Abstract
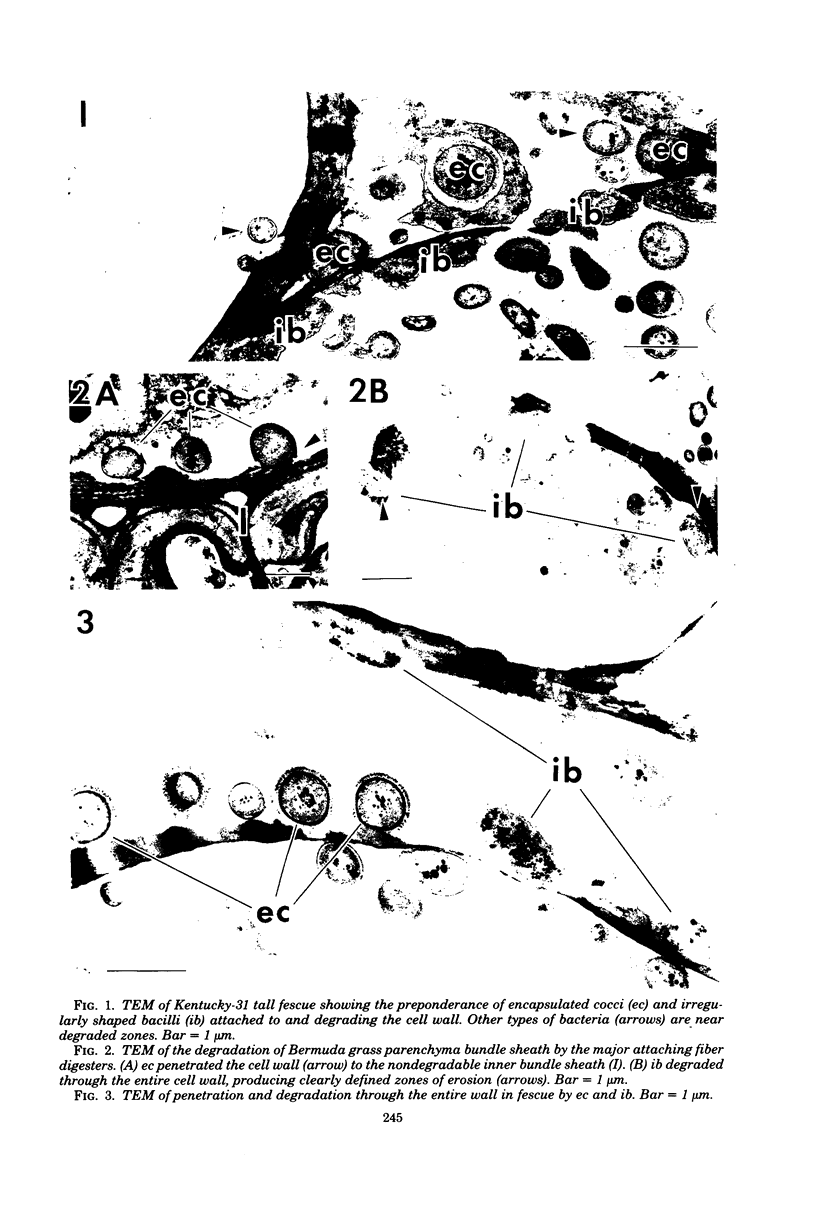
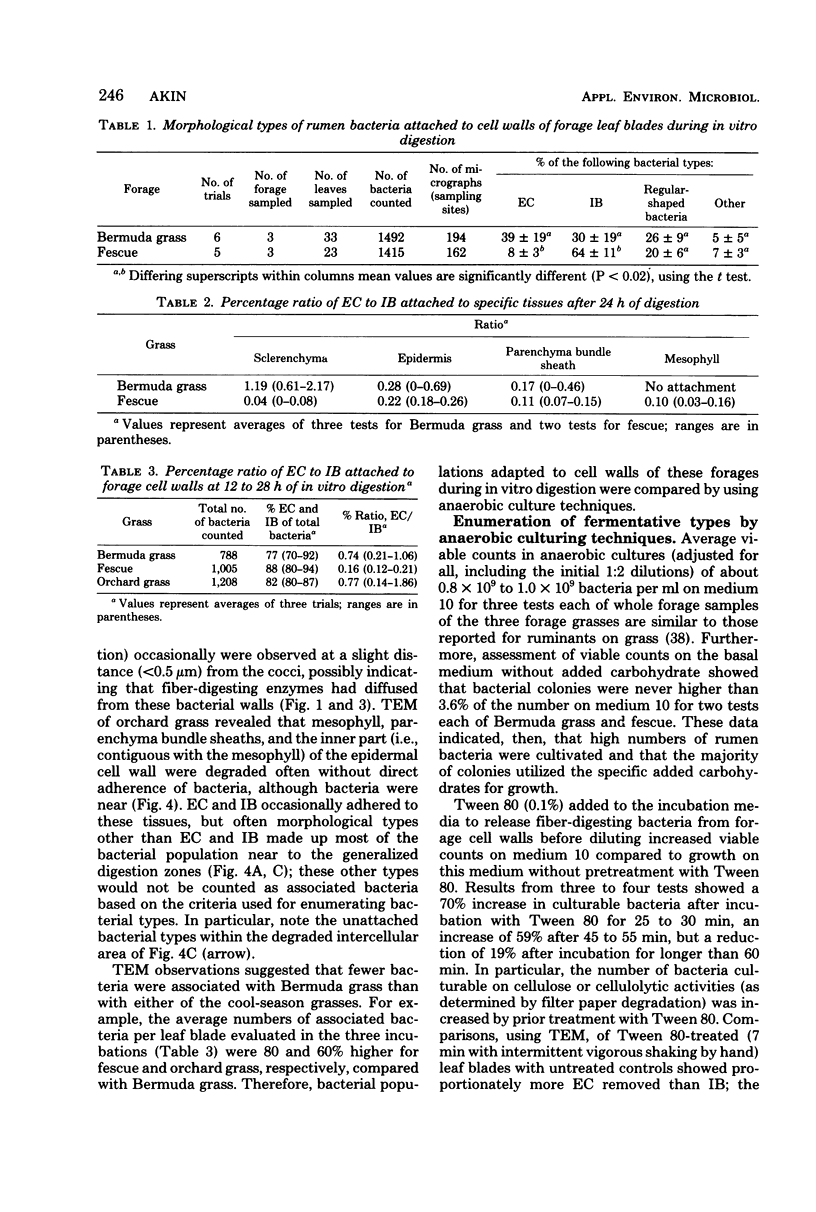
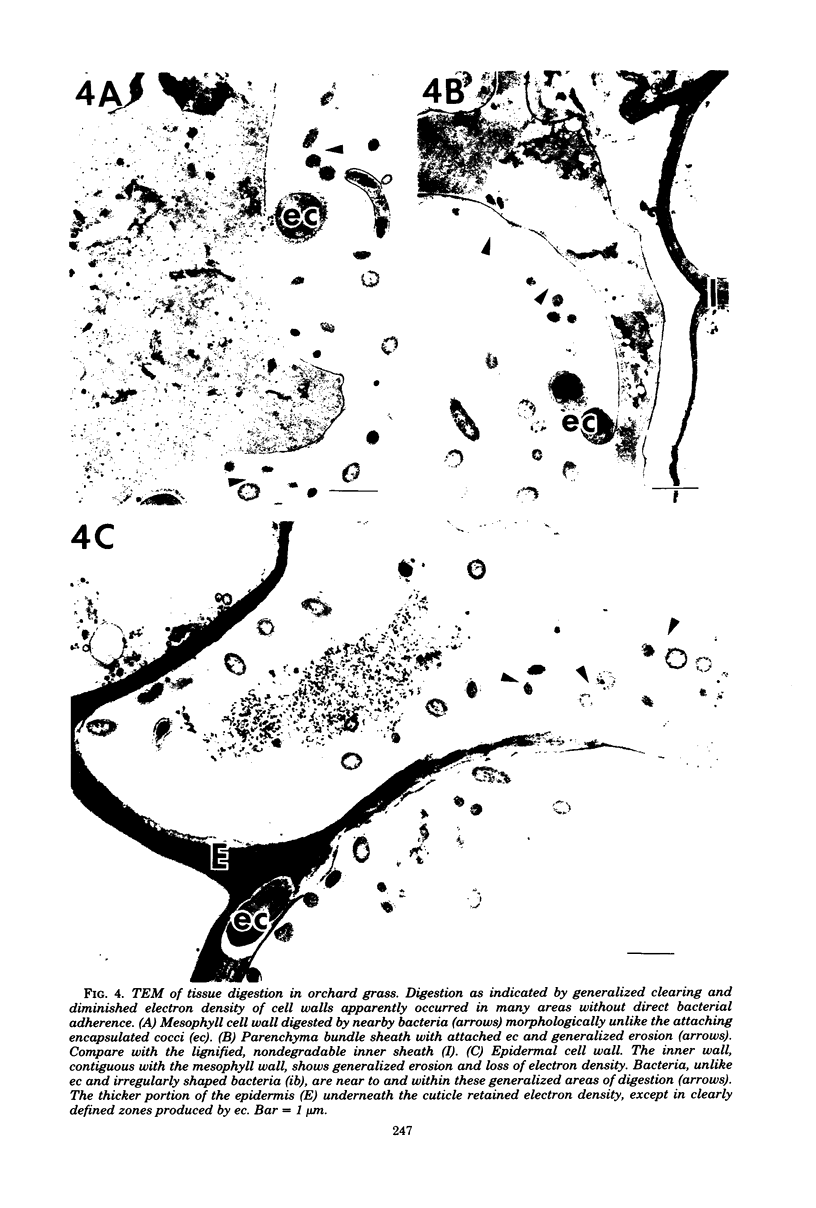
Different morphological types of rumen bacteria which degraded cell walls of forage grasses with various in vitro digestibilities were evaluated with electron microscopy. The majority of these bacteria (i.e., about 70% or more) consisted of two distinct types: (i) encapsulated cocci and (ii) irregularly shaped bacteria, resembling major fiber digesters found in the rumen. Each type was capable of degrading structurally intact cell walls. Differences (P less than or equal to 0.02) in the percent ratio of encapsulated cocci to irregularly shaped bacteria were observed between Bermuda grass and fescue; the ratio of encapsulated cocci to irregularly shaped bacteria between Bermuda grass and orchard grass was similar and variations were high. The proportion of irregularly shaped bacteria usually increased with increased time of digestion. Differences (P greater than 0.1) were not found in the percentage ratio of encapsulated cocci to irregularly shaped bacteria attached to specific tissue types in either Bermuda grass or fescue. However, encapsulated cocci tended to be more prevalent on sclerenchyma than other tissues in Bermuda grass, but less prevalent on sclerenchyma than other tissues in fescue. Transmission electron microscopy of tissue digestion of rapidly degraded orchard grass blades revealed that mesophyll, parenchyma bundle sheath, and parts of the epidermal cell wall apparently were degraded without direct attachment of bacteria although bacteria were near the cell walls undergoing digestion. Anaerobic growth studies showed that the total culturable bacteria developing on medium 10 and media containing carbohydrates similar to those in forage cell walls (i.e., pectin, xylan, and cellobiose) were 80% higher from rumen bacterial populations adapted in vitro to cell walls of orchard grass compared to those from Bermuda grass; the number of colonies from the orchard grass-adapted population was significantly (P less than or equal to 0.05) greater on the medium containing xylan. Filter paper tests showed that the cellulolytic activity of populations adapted to fescue was greater than that of orchard grass or Bermuda grass.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Amos H. E. Mode of attack on orchardgrass leaf blades by rumen protozoa. Appl Environ Microbiol. 1979 Feb;37(2):332–338. doi: 10.1128/aem.37.2.332-338.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Amos H. E. Rumen bacterial degradation of forage cell walls investigated by electron microscopy. Appl Microbiol. 1975 May;29(5):692–701. doi: 10.1128/am.29.5.692-701.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Microscopic evaluation of forage digestion by rumen microorganisms--a review. J Anim Sci. 1979 Mar;48(3):701–710. doi: 10.2527/jas1979.483701x. [DOI] [PubMed] [Google Scholar]
- Akin D. E. Ultrastructure of rumen bacterial attachment to forage cell walls. Appl Environ Microbiol. 1976 Apr;31(4):562–568. doi: 10.1128/aem.31.4.562-568.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos H. E., Akin D. E. Rumen protozoal degradation of structurally intact forage tissues. Appl Environ Microbiol. 1978 Sep;36(3):513–522. doi: 10.1128/aem.36.3.513-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY R. W., CLARKE R. T., WRIGHT D. E. Carbohydrases of the rumen ciliate Epidinium ecaudatum (Crawley). Biochem J. 1962 Jun;83:517–523. doi: 10.1042/bj0830517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Basal medium for the selective enumeration of rumen bacteria utilizing specific energy sources. Appl Environ Microbiol. 1976 Nov;32(5):703–710. doi: 10.1128/aem.32.5.703-710.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Dehority B. A. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968 May;16(5):781–786. doi: 10.1128/am.16.5.781-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale D., Morris E. J., Bacon J. S. Electron microscopy of the microbial populations present and their modes of attack on various cellulosic substrates undergoing digestion in the sheep rumen. Appl Environ Microbiol. 1978 Jul;36(1):160–168. doi: 10.1128/aem.36.1.160-168.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. L., Gawthorne J. M., Storer G. B. Factors affecting the activity of cellulases isolated from the rumen digesta of sheep. Appl Environ Microbiol. 1978 Nov;36(5):643–649. doi: 10.1128/aem.36.5.643-649.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL G., BRYANT M. P. THE CELLULOLYTIC ACTIVITY OF PURE STRAINS OF BACTERIA FROM THE RUMEN OF CATTLE. J Gen Microbiol. 1963 Sep;32:441–448. doi: 10.1099/00221287-32-3-441. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall E. I. Studies on ruminant saliva. 1. The composition and output of sheep's saliva. Biochem J. 1948;43(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Morrison I. M. Structural invesiigations on the lignin-carbohydrate complexes of Lolium perenne. Biochem J. 1974 Apr;139(1):197–204. doi: 10.1042/bj1390197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKETT L. V., PLUMLEE M. L., BARNES R., MOTT G. O. INFLUENCE OF HEMICELLULOSE A AND B ON CELLULOSE DIGESTION, VOLATILE FATTY ACID PRODUCTION AND FORAGE NUTRITIVE EVALUATION. J Nutr. 1965 Jan;85:89–101. doi: 10.1093/jn/85.1.89. [DOI] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R., Yu I., Hungate R. E. Factors affecting cellulolysis by Ruminococcus albus. J Bacteriol. 1973 May;114(2):729–737. doi: 10.1128/jb.114.2.729-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]