Boucheron S, Gentil-Perret A, Mosnier JF, Fabiani B, Michenet P, Patey M, Eric Keddari, Fonck A, Memeteau F, Kambouchner M, Lagorce, Marck MF, Raymond-Gellé MF, Tas P, Chenard-Neu MP, Eloit S, Corvasier P, Julié C, Lhuintre P, Petit J, Fabre-Bocquentin B, Cazal-Hatem D, Rousset T, Lemaire JC, Akpo-Alavo B, Douvin D. We thank particularly the colleagues of the pathological review board: Dr Fanny Gaillard, Dr Josette Brière, Dr Martine Raphael (for the first trial) and the assistance of Dr S Saïkali. We are very grateful to the data managers of the GOELAMS group, in particular Valérie Rolland. We thank Dr. Mary Callanan, Prof. Marie-Christine Béné, and Prof. Jean-Yves Cahn for critically reviewing the manuscript and for English editing. We express sincere thanks to Prof. Jean-Jacques Sotto for his constant support and encouragement of this work.
Footnotes
Authorship and Disclosures
RG designed the trial; SC-M reviewed all the pathologic samples; RG, ED, OT, EG, MP, AE-Y, JC, JFR, SLG, GL, GD, PSC, HM, BC, JPV, PC, TL and PC were involved in the care of patients, the collections and the validation of the clinical data with the clinical research associates of the Goelams group; MC has provided statistical analysis; RG, SLG, TL and PC wrote the manuscript; all the authors have checked the final version of the manuscript.
JFR received honooraria from HOSPIRA; RG and TLdLC received honoraria from CELGENE.
The other authors reported no potential conflicts of interest.
References
- 1.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting--Airlie House, Virginia, November, 1997. Hematol J. 2000;1(1):53–66. doi: 10.1038/sj.thj.6200013. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–8. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 3.Bernard M, Gressin R, Lefrere F, Drenou B, Branger B, Caulet-Maugendre S, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15(11):1785–91. doi: 10.1038/sj.leu.2402272. [DOI] [PubMed] [Google Scholar]
- 4.Meusers P, Engelhard M, Bartels H, Binder T, Fulle HH, Gorg K, et al. Multicentre randomized therapeutic trial for advanced centrocytic lymphoma: anthracycline does not improve the prognosis. Hematol Oncol. 1989;7(5):365–80. doi: 10.1002/hon.2900070505. [DOI] [PubMed] [Google Scholar]
- 5.Zucca E, Roggero E, Pinotti G, Pedrinis E, Cappella C, Venco A, et al. Patterns of survival in mantle cell lymphoma. Ann Oncol. 1995;6(3):257–62. doi: 10.1093/oxfordjournals.annonc.a059155. [DOI] [PubMed] [Google Scholar]
- 6.Majlis A, Pugh WC, Rodriguez MA, Benedict WF, Cabanillas F. Mantle cell lymphoma: correlation of clinical outcome and biologic features with three histologic variants. J Clin Oncol. 1997;15(4):1664–71. doi: 10.1200/JCO.1997.15.4.1664. [DOI] [PubMed] [Google Scholar]
- 7.Hiddemann W, Unterhalt M, Herrmann R, Woltjen HH, Kreuser ED, Trumper L, et al. Mantle-cell lymphomas have more widespread disease and a slower response to chemotherapy compared with follicle-center lymphomas: results of a prospective comparative analysis of the German Low-Grade Lymphoma Study Group. J Clin Oncol. 1998;16(5):1922–30. doi: 10.1200/JCO.1998.16.5.1922. [DOI] [PubMed] [Google Scholar]
- 8.Lefrere F, Delmer A, Levy V, Delarue R, Varet B, Hermine O. Sequential chemotherapy regimens followed by high-dose therapy with stem cell transplantation in mantle cell lymphoma: an update of a prospective study. Haematologica. 2004;89(10):1275–6. [PubMed] [Google Scholar]
- 9.Khouri IF, Romaguera J, Kantarjian H, Palmer JL, Pugh WC, Korbling M, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16(12):3803–9. doi: 10.1200/JCO.1998.16.12.3803. [DOI] [PubMed] [Google Scholar]
- 10.Lefrere F, Delmer A, Suzan F, Levy V, Belanger C, Djabarri M, et al. Sequential chemotherapy by CHOP and DHAP regimens followed by high-dose therapy with stem cell transplantation induces a high rate of complete response and improves event-free survival in mantle cell lymphoma: a prospective study. Leukemia. 2002;16(4):587–93. doi: 10.1038/sj.leu.2402406. [DOI] [PubMed] [Google Scholar]
- 11.Milpied N, Gaillard F, Moreau P, Mahe B, Souchet J, Rapp MJ, et al. High-dose therapy with stem cell transplantation for mantle cell lymphoma: results and prognostic factors, a single center experience. Bone Marrow Transplant. 1998;22(7):645–50. doi: 10.1038/sj.bmt.1701400. [DOI] [PubMed] [Google Scholar]
- 12.Freedman AS, Neuberg D, Gribben JG, Mauch P, Soiffer RJ, Fisher DC, et al. High-dose chemoradiotherapy and anti-B-cell monoclonal antibody-purged autologous bone marrow transplantation in mantle-cell lymphoma: no evidence for long-term remission. J Clin Oncol. 1998;16(1):13–8. doi: 10.1200/JCO.1998.16.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Andersen NS, Pedersen L, Elonen E, Johnson A, Kolstad A, Franssila K, et al. Primary treatment with autologous stem cell transplantation in mantle cell lymphoma: outcome related to remission pre-transplant. Eur J Haematol. 2003;71(2):73–80. doi: 10.1034/j.1600-0609.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 14.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, Conde E, Lopez-Guillermo A, Gisselbrecht C, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120(5):793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 15.Mangel J, Leitch HA, Connors JM, Buckstein R, Imrie K, Spaner D, et al. Intensive chemotherapy and autologous stem-cell transplantation plus rituximab is superior to conventional chemotherapy for newly diagnosed advanced stage mantle-cell lymphoma: a matched pair analysis. Ann Oncol. 2004;15(2):283–90. doi: 10.1093/annonc/mdh069. [DOI] [PubMed] [Google Scholar]
- 16.Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677–84. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 17.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687–93. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gressin R, Legouffe E, Leroux D, Jacob MC, Swiercz P, Peoch M, et al. Treatment of mantle-cell lymphomas with the VAD +/− chlorambucil regimen with or without subsequent high-dose therapy and peripheral blood stem-cell transplantation. Ann Oncol. 1997;8 (Suppl 1):103–6. [PubMed] [Google Scholar]
- 19.Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 22.Lenz G, Dreyling M, Hoster E, Wormann B, Duhrsen U, Metzner B, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23(9):1984–92. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 23.Howard OM, Gribben JG, Neuberg DS, Grossbard M, Poor C, Janicek MJ, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20(5):1288–94. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- 24.Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013–23. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 25.de Guibert S, Jaccard A, Bernard M, Turlure P, Bordessoule D, Lamy T. Rituximab and DHAP followed by intensive therapy with autologous stem-cell transplantation as first-line therapy for mantle cell lymphoma. Haematologica. 2006;91(3):425–6. [PubMed] [Google Scholar]
- 26.Gianni AM, Magni M, Martelli M, Di Nicola M, Carlo-Stella C, Pilotti S, et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen) Blood. 2003;102(2):749–55. doi: 10.1182/blood-2002-08-2476. [DOI] [PubMed] [Google Scholar]
- 27.Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(15):3383–9. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 28.Rummel MJ, Niederle N, Maschmayer G, Banat A, von Grunhagen U, Losem C, et al. Bendamustine Plus Rituximab Is Superior in Respect of Progression Free Survival and CR Rate When Compared to CHOP Plus Rituximab as First-Line Treatment of Patients with Advanced Follicular, Indolent, and Mantle Cell Lymphomas: Final Results of a Randomized Phase III Study of the StiL (Study Group Indolent Lymphomas, Germany) Blood. 2009;114:168. [Google Scholar]
- 29.Tam CS, Khouri IF. Autologous and allogeneic stem cell transplantation: rising therapeutic promise for mantle cell lymphoma. Leuk Lymphoma. 2009:1–10. doi: 10.1080/10428190903026518. [DOI] [PubMed] [Google Scholar]
- 30.Dreger P, Rieger M, Seyfarth B, Hensel M, Kneba M, Ho AD, et al. Rituximab-augmented myeloablation for first-line autologous stem cell transplantation for mantle cell lymphoma: effects on molecular response and clinical outcome. Haematologica. 2007;92(1):42–9. doi: 10.3324/haematol.10608. [DOI] [PubMed] [Google Scholar]
- 31.Murali S, Winton E, Waller EK, Heffner LT, Lonial S, Flowers C, et al. Long-term progression-free survival after early autologous transplantation for mantle-cell lymphoma. Bone Marrow Transplant. 2008;42(8):529–34. doi: 10.1038/bmt.2008.201. [DOI] [PubMed] [Google Scholar]
- 32.Andersen NS, Pedersen LB, Laurell A, Elonen E, Kolstad A, Boesen AM, et al. Preemptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol. 2009;27(26):4365–70. doi: 10.1200/JCO.2008.21.3116. [DOI] [PubMed] [Google Scholar]
- 33.Klapper W, Hoster E, Determann O, Oschlies I, van der Laak J, Berger F, et al. Ki-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL Network. J Hematop. 2009 Jun 16; doi: 10.1007/s12308-009-0036-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Determann O, Hoster E, Ott G, Wolfram Bernd H, Loddenkemper C, Leo Hansmann M, et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood. 2008;111(4):2385–7. doi: 10.1182/blood-2007-10-117010. [DOI] [PubMed] [Google Scholar]
- 35.Khouri IF, Lee MS, Saliba RM, Jun G, Fayad L, Younes A, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol. 2003;21(23):4407–12. doi: 10.1200/JCO.2003.05.501. [DOI] [PubMed] [Google Scholar]
- 36.Maris MB, Sandmaier BM, Storer BE, Chauncey T, Stuart MJ, Maziarz RT, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535–42. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 37.Ganti AK, Bierman PJ, Lynch JC, Bociek RG, Vose JM, Armitage JO. Hematopoietic stem cell transplantation in mantle cell lymphoma. Ann Oncol. 2005;16(4):618–24. doi: 10.1093/annonc/mdi107. [DOI] [PubMed] [Google Scholar]
- 38.Coleman M, Martin P, Ruan J, Furman R, Niesvizky R, Elstrom R, et al. Low-dose metronomic, multidrug therapy with the PEP-C oral combination chemotherapy regimen for mantle cell lymphoma. Leuk Lymphoma. 2008;49(3):447–50. doi: 10.1080/10428190701837330. [DOI] [PubMed] [Google Scholar]