Abstract
Background
Natural killer cells have been demonstrated to exert remarkable graft-versus-leukemia effects after haploidentical transplantation. Acquisition of both, inhibiting and activating, receptors on developing natural killer cells is an important step in their functional maturation. Here, we report on the reconstitution of natural killer receptors after haploidentical transplantation of T-and B-cell (CD3/CD19) depleted grafts with co-transfusion of natural killer cells in children and its influence on natural killer cell activity and clinical outcome.
Design and Methods
We analyzed reconstitution patterns of natural killer receptors at different time intervals after haploidentical transplantation by multi-color flow cytometry. Natural killer cell activity and antibody-dependent cellular cytotoxicity was tested against cell lines and leukemic blasts in vitro. Survival was analyzed using Kaplan-Meier estimates.
Results
Recovery of CD56+/CD16+ cells was fast with high cytolytic activity against K562 and strong antibody-dependent cellular cytotoxicity activity against neuroblastoma and leukemic blasts as early as day 14 posttransplant. KIR reconstitution showed a predominance of KIR negative natural killer cells early after transplantation and an early reconstitution of CD158b compared to CD158a and CD158e. These differences were independent of presence or absence of the corresponding KIR ligands in donors or recipients. This reconstitution pattern was associated with a higher relapse probability of patients homozygous for HLA-C1-alleles compared to patients homozygous or even heterozygous for HLA-C2-alleles.
Conclusions
Our results indicate a fast recovery of functional and alloreactive natural killer cells with a constant KIR pattern after haploidentical transplantation with T- and B-cell depleted grafts. Moreover, these natural killer cells can mediate antibody-dependent cellular cytotoxicity and therefore may allow for an early use of antibodies against residual malignant cells.
Keywords: NK cells, haploidentical transplantation, multi-color flow cytometry
Introduction
Haploidentical stem cell transplantation (SCT) from mismatched related donors has become an established procedure for the treatment of children with high-risk and relapsed leukemia.1–3 Natural killer (NK) cells are the lymphocyte subset showing the fastest reconstitution in vivo. Therefore, NK cells are the predominant lymphocyte subset which may exert antileukemic effects early after haploidentical stem cell transplantation due to delayed reconstitution of a functional T-cell repertoire. The function of NK cells is regulated by the balance of activating and inhibitory signals transmitted by different cell surface receptors. Most inhibitory receptors bind to HLA class I molecules. Importantly, members of the killer immunoglobulin like receptors (KIR) only bind to certain HLA-allele groups, e.g. the KIR receptor CD158a (KIR2DL1) binds to HLA-C2-alleles, CD158b (KIR2DL2/3) to HLA-C1-alleles and CD158e (KIR3DL1) to Bw4-alleles, therefore sensing the lack of certain HLA-alleles on target cells. Other inhibitory receptors like NKG2A and CD85j bind to a broader range of HLA molecules and are sensing overall HLA expression of target cells.4 In the setting of allogeneic transplantation, NK cells which are not inhibited by HLA class I molecules of the recipient are called “alloreactive NK cells”. Alloreactive NK cells have been demonstrated to exert remarkable graft-versus-leukemia (GvL) effects both in adult patients with acute myeloid leukemia (AML) and in pediatric patients with acute lymphoblastic leukemia (ALL).5–7 Based on these clinical findings, different models to define NK alloreactive donors have been proposed.
During immune reconstitution after stem cell transplantation, NK cells start to express different NK receptors sequentially and different stages of NK cell differentiation can be distinguished.8–13 The expression of KIR receptors and CD16, as well as the transition from the CD56bright to the CD56dim phenotype, have been considered important steps for the acquisition of cytolytic function, which occur in a very late phase of NK cell maturation.14,15 Therefore, a detailed analysis of phenotype and function of regenerating NK cells after stem cell transplantation is necessary to assess their functionality and their contribution to GvL effects.
After allogeneic stem cell transplantation with purified stem cells, donor derived NK cells develop from CD34+ hematopoietic progenitor cells.16,17 In contrast to conventional enrichment of CD34+ stem cells, the T- and B-cell depletion of grafts with antiCD3+ and antiCD19+ coated microbeads leads to co-transfusion of large numbers of mature donor NK cells with stem cells at day zero of stem cell transplantation. In this study, we analyzed the reconstitution and maturation of NK cells after haploidentical transplantation with CD3/CD19 depleted grafts in children. We investigated the expression of inhibitory and activating NK receptors as well as NK cell cytotoxicity and NK cell mediated antibody-dependent cellular cytotoxicity (ADCC) against different cell lines and leukemic blasts. In contrast to published results in adult patients after haploidentical transplantation of CD34 selected grafts, we found a fast recovery of CD56+/CD16+ NK cells showing good ADCC activity in vitro, which encourages an early posttransplant use of antibodies directed against residual malignant cells. We observed an association between predominant reconstitution of CD158b positive cells and higher relapse rate in patients homozygous for the corresponding C1-alleles. No correlation between NK alloreactivity defined by the described models5–7 and survival was found.
Design and Methods
Patients
NK cell reconstitution was analyzed in 59 patients transplanted with CD3/CD19 depleted grafts at our clinic between December 2003 and December 2007. These patients participated in an ongoing trial which will be described in detail elsewhere. Diagnoses were mainly relapsed acute lymphoblastic leukemia (n=16) and acute myeloid leukemia (n=10), advanced myelodysplastic syndromes (MDS) (n=6), chronic myeloid leukemia (n=1), solid tumors (n=17) and non-malignant diseases (n=9). Median age at stem cell transplantation was 8.4 years (range 0.4–26.6 years). Thirteen patients (40%) with leukemias/MDS had active disease (morphological relapse) prior to transplantation; 10 patients had received a previous transplantation with matched donors. A detailed analysis of NK receptors by flow cytometry was carried out in 26 patients of this cohort (Table 1). For correlation of event free survival (EFS) and cumulative incidence of relapse to KIR ligand expression all above mentioned patients with malignant diseases were included. Median age at transplant in this subgroup was 8.5 years (range 0.4–26.6 years). Patients and donors gave their written informed consent, and the study was approved by the institutional ethical review board according to the declaration of Helsinki.
Table 1.
Characteristics of patients included in FACS-analysis of natural killer receptors after transplantation.
Regeneration of NK cells was compared to a cohort of 42 patients transplanted between 1997 and 2001 who received CD34 enriched grafts from mismatched donors. Median age in this control group was 8.8 years (range 0.4–18.28 years), Diagnoses were acute lymphoblastic leukemia (n=22), acute myeloid leukemia (n=5), myelodysplastic syndromes (n=3), chronic myeloid leukemia (CML) (n=4), non-Hodgkin’s lymphoma (n=3) and non-malignant diseases (n=5). Both groups were compared to the absolute NK cell count/μl from stem cell donors (n=34) during evaluation before donation.
Conditioning regimens and transplantation
The conditioning regimens for haploidentical transplantation comprised fludarabine/clofarabine, thiotepa and melphalan or total body irradiation (TBI)/busulphan based standard protocols. OKT3 was given as rejection prophylaxis. In detail, most patients with CD3/CD19 depleted grafts received a melphalan based, reduced intensity regimen (melphalan 2×70 mg/m2, fludarabine 4×40 mg/m2, thiotepa 10 mg/kg and OKT3 0.1 mg/kg, days −8 to +14). Patients received CD3/CD19 depleted haploidentical grafts with a median stem cell number (CD34+) of 12.6×106/kg, a median T-cell number (CD3+) of 56.8×103/kg and a median B-cell number (CD20+) of 24.8×103/kg. The stem cell grafts contained a median NK cell number (CD56+/CD3−) of 106×106/kg and a median number of myeloid cells (CD14+/CD33+) of 413.7×106. Mycophenolate-mofetil was given as graft-versus-host disease (GvHD) prophylaxis starting on day -1 at a dose of 1,200 mg/m2/d and stopped between days +60 and +100. All patients in the control group received CD34 enriched grafts after total body irradiation or busulphan based standard myeloablative conditioning regimens. Anti-thymocyte globulin (ATG) was given as rejection prophylaxis from days −4 to −1. No GvHD prophylaxis was given posttransplant.
Flow cytometry
FACS analysis of NK cell receptors was carried out at different time points (day 30, day 60, day 100, day 200) after transplantation. The following mouse anti-human monoclonal antibodies were used: CD2 FITC, CD3 PerCP, CD11a FITC, CD16 PE, CD56 FITC and APC, CD69 FITC, CD94 FITC, CD158a FITC, CD158b PE, CD158e Biotin, CD226 PE, CD244 FITC, Streptavidin PerCP (all from Beckton Dickinson/BD Pharmingen, Heidelberg, Germany), CD54 FITC (Dako cytomation, Hamburg, Germany), CD85j PE, NKG2A PE, NKp30 PE, NKp44 PE, NKp46 PE (all from Beckman Coulter, Krefeld, Germany), NKG2D APC (R&D Systems, Wiesbaden-Nordenstadt, Germany). Peripheral blood mononuclear cells from patients were incubated with the corresponding mAb for 30min at 4°C. Control aliquots were stained with non-binding isotype IgG (Becton-Dickinson, Heidelberg, Germany). Samples were analyzed on a FACSCalibur flow cytometer (Becton-Dickinson, Heidelberg, Germany) using CELLQUEST software (BD). A minimum of 10,000 events were used for assessment.
Cytotoxicity assay
Cytolytic activity of NK cells was tested in a 2-hour BATDA [bis (acetoxymethyl) 2,2′:6′,2″-terpyridine-6,6″-dicarboxylate] europium release assay as described elsewhere.18 K562 (erythroleukemia cell line), SK-N-BE (neuroblastoma cell line) or leukemic blasts from our patients (if possible autologous blasts) were used as target cells. For testing antibody-dependent cellular cytotoxicity, target cells were preincubated for 30 min either with humanized anti-GD2-mAb (provided by Dr. Barfield, St. Jude Children’s Research Hospital, Memphis, TN, USA) when using SK-N-BE as targets or with a humanized anti-CD19-mAb (Lexigen, Massachusetts, USA) when using leukemic blasts. Four different effector to target cell ratios were tested. Specific lysis was calculated as follows:
Statistical analysis
Student’s t-tests, survival analysis (using Kaplan-Meyer estimates) and cumulative incidence of relapse were performed using GraphPad Prism version 4.01 for Windows, GraphPad Software, San Diego California USA (www.graphpad.com).
Results
Reconstitution of CD56+/CD3− cells and killer immunoglobulin-like receptors (KIRs)
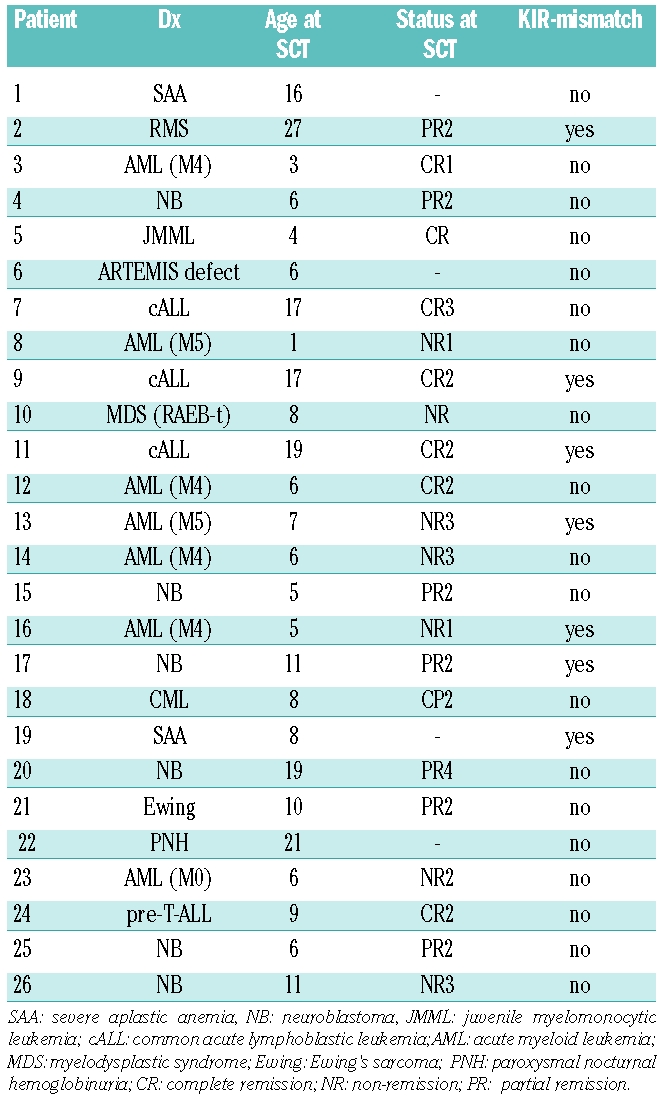
The CD3/CD19 depleted grafts contained a median NK cell number of 106×106/kg bodyweight, but infused NK cells were detectable in peripheral blood by FACS analysis only to a low extent and a few days after infusion (data not shown). Patients with CD3/CD19 depleted grafts showed a very fast reconstitution of NK cells (defined as CD56+/CD3−) which reached a normal level already at day +14 (t-test, P=0.91 compared to healthy donors). We found significantly higher numbers of NK cells at this time point compared to patients with CD34 enriched grafts whereas no significant difference was found at later time points (Figure 1A). Analysis of KIR receptors was only carried out in the CD3/CD19 depleted group. We found higher levels for CD158b/j positive NK cells compared to CD158a/h and CD158e positive cells (Figure 1B). These observations were independent of the presence or absence of HLA-Cw3 alleles in donors or recipients (data not shown).
Figure 1.
Reconstitution of NK cells and KIRs. (A) Absolute CD56+/CD3− cell counts/μl blood from patients in different periods after SCT with CD3/19 depleted or CD34 enriched grafts compared to healthy donors (h.d., n = 34). Bars show mean values + standard error of the mean (SEM). Numbers of patients at different time points were 18 (CD3/19) and one (CD34) at day +7, 54 and 11 at day +14, 57 and 27 at day +21, 56 and 33 at day +30, 54 and 31 at day +60, 49 and 32 at day +90. We found a faster reconstitution of NK cells after CD3/CD19 depletion which showed a significant difference at day +14 (t-test, *P=0.0002), whereas no significant difference was found at other time points. (B) Reconstitution of KIR negative and CD158a, CD158b and CD158e positive NK cells in various periods post SCT. P values from Student’s t-tests comparing CD158b+ and CD158a+ cells are indicated for each period. Number of patients in the different time periods were 21 (day 0–50), 20 (day 50–100), 19 (day 100–200) and 17 (day >200). (C) Reconstitution of CD158a/h and CD158b/j single positive NK cells in the first 100 days posttransplant according to KIR ligand expression of the recipients. Number of measurements were 11 (C1/C1 recipients), 20 (C1/C2 recipients) and 8 (C2/C2 recipients).
In a second step, we focused on KIR single positive NK cells. These are an interesting population for studying potential alloreactive responses because these cells are only inhibited by a certain group of HLA-alleles. Analysis of NK cell subpopulations with exclusive expression of one KIR also showed a predominance of CD158b/j+ 158a/h− 158e− NK cells. Interestingly, this predominance again was not influenced by donor or recipient KIR ligand expression in our group of patients (Figure 1C). Instead, C2 homozygous patients showed a tendency to a lower expression of both CD158b/j and CD158a, which was significant for CD158a single positive NK cells compared to C1 homozygous patients (t-test, P=0.02) but not for CD158b single positive NK cells (P=0.16).
Reconstitution of other inhibitory and activating natural killer receptors
In addition to KIR receptors we analyzed the expression of other inhibitory and activating receptors as well as different adhesion molecules on NK cells in the patients with CD3/CD19 depleted grafts. Patients after transplantation showed a higher percentage of NKG2A and CD62L positive NK cells compared to their donors which declined to normal levels after day +200. CD244 and CD85j were expressed at slightly decreased levels. Percentages of NKp30, NKp46 and CD226 positive NK cells reached a normal range early. Patients showed significantly decreased percentages of NKG2D positive NK cells in the first 100 days after transplantation. However, percentages of NKp44 and CD69 positive NK cells were slightly increased. Adhesion molecules were either expressed in the same range (CD11a, CD2) or even in a slightly higher percentage (CD54) compared to healthy donors (data not shown).
Reconstitution of CD56dim and CD56bright subsets
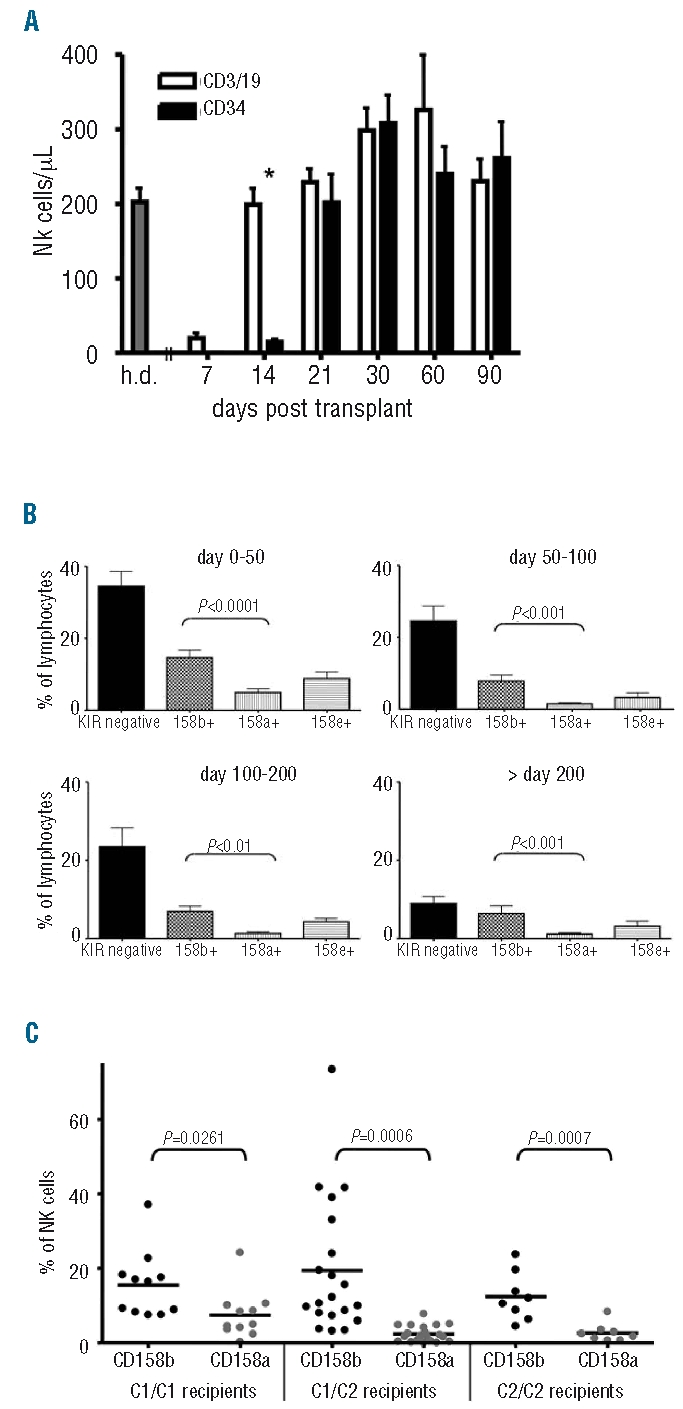
Two subsets of NK cells can be distinguished by their expression of CD56 and CD16, CD56dim NK cells, which are mainly CD16 positive, and CD56bright NK cells, which are mainly CD16 negative. In the donors, 87.5% of NK cells (defined as CD56+/CD3−) showed a CD56dim phenotype and 70% were positive for CD16. In patients after transplantation, 79.11% of NK cells showed a CD56dim phenotype (P=0.07) and 63.0% were positive for CD16 (P=0.17) in the first 50 days after transplantation and showed a slight decrease over time (Figure 2A). However, these CD56dim NK cells showed several phenotypic differences to CD56dim NK cells from healthy donors. Specifically, expression of NKG2A (P<0.0001) and CD62L (P=0.037) was increased and expression of NKG2D (P=0.0006) was reduced, indicating rather a maturation from CD56bright NK cells than an expansion from infused CD56dim NK cells (Figure 2B). As in the donors, regenerating CD56bright/CD16− NK cells showed higher expression of NKG2A, NKp46, NKp44 and CD62L and lower expression of CD85j compared to CD56dim (data not shown).
Figure 2.
Reconstitution of CD56dim/CD16+ NK cells. (A) Percentage of CD56dim/CD16+ and CD56high/CD16− NK cells from healthy donors and patients in various periods post SCT. Patients showed nearly the same level of CD56dim/CD16+ NK cells in the first 50 days post SCT compared to healthy donors. (B) Expression of different NK receptors on CD56dim NK cells in different periods post SCT. A comparison with healthy donors (first column) showed significantly higher expression of NKG2A and CD62L and reduced expression of NKG2D indicating rather a maturation from CD56bright NK cells than an expansion from infused CD56dim NK cells (n=15 in time periods 0–50 and 50–100, n=8 in time periods 100–200 and >200).
Cytolytic activity of reconstituting natural killer cells
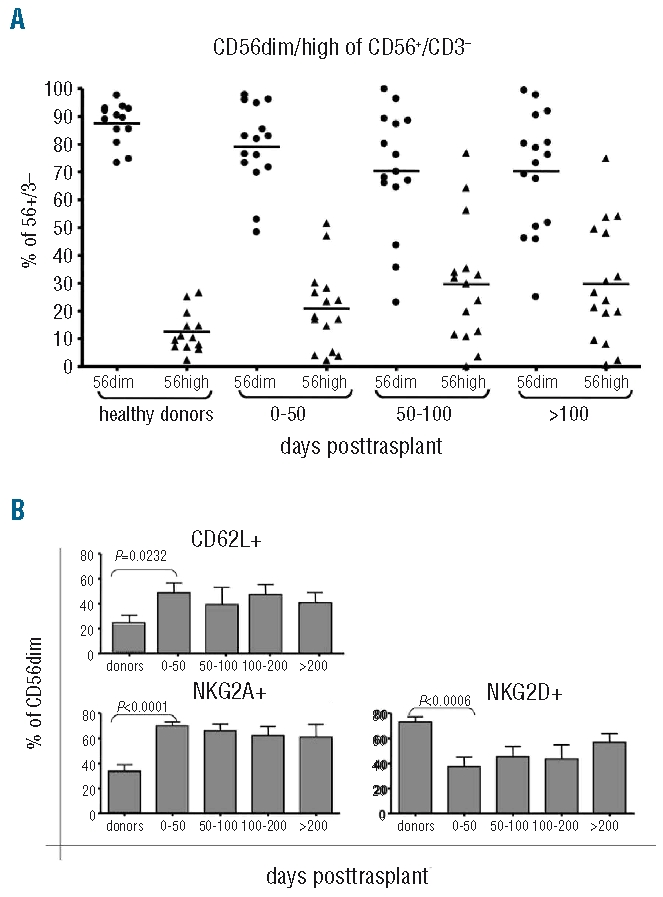
For functional analysis of reconstituting NK cells, we measured cytotoxic activity against K562. In selected patients cytotoxic activity was also analyzed against primary leukemic blasts or a neuroblastoma cell line. Cytotoxic activity against K562 of both resting and IL-2 activated NK cells reached high levels already within the first 50 days after transplantation (Figure 3A), indicating an early cytolytic potential posttransplant. Activity of resting NK cells against neuroblastoma and leukemic blasts was rather low, but could be enhanced against neuroblastoma by overnight incubation with IL-2 (data not shown).
Figure 3.
Cytotoxic activity of reconstituted natural killer (NK) cells post SCT. (A) Cytotoxic activity against the cell line K562. Resting NK cells from patients showed good activity against K562 early post SCT (gray diamonds), which could be further enhanced by overnight stimulation with IL-2 (black rectangles). Cytotoxic activity against this target in the first 50 days post SCT was in the same range as in later time periods. (B) ADCC activity at early time points post SCT against cryopreserved leukemic blasts (at day +14) or a neuroblastoma cell line (between day +14 and +21). Cytotoxic activity was significantly enhanced against both targets using appropriate antibodies (P= 0.03 for leukemic blasts, P=0.01 for neuroblastoma). (C) Activity of patients’ cells post SCT against autologous blasts. Both resting (gray diamonds) and IL-2 activated cells (black rectangles) showed low but significant specific lysis. Exploiting ADCC with the CD19 antibody significantly enhanced specific lysis of both resting and activated NK cells (E:T=20:1, P=0.02 for resting and P=0.0004 for activated NK cells).
A possibility to enhance NK activity is the exploitation of antibody dependent cellular cytotoxicity by the use of appropriate antibodies. We, therefore, used antibodies optimized for induction of antibody dependent cellular cytotoxicity by NK cells against disialoganglioside (GD2) for neuroblastoma and CD19 for pediatric leukemic blasts. Lysis of the respective target was significantly enhanced with both antibodies. Since we found a fast reconstitution of CD16+ NK cells in our patients with CD3/CD19 depleted grafts, we tested the induction of antibody dependent cellular cytotoxicity at very early time points after transplantation. With both antibodies we found a significant increase in specific lysis as early as day 14 after transplantation (Figure 3B). In 6 patients, NK activity could be tested against autologous cryopreserved primary blasts. All 6 patients showed a weak but significant (>10% at an E:T ratio of 20:1) specific lysis against autologous blasts and a slight increase after stimulation with IL-2. Using the CD19 antibody, remarkable antibody dependent cellular cytotoxicity activity was obtained also against these targets (Figure 3C).
Correlation of different models of natural killer alloreactivity and outcome after transplantation
Different models for predicting NK alloreactivity after stem cell transplantation have been established which show heterogeneous results in different patient cohorts, diseases and transplantation settings. We tested correlations between predicted NK alloreactivity and overall survival or relapse probability in our group of patients after haploidentical transplantations with CD3/CD19 depleted grafts according to different models. Grouping all patients or patients with leukemias and advanced myelodysplastic syndromes (ALL, AML, MDS) according either to the “KIR ligand model”, the “KIR receptor ligand model” or the “missing KIR ligand model” did not show any survival advantage for patients with a supposed NK alloreactive donor. The faster and higher reconstitution of CD158b on NK cells independent of KIR ligand expression in recipients prompted us to group patients according to their KIR ligand expression. We assumed that patients homozygous for HLA-C1-alleles block CD158b+ regenerating donor NK cells most effectively while patients homozygous for HLA-C2-alleles fail to block these NK cells. Indeed, all patients with leukemias or advanced myelodysplastic syndromes irrespective of remission status who were homozygous or even heterozygous for HLA-C2-alleles showed a better event free survival than did patients homozygous for HLA-C1-alleles (Figure 4A and B). For patients with acute leukemia who were in any complete remission prior to haploidentical transplantation this difference was even more pronounced (Figure 4C). Furthermore, relapse probability for patients with acute leukemias was significantly lower in the homozygous/heterozygous HLA-C2 group (Figure 4D and E).
Figure 4.
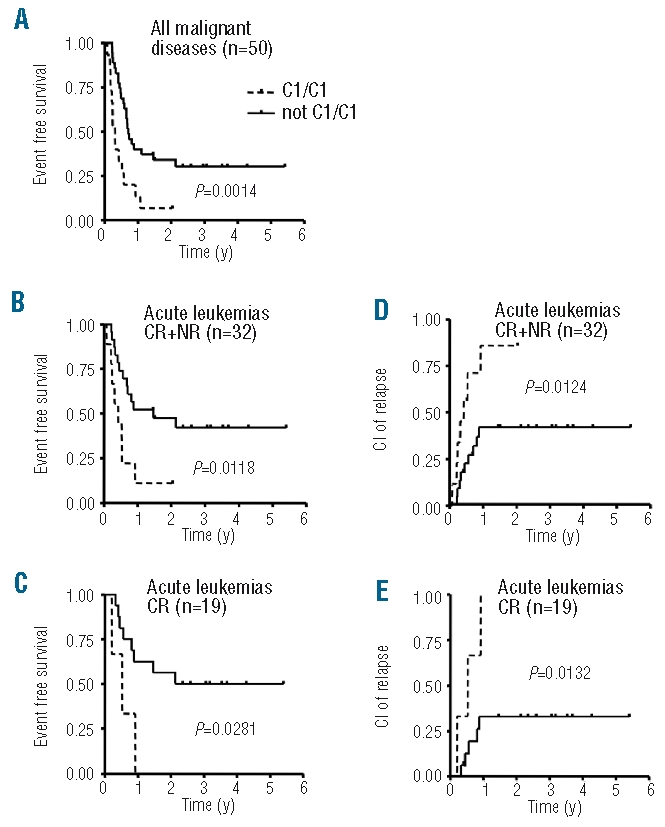
Influence of KIR ligand expression on event free survival (EFS) and relapse probability. The first three panels show the influence of KIR ligand expression on event free survival. (A) Event free survival (EFS) of patients with malignant diseases transplanted with CD3/CD19 depleted haploidentical grafts between 12/2003 and 12/2007 (n=50; n=15 for the “C1/C1” group and n=35 for the “not C1/C1” group). (B) EFS of patients with acute leukemias and advanced myelodysplastic syndromes (MDS) irrespective of remission status (CR and NR, n=32; n=9 for the “C1/C1” group and n = 23 for the “not C1/C1” group). (C) EFS of patients with acute leukemias/MDS in any complete remission prior to SCT (n=19; n=3 for the “C1/C1” group and n = 16 for the “not C1/C1” group). Patients homozygous for C1-alleles (dotted line) showed significantly worse survival compared to patients not homozygous for C1-alleles (solid line). The following panels show the influence of KIR ligand expression on cumulative incidence (CI) of relapse. (D) Patients with acute leukemias irrespective of remission status [CR and NR, patient numbers see (B)]. (E) Patients with acute leukemias in any complete remission prior SCT [patient numbers see (C)]. All subgroups showed a lower relapse probability for patients not homozygous for C1-alleles.
Discussion
In the present study we analyzed, for the first time, the regeneration and function of NK cells and their receptors after haploidentical stem cell transplantation with CD3/CD19 depleted grafts. CD3/CD19 depletion of stem cell grafts leads to co-transfusion of large numbers of NK cells and myeloid cells at day zero of transplant with the intention of an improved graft-versus-leukemia effect posttransplant and a facilitated engraftment. NK cells have been shown to mediate antileukemic effects after haploidentical transplantation,5–7 and acquisition of both inhibiting and activating receptors on developing NK cells is an important step in their functional maturation after transplantation. NK cell reconstitution has been well described after haploidentical transplantation with CD34 enriched hematopoietic stem cells. Substantial differences are apparent comparing transplantation procedures with CD3/CD19 depleted cells and CD34 enriched cells. Our patients mainly received an intensity reduced conditioning regimen and high numbers of NK cells as well as myeloid cells in the graft. In the mouse model, donor NK cells contribute to a better engraftment and, therefore, may accelerate de novo regeneration of NK cells from hematopoietic stem cells.19
We found a very fast recovery of CD56+/CD3− NK cells, which may exert antileukemic effects, with CD3/CD19 depleted grafts. Absolute NK cell numbers/μl reached a normal range compared to healthy donors already at day +14. At this time point, recovery of NK cells with CD3/19 depleted grafts was significantly faster compared to CD34 enriched grafts, whereas no significant difference was found at later time points. This may be due to co-transfused NK cells, while also the influence of intensity reduced conditioning and the use of OKT3 instead of anti-thymocyte globulin in patients with CD3/19 depleted grafts have to be considered. The use of mycophenolate-mofetil as graft-versus-host disease prophylaxis, which was given after CD3/19 depletion but not in patients with CD34 enriched grafts did not counterbalance this effect.
We also found a faster recovery of CD56dim/CD16+ NK cells compared to published results after haploidentical transplantation with CD34 enriched grafts.20,21 In these studies, CD56brigth/CD16− NK cells represented the main NK subpopulation over several months post stem cell transplantation. A faster reconstitution of the CD56dim/CD16+ subset might be an advantage in regard to graft-versus-leukemia or antiviral activity as this is the more mature and more cytotoxic NK subset compared to the CD56brigth/CD16− subset. It is not clear if the faster reconstitution of CD56dim+CD16+ NK cells is an effect of CD3/CD19 depletion or also a general difference between children and adults. Unfortunately, no data about CD16 expression is available from our historical group of patients with CD34 enriched grafts. It has recently been shown that trans-presented IL-15 promotes NK cell differentiation in vivo from CD56high/CD16−/KIR− to CD56dim/CD16+/KIR− and finally CD56dim/CD16+/KIR+22 and it is postulated that this trans-presentation is done by myeloid cells.23 Since our patients received high numbers of myeloid cells with the CD3/CD19 depleted grafts, these infused myeloid cells may have contributed to the faster reconstitution of CD56dim/CD16+ cells by trans-presentation of IL-15.
The NK cells infused with the CD3/19 depleted grafts did not expand to a higher extent in peripheral blood in our patients in the first days after transplantation and the reconstituting CD56dim NK cells showed higher expression of CD62L and NKG2A compared to CD56dim NK cells from healthy donors. Therefore, we speculate that the CD56dim NK cells differentiated from CD56bright NK cells rather than expanded from transplanted CD56dim NK cells. Higher expression of CD62L and NKG2A on CD56dim NK cells has also been shown after transplantation with CD34 enriched grafts.20 High expression of NKG2A on regenerating NK cells is known to contribute to weak lysis of acute myeloid leukemia blasts21 and weak cytokine production20 by blocking of NKG2A-HLA-E interaction. By contrast, a strong lysis of HLA class I negative targets has been observed by these NK cells. We found the same pattern in our study with good lysis of K562 which could be further enhanced by stimulation with IL-2 and a weaker, but still significant, lysis of acute lymphoblastic leukemia blasts by resting or IL-2 stimulated NK cells. The low lysis of the neuroblastoma cell line SK-N-BE, which expresses only low amounts of HLA class I, by resting NK cells indicates that there are additional mechanisms responsible for the functional immaturity of the reconstituting NK cells. Furthermore it has been shown that many pediatric acute lymphoblastic leukemia blasts show a reduced expression of HLA class I and HLA-E compared to healthy B cells.24 NK clones positive for NKG2A and negative for CD158a, b and e showed strong lysis of pediatric blasts with reduced HLA-expression indicating that NKG2A-HLA-E interaction may not be the main inhibiting signal in these target cells.25
However, the lysis of neuroblastoma and acute lymphoblastic leukemia blasts mediated by reconstituting NK cells could be clearly improved by antibody dependent cellular cytotoxicity using appropriate antibodies. We used optimized chimeric antibodies against CD19 for antibody dependent cellular cytotoxicity in acute lymphoblastic leukemia blasts and against GD2 in neuroblastoma and found a significant increase in specific lysis both by resting and IL-2 activated NK cells. As such antibodies have also been shown to mediate effective anti-tumor reactions in vivo,26,27 it could be a promising approach to use them early after transplantation with CD3/CD19 depleted grafts taking advantage of the fast reconstitution of CD16+ NK cells.
In several retrospective analyses of haploidentical transplantations, different models have been established to predict NK alloreactivity and their influence on relapse rate and overall survival.5–7 In contrast, other studies did not show a positive effect of potential NK alloreactivity.21,28 In transplantations from unrelated donors, different results of KIR ligand incompatibility have also been reported.29–32 These conflicting results might partly be due to differences in the conditioning regimen, degree of T-cell depletion or different diseases. Grouping our patients with leukemia and advanced myelodysplastic syndromes (ALL, AML, MDS) according either to the “KIR ligand model”, the “KIR receptor ligand model” or the “missing KIR ligand model” did not show any survival advantage for patients with a supposed NK alloreactive donor in univariate analysis. However, we found a better overall and event free survival and a lower relapse probability in patients homozygous or even heterozygous for HLA-C2-alleles compared to patients homozygous for HLA-C1-alleles. This effect could be caused by the faster and significantly higher reconstitution of CD158b positive NK cells which should be blocked most efficiently in patients homozygous for HLA-C1-alleles. According to the “licensing” model, one can assume that CD158b single positive NK cells would not acquire functional competence in recipients homozygous for HLA-C2-alleles. However, the hyporesponsive state of these “unlicensed” NK cells is not permanent as they can be activated in response to proinflammatory cytokines.33 A clinical effect for such “unlicensed” NK cells has been shown in autologous stem cell transplantation for solid tumors and lymphoma34 indicating that this setting can provide an environment promoting activation of “unlicensed” NK cells. Furthermore, Yu et al.35 showed that “unlicensed” NK cells exhibit intracellular IFN-γ and cytotoxic response to target cells lacking cognate ligands early after T-cell depleted stem cell transplantation. These NK cells become gradually tolerized to self by day 100 posttransplant. Recently, Pende et al. showed a differentially pronounced inhibition of KIR2DL2/3 positive NK clones also by HLA-C2-alleles, although binding of KIR2DL2/3 to HLA-C2-alleles was weaker compared to HLA-C1-alleles.36 On the other hand, they also showed a contribution of KIR2DS1 to the alloreactive potential of NK cells which could override inhibition by NKG2A or KIR2DL2/3 against targets homozygous for HLA-C2-alleles. Unfortunately, we do not have data on KIR2DS1 expression but one can speculate that recognition of HLA-C2 positive target cells by the activating KIR2DS1 also contributes to the lower relapse rate observed in our patients positive for HLA-C2-alleles.
Our clinical observation is in contrast to findings in adult patients after unrelated donor hematopoietic stem cell transplantation where patients with chronic myeloid leukemia37 or chronic myeloid leukemia, acute myeloid leukemia, acute lymphoblastic leukemia and myelodysplastic syndromes,38 which were homozygous for HLA-C2-alleles had an increased risk of relapse. This difference again could be caused by differences in the transplant setting including graft manipulation, haploidentical compared with HLA-matched unrelated donors, as well as differences in the underlying diseases.
Unfortunately, the low number of patients in our study did not allow analysis in more homogenous subgroups. Therefore, larger cohorts should be analyzed to validate our observation in pediatric patients after haploidentical transplantation.
In summary, reconstitution of KIR receptors followed an already known pattern with higher and faster reconstitution of CD158b which was associated with an increased relapse risk in patients homozygous for HLA-C1-alleles. NK reconstitution in the early posttransplant period was faster after CD3/CD19 depletion compared to a control cohort with CD34 enriched grafts. Additionally, we found a fast recovery of CD56dim/CD16+ NK cells after haploidentical transplantation with CD3/CD19 depleted grafts. This observation is in contrast to published results in adult patients after haploidentical transplantation with CD34-selected grafts. Furthermore, we could show that these NK cells, despite a limited “natural cytotoxicity” against acute lymphoblastic leukemia blasts, mediate excellent antibody dependent cellular cytotoxicity activity in vitro as early as day 14 after transplantation. These results encourage an early posttransplant use of cytokine stimulation and antibodies to target residual malignant cells after haploidentical transplantation with CD3/CD19 depleted grafts, especially in patients homozygous for C1-alleles.
Acknowledgments
we thank Anni Babarin-Dorner for excellent technical assistance and David Martin for proof-reading the manuscript. We thank Dr Raymond Barfield, St. Jude Children’s Research Hospital, Memphis, for providing the humanized anti-GD2 antibody.
Footnotes
Funding: this work was supported by grants (SFB 685 and TRANSARNET) from the Deutsche Forschungsgemeinschaft (DFG), the Bundesministerium für Bildung und Forschung (BMBF) and from the Reinhold Beitlich Stiftung Tuebingen to PL, and from the Jung-Stiftung für Wissenschaft und Forschung and the Deutsche Jose Carreras Leukämie-Stiftung to MP.
Authorship and Disclosures
MMP and TF contributed equally to this work; MMP carried out the scientific work; PL and MMP carried out the experimental work; IM, RH and PL took care of patients and provided selected samples; RH directs the transplantation program; RH, MS and PL supervised research; MMP, TF, HMT and PL carried out data analysis; MMP and TF wrote the manuscript which was critically reviewed by all authors.
The authors reported no potential conflicts of interest.
References
- 1.Handgretinger R, Klingebiel T, Lang P, Schumm M, Neu S, Geiselhart A, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27(8):777–83. doi: 10.1038/sj.bmt.1702996. [DOI] [PubMed] [Google Scholar]
- 2.Lang P, Handgretinger R, Niethammer D, Schlegel PG, Schumm M, Greil J, et al. Transplantation of highly purified CD34+ progenitor cells from unrelated donors in pediatric leukemia. Blood. 2003;101(4):1630–6. doi: 10.1182/blood-2002-04-1203. [DOI] [PubMed] [Google Scholar]
- 3.Marks DI, Khattry N, Cummins M, Goulden N, Green A, Harvey J, et al. Haploidentical stem cell transplantation for children with acute leukaemia. Br J Haematol. 2006;134(2):196–201. doi: 10.1111/j.1365-2141.2006.06140.x. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 6.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–61. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172(1):644–50. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 8.Silva MR, Hoffman R, Srour EF, Ascensao JL. Generation of human natural killer cells from immature progenitors does not require marrow stromal cells. Blood. 1994;84(3):841–6. [PubMed] [Google Scholar]
- 9.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87(7):2632–40. [PubMed] [Google Scholar]
- 10.Carayol G, Robin C, Bourhis JH, Bennaceur-Griscelli A, Chouaib S, Coulombel L, et al. NK cells differentiated from bone marrow, cord blood and peripheral blood stem cells exhibit similar phenotype and functions. Eur J Immunol. 1998;28(6):1991–2002. doi: 10.1002/(SICI)1521-4141(199806)28:06<1991::AID-IMMU1991>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Mingari MC, Vitale C, Cantoni C, Bellomo R, Ponte M, Schiavetti F, et al. Interleukin-15-induced maturation of human natural killer cells from early thymic precursors: selective expression of CD94/NKG2-A as the only HLA class I-specific inhibitory receptor. Eur J Immunol. 1997;27(6):1374–80. doi: 10.1002/eji.1830270612. [DOI] [PubMed] [Google Scholar]
- 12.Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulinlike receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIR2DL2/L3/S2. Blood. 2001;98(3):705–13. doi: 10.1182/blood.v98.3.705. [DOI] [PubMed] [Google Scholar]
- 13.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203(4):1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3(5):413–25. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 15.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 16.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3(4):459–73. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 17.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83(9):2594–601. [PubMed] [Google Scholar]
- 18.Pfeiffer M, Stanojevic S, Feuchtinger T, Greil J, Handgretinger R, Barbin K, et al. Rituximab mediates in vitro antileukemic activity in pediatric patients after allogeneic transplantation. Bone Marrow Transplant. 2005;36(2):91–7. doi: 10.1038/sj.bmt.1705014. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202–18. doi: 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Vago L, Forno B, Sormani MP, Crocchiolo R, Zino E, Di Terlizzi, et al. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood. 2008;112(8):3488–99. doi: 10.1182/blood-2007-07-103325. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105(10):4135–42. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 22.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer M, Schumm M, Feuchtinger T, Dietz K, Handgretinger R, Lang P. Intensity of HLA class I expression and KIR-mismatch determine NK-cell mediated lysis of leukaemic blasts from children with acute lymphatic leukaemia. Br J Haematol. 2007;138(1):97–100. doi: 10.1111/j.1365-2141.2007.06631.x. [DOI] [PubMed] [Google Scholar]
- 25.Feuchtinger T, Pfeiffer M, Pfaffle A, Teltschik HM, Wernet D, Schumm M, et al. Cytolytic activity of NK cell clones against acute childhood precursor-B-cell leukaemia is influenced by HLA class I expression on blasts and the differential KIR phenotype of NK clones. Bone Marrow Transplant. 2009;43(11):875–81. doi: 10.1038/bmt.2008.398. [DOI] [PubMed] [Google Scholar]
- 26.Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68(19):8049–57. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 27.Otto M, Barfield RC, Martin WJ, Iyengar R, Leung W, Leimig T, et al. Combination immunotherapy with clinical-scale enriched human gammadelta T cells, hu14.18 antibody, and the immunocytokine Fc-IL7 in disseminated neuroblastoma. Clin Cancer Res. 2005;11(23):8486–91. doi: 10.1158/1078-0432.CCR-05-1184. [DOI] [PubMed] [Google Scholar]
- 28.Bishara A, De Santis D, Witt CC, Brautbar C, Christiansen FT, Or R, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63(3):204–11. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 29.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814–9. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 30.Lowe EJ, Turner V, Handgretinger R, Horwitz EM, Benaim E, Hale GA, et al. T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Br J Haematol. 2003;123(2):323–6. doi: 10.1046/j.1365-2141.2003.04604.x. [DOI] [PubMed] [Google Scholar]
- 31.Cooley S, McCullar V, Wangen R, Bergemann TL, Spellman S, Weisdorf DJ, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106(13):4370–6. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroger N, Binder T, Zabelina T, Wolschke C, Schieder H, Renges H, et al. Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation. 2006;82(8):1024–30. doi: 10.1097/01.tp.0000235859.24513.43. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 34.Leung W, Handgretinger R, Iyengar R, Turner V, Holladay MS, Hale GA. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97(4):539–42. doi: 10.1038/sj.bjc.6603913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Venstrom JM, Liu XR, O’Reilly R, Pring J, Hasan RS, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function following T-cell depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113(16):3875–84. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113(13):3119–29. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 37.Fischer JC, Ottinger H, Ferencik S, Sribar M, Punzel M, Beelen DW, et al. Relevance of C1 and C2 epitopes for hemopoietic stem cell transplantation: role for sequential acquisition of HLA-C-specific inhibitory killer Ig-like receptor. J Immunol. 2007;178(6):3918–23. doi: 10.4049/jimmunol.178.6.3918. [DOI] [PubMed] [Google Scholar]
- 38.Giebel S, Locatelli F, Wojnar J, Velardi A, Mina T, Giorgiani G, et al. Homozygosity for human leucocyte antigen-C ligands of KIR2DL1 is associated with increased risk of relapse after human leucocyte antigen-C-matched unrelated donor haematopoietic stem cell transplantation. Br J Haematol. 2005;131(4):483–6. doi: 10.1111/j.1365-2141.2005.05797.x. [DOI] [PubMed] [Google Scholar]


