Abstract
Background and purpose:
P2Y receptors evoke Ca2+ signals in vascular smooth muscle cells and regulate contraction and proliferation, but the roles of the different P2Y receptor subtypes are incompletely resolved.
Experimental approach:
Quantitative PCR was used to define expression of mRNA encoding P2Y receptor subtypes in freshly isolated and cultured rat aortic smooth muscle cells (ASMC). Fluorescent indicators in combination with selective ligands were used to measure the changes in cytosolic free [Ca2+] in cultured ASMC evoked by each P2Y receptor subtype.
Key results:
The mRNA for all rat P2Y receptor subtypes are expressed at various levels in cultured ASMC. Four P2Y receptor subtypes (P2Y1, P2Y2, P2Y4 and P2Y6) evoke Ca2+ signals that require activation of phospholipase C and comprise both release of Ca2+ from stores and Ca2+ entry across the plasma membrane.
Conclusions and implications:
Combining analysis of P2Y receptor expression with functional analyses using selective agonists and antagonists, we isolated the Ca2+ signals evoked in ASMC by activation of P2Y1, P2Y2, P2Y4 and P2Y6 receptors.
Keywords: vascular smooth muscle, ATP, ADP, UTP, P2Y receptor, Ca2+ signal
Introduction
P2Y receptors comprise a family of G protein-coupled receptors that are activated by adenine and uridine nucleotides, many of which evoke increases in cytosolic Ca2+ concentration (Abbracchio et al., 2006) (Figure 1). The seven P2Y receptor subtypes known to be expressed in rats have overlapping ligand-recognition properties; they differ in the G proteins that they preferentially activate, and in whether they regulate phospholipase C, adenylyl cyclase or both. This complexity and the limited range of subtype-selective antagonists make it difficult to discriminate the role of each P2Y receptor subtype in native tissues. In vascular smooth muscle, for example, many functional responses are regulated by the Ca2+ signals evoked by purinoceptors (Erlinge, 1998; Burnstock, 2002; Hou et al., 2002; Vial and Evans, 2002). These receptors are activated by extracellular nucleotides released by many different cells, including activated platelets, and they can be further metabolized by extracellular nucleotidases (Abbracchio et al., 2006). The Ca2+ signals regulate contraction (Horowitz et al., 1996) and thereby contribute to control of blood pressure. They also regulate proliferation and are thereby important in mediating responses to vascular injury during restenosis (Inoue and Node, 2009) and atherosclerosis (Owens et al., 2004). Analyses of the roles of specific P2Y receptors in evoking Ca2+ signals in vascular smooth muscle has, however, been hampered by the lack of selective ligands (Harper et al., 1998; Pediani et al., 1999; Kumari et al., 2003). It is important to resolve the roles of each P2Y receptor subtype in mediating the Ca2+ signals evoked by nucleotides and so perhaps to identify receptors that might selectively contribute to control of contractility or proliferation.
Figure 1.
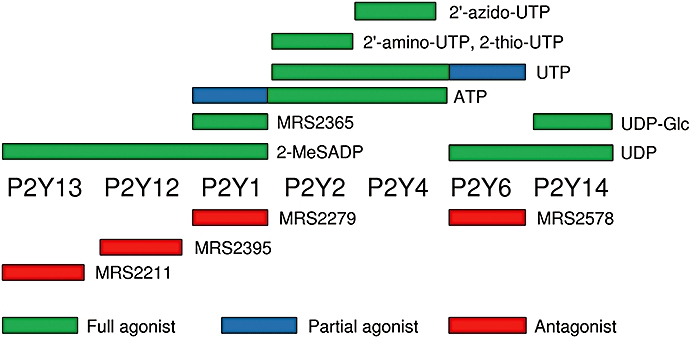
Selectivity of ligands for the P2Y receptors expressed in rat cultured ASMC. (Abbracchio et al., 2006). The structures of the ligands are shown in Figure S1. 2′-amino-UTP, 2′-amino-2′-deoxyuridine-5′-triphosphate; 2′-azido-UTP, 2′-azido-2′-deoxyuridine-5′-triphosphate; 2-MeSADP, 2-methylthio-adenosine-5′-diphosphate; 2-thio-UTP, 2-thio-uridine-5′-triphosphate; MRS2211, 2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde; MRS2279, 2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosione-3′,5′-bisphosphate; MRS2365, (N)-methanocarba-2-MeSADP; MRS2395, 2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester; MRS2578, N,N″-1,4-butanediylbis [N′-(3-isothiocyanatophenyl)thio] urea; UDP-Glc, uridine-5′-diphospho-α-d-glucose.
In culture, vascular smooth muscle cells develop a proliferative phenotype that is more reminiscent of cells contributing to vascular remodelling than the contractile phenotype of fully differentiated cells (Schwartz et al., 1986). Although cultured cells are not contractile, they continue to express several vascular smooth muscle-specific proteins, including smooth muscle α-actin (Chamley-Campbell et al., 1979), and in common with intact tissues they express a variety of functional purinoceptors (Erlinge, 1998; Hou et al., 2002; Vial and Evans, 2002; Burnstock, 2009). Cultured vascular smooth muscle cells are thus a useful model for the proliferative state and they afford opportunities for quantitative analysis of the Ca2+ signals evoked by different purinoceptors.
Methods
Isolation and culture of rat aortic smooth muscle cells
All animal care and experimental procedures complied with UK Home Office policy. Smooth muscle cells were isolated from the aortas of adult male Wistar rats (250–300 g) by enzymatic digestion (Orallo, 1997). Briefly, rats were humanely killed by cervical dislocation. The aorta was isolated under sterile conditions in Hank's balanced salt solution (HBSS) supplemented with penicillin (100 U·mL−1) and streptomycin (100 µg·mL−1), and cleared of adhering fat, connective tissue and the inner endothelial layer. After the first digestion (37°C, 12 min) in HBSS supplemented with antibiotics and collagenase B (0.015 units·mL−1), the adventitia were removed. After a second digestion (37°C, 90 min) in HBSS containing antibiotics, collagenase B (0.075 U·mL−1) and elastase (8 U·mL−1), single cells were dispersed by trituration with a sterile Pasteur pipette. The cells were washed with Dulbecco's modified Eagle's medium (DMEM) (650 g, 5 min), resuspended in DMEM with antibiotics and fetal calf serum (10%) and grown at 37°C in a humidified atmosphere of 95% air and 5% CO2. The aortic smooth muscle cells (ASMC) were either passaged or frozen when they reached confluence. Cells were used between passages 2 and 8, throughout which they retained the appearance of smooth muscle cells and the characteristic ‘hill and valley’ growth pattern (Chamley-Campbell et al., 1979) and, assessed by immunostaining, they expressed smooth muscle α-actin (Figure 2A and B).
Figure 2.
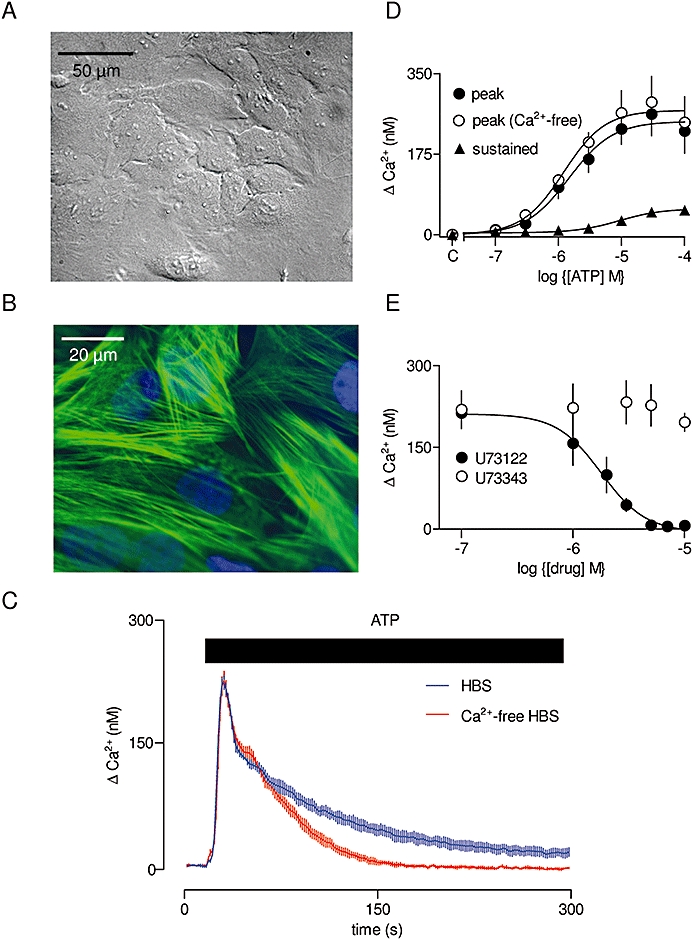
Ca2+ signals evoked by ATP in cultured ASMC. Typical field of cultured rat ASMC at passage 5 (A). Field of cells immunostained for smooth muscle α-actin (B). Ca2+ signals recorded from a population of fluo-4-loaded ASMC stimulated with ATP (100 µM, bar) in the presence or absence of extracellular Ca2+ (C). Results are means ± SEM from three wells on a single plate and are typical of results from three independent plates. Concentration-dependent effects of ATP on the peak increase in [Ca2+]i in the presence or absence of extracellular Ca2+, and on the sustained Ca2+ entry signal (measured 250–300 s after addition of ATP) (D). C denotes control. Peak responses to ATP (100 µM) with the indicated concentrations of U73122 or U73343 (each added for 5 min before and then during the stimulation) (E). [Ca2+]i, cytosolic free Ca2+ concentration; ASMC, aortic smooth muscle cell; HBS, HEPES-buffered saline; U73122, 1-[6-[[(17β)-3-methoxyestra-1,3,5 (10)-trien-17-yl] amino] hexyl]-1H-pyrrole-2,5-dione; U73343, 1-[6-[((17β)-3-methoxyestra-1,3,5 (10)-trien-17-yl)amino] hexyl]-2,5-pyrrolidinedione.
Quantitative PCR
Confluent cultures of cells in 96-well plates were lysed with Fastlane cell cDNA kit (50 µL·well−1, Qiagen, Crawley, West Sussex, UK), and cDNA was then synthesized from the extract (4 µL) in a final volume of 20 µL and diluted 10-fold in water. For quantitative PCR, each reaction included primers specific for P2Y receptor subtypes or, for calibration, primers for β-actin. The primers used are listed in Table S1. Each reaction (15 µL) included cDNA (0.5 µL), P2Y receptor-specific primers (Quantitect Primer Assay, Qiagen, 1.5 µL) or β-actin primers (1.5 µL, 500 nM final concentration), and Bioline Sensimix (Bioline, London, UK, 7.5 µL). For PCR (Rotor-Gene 6000, Qiagen), an initial denaturation (95°C, 10 min) was followed by 40 cycles of amplification (95°C for 10 s, 60°C for 30 s and 72°C for 20 s), with fluorescence measured at the end of each cycle. For each PCR, amplification efficiency (E) was calculated as 10m, where m is the average increase in fluorescence for four cycles after the cycle threshold (CT). Expression levels relative to the housekeeping product (β-actin) were calculated from: relative expression =E-CT(P2Y)/E-CT(β-actin). Each QPCR reaction was performed in duplicate with samples from three different wells. The authenticity of each PCR product was assessed by melting-curve analysis (72°C for 2 min, then 1°C steps at 5 s intervals to 99°C). Each reaction produced a single amplification product with a melting point above 80°C. Control reactions in which the template cDNA was omitted from the PCR reaction, or reverse-transcriptase omitted from the reverse transcription reaction, failed to produce any specific amplification products. Similar methods were used for analyses of cells freshly isolated from aorta (30 mg) immediately before the second enzyme digestion, as described above. For these cells, RNA was extracted using an Illustra RNA spin mini-RNA isolation kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK), and cDNA was synthesized using Bioscript reverse transcriptase (Bioline).
Measurement of [Ca2+]i in cell populations
Confluent cultures of cells grown in 96-well plates were loaded with fluo-4 by incubation in HEPES-buffered saline (HBS) supplemented with probenecid (2.5 mM) and fluo-4 acetoxymethyl ester [4 µM in fresh dimethyl sulphoxide (DMSO); final DMSO concentration, 0.4%]. After 1 h at 20°C, cells were washed with HBS and after 30 min (to allow complete de-esterification of fluo-4AM), they were used for experiments. HBS had the following composition: 135 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 11.5 mM glucose and 11.6 mM HEPES, pH 7.3, at 20°C. All experiments were performed in HBS (or nominally Ca2+-free HBS) at 20°C. For fluorescence measurements, the 96-well plate containing fluo-4-loaded cells was mounted in a FlexStation III (MDS Analytical Technologies, Wokingham, Berkshire, UK), which allows up to three automated additions to each well, while recording fluorescence (excitation at 485 nm; emission at 525 nm). Six simultaneous readings were taken from each well at intervals of 1.52 s. At the end of each experiment, fluorescence of Ca2+-saturated indicator (Fmax) was determined for each well by addition of Triton X-100 (0.05%) and CaCl2 (10 mM). Fluorescence from cells containing only Ca2+-free indicator (equivalent to background fluorescence because Ca2+-free fluo-4 is not fluorescent) was measured in parallel wells treated with Triton X-100 (0.05%) and BAPTA (10 mM). This background fluorescence was subtracted from all measurements. The corrected fluorescence (F) was then calibrated to cytosolic free Ca2+ concentration ([Ca2+]i) from: [Ca2+]i=KDF/(Fmax−F), where the KD of fluo-4 for Ca2+ is 345 nM (Gee et al., 2000).
Immunostaining
Cells grown on poly-l-lysine-coated coverslips under conditions identical to those used for measurements of [Ca2+]i were washed twice with phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4 and 1.76 mM KH2PO4, pH 7.4) and fixed with paraformaldehyde (4%) in PBS for 20 min at 20°C. Cells were then permeabilized with Triton X-100 (0.1%) in PBS for 5 min. Non-specific binding was blocked by incubating with BSA (5%) in PBS for 10 min at 20°C, before staining with a FITC-conjugated monoclonal antibody to anti-smooth muscle α-actin (1:200) for 2.5 h at 20°C. Cells were then washed three times with PBS, and the nuclei stained with Hoechst 33258 (1 µg·mL−1). Coverslips were mounted in Prolong Gold (Invitrogen, Paisley, Scotland) and stored at −20°C before confocal imaging.
Statistical analysis
Results are presented as means ± SEM from n independent experiments (n= 3, unless otherwise stated). Statistical comparisons used paired or unpaired Student's t-test, as appropriate, with P < 0.05 considered significant (GraphPad Prism, version 5, La Jolla, CA, USA).
Materials
Collagenase B and hexokinase were from Roche Applied Science (Burgess Hill, West Sussex, UK). Elastase and FITC-conjugated α-actin antiserum were from Sigma (Poole, Dorset, UK). MRS2211 (2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde), MRS2279 (2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosione-3′,5′-bisphosphate), MRS2365 [(N)-methanocarba-2-MeSADP], MRS2578 (N,N″-1,4-butanediylbis [N′-(3-isothiocyanatophenyl)thio] urea), MRS2690 (diphosphoric acid 1-α-d-glucopyranosyl ester 2-[(4′-methylthio)uridin-5″-yl] ester), 2-MeSADP (2-methylthio-adenosine-5′-diphosphate) and UDP were from Tocris (Bristol, Avon, UK). Cell culture materials were from Invitrogen, except for fetal bovine serum (Sigma). 2′-amino-UTP (2′-amino-2′-deoxyuridine-5′-triphosphate), 2′-azido-UTP (2′-azido-2′-deoxyuridine-5′-triphosphate) and 2-thio-UTP (2-thio-uridine-5′-triphosphate) were from Trilink Biotechnologies (San Diego, CA, USA). The structures of the ligands are shown in Figure S1. Fluo-4AM was from Invitrogen. Pertussis toxin was from List Biological Laboratories (Campbell, CA, USA). Other reagents, including MRS2395 (2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester), were from Sigma unless stated otherwise. The nomenclature of receptors and ligands follows Alexander et al. (2009).
Results
Ca2+ signals evoked by ATP in rat aortic smooth muscle cells
ATP evoked a concentration-dependent increase in [Ca2+]i in populations of rat ASMC. Both the initial peak response and its sensitivity to ATP were unaffected by removal of extracellular Ca2+, but a small sustained Ca2+ signal was abolished in Ca2+-free medium (Figure 2C and D; Table 1). There was no response to αβ-meATP (αβ-methylene-adenosine-5′-triphosphate), a selective agonist of P2X1 and P2X3 receptors (not shown); the former, with P2X7 receptors, predominate in vascular smooth muscle (North, 2002). The lack of responses mediated by P2X receptors is consistent with previous studies indicating that P2X receptors are down-regulated in cultured vascular smooth muscle cells (Erlinge et al., 1998). The Ca2+ signals and their sensitivity to ATP were unaffected by treatment of the ATP and cells with an ATP-regenerating system (2 U·mL−1, creatine phosphokinase, 1 mM phosphocreatine and 10 mM LiCl in HBS for 10 min) (Figure S2A). This confirms that the Ca2+ signals are evoked by ATP rather than by contaminating ADP. U73122 (1-[6-[[(17β)-3-methoxyestra-1,3,5 (10)-trien-17-yl] amino] hexyl]-1H-pyrrole-2,5-dione), an inhibitor of phospholipase C (Bleasdale et al., 1990), caused a concentration-dependent and complete inhibition (pIC50= 5.78 ± 0.07) of the Ca2+ signals evoked by ATP; the inactive analogue, U73343 (1-[6-[((17β)-3-methoxyestra-1,3,5 (10)-trien-17-yl)amino] hexyl]-2,5-pyrrolidinedione), had no effect (Figure 2E). These results demonstrate that activation of P2Y receptors by ATP in rat ASMC evokes both Ca2+ release and Ca2+ entry via activation of phospholipase C.
Table 1.
Ca2+ signals evoked by activation of P2Y receptors
|
Δ[Ca2+]i (nM) |
pEC50 (h) |
||||
|---|---|---|---|---|---|
| Peak | Sustained | Peak (Ca2+-free) | Peak (with Ca2+) | Ca2+entry | |
| ATP | 248 ± 48 | 64 ± 20 | 5.95 ± 0.10 (1.1 ± 0.2) | 5.83 ± 0.03 (1.4 ± 0.2) | 5.01 ± 0.13 (1.8 ± 0.4) |
| MRS2365 | 154 ± 5 | 18 ± 7 | 8.56 ± 0.06 (0.9 ± 0.1) | 8.68 ± 0.16 (0.8 ± 0.2) | 8.11 ± 0.32 (0.7 ± 0.2) |
| UTP | 254 ± 47 | 67 ± 5 | 5.39 ± 0.15 (1.1 ± 0.1) | 5.23 ± 0.22 (1.0 ± 0.2) | 4.64 ± 0.32 (0.7 ± 0.2) |
| UDP | 94 ± 20 | 25 ± 6 | 6.16 ± 0.43 (0.6 ± 0.2) | 6.45 ± 0.45 (0.6 ± 0.2) | 6.83 ± 0.35 (0.7 ± 0.1) |
| 2′-amino-UTP | 301 ± 34 | 84 ± 36 | ND | 5.58 ± 0.37 (1.1 ± 0.1) | 5.40 ± 0.23 (1.1 ± 0.1) |
| 2-thio-UTP | 327 ± 42 | 88 ± 26 | ND | 5.89 ± 0.29 (1.1 ± 0.3) | 5.11 + 0.18 (1.1 ± 0.2) |
| 2′-azido-UTP | 142 ± 29 | 55 ± 20 | ND | 4.67 ± 0.32 (1.3 ± 0.1) | 4.67 ± 0.06 (1.1 ± 0.1) |
| 2-MeSADP | 122 ± 30 | 18 ± 4 | ND | 7.23 ± 0.53 (0.8 ± 0.1) | 5.58 ± 0.16 (1.8 ± 0.7) |
The results in the Table show the peak and sustained increase (measured between 250 and 300 s) in [Ca2+]i in populations of cultured ASMC stimulated with the indicated ligands. pEC50 values with Hill coefficients (h) are shown for the responses in HBS and Ca2+-free HBS, and for the Ca2+ entry.
[Ca2+]i, cytosolic free Ca2+ concentration; 2′-amino-UTP, 2′-amino-2′-deoxyuridine-5′-triphosphate; 2′-azido-UTP, 2′-azido-2′-deoxyuridine-5′-triphosphate; 2-MeSADP, 2-methylthio-adenosine-5′-diphosphate; 2-thio-UTP, 2-thio-uridine-5′-triphosphate; HBS, HEPES-buffered saline; MRS2365, (N)-methanocarba-2-MeSADP; ND, not determined.
Expression of P2Y receptor subtypes in rat aortic smooth muscle cells
ATP is an agonist of several subtypes of P2Y receptor (Abbracchio et al., 2006) (Figure 1). We therefore used PCR to quantify the transcripts for all known rat P2Y receptor subtypes in cultures of ASMC before attempting to delineate the subtypes responsible for ATP-evoked Ca2+ signals. The results demonstrate the presence of transcripts for seven different P2Y receptor subtypes, including three for which ATP is an agonist (Figure 3A). These results establish which P2Y receptors may be expressed in rat ASMC and thereby dictate our choice of ligands to resolve, in functional assays, the receptor subtypes that mediate Ca2+ signals (Figure 1). Similar analyses of freshly isolated ASMC established a different pattern of expression with mRNA for only three P2Y receptors predominating (P2Y2, P2Y12 and P2Y14) (Figure 3B). It is noteworthy that the predominant P2Y receptor subtype in cultured ASMC is P2Y6, which is involved in regulating proliferation (Hou et al., 2002), whereas P2Y12 is the major subtype in freshly isolated ASMC (Figure 3). The substantial effects of cell culture on the expression of mRNA for P2Y6 receptors differ from a previous study of a more limited range of P2Y receptors, in which relative levels of expression of the P2Y receptor subtypes were similar in fresh and cultured ASMC (Erlinge et al., 1998). Here, we focus on analyses of cultured ASMC because they are more amenable than primary cells to quantitative analyses of the Ca2+ signals evoked by different P2Y receptors.
Figure 3.
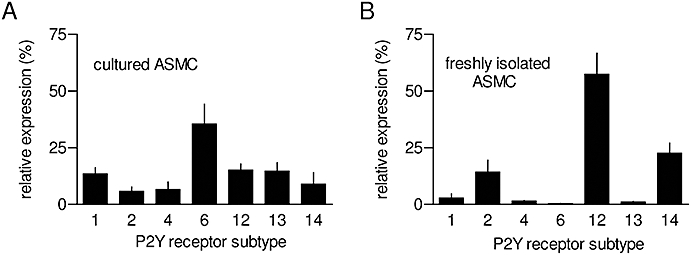
Expression of P2Y receptor subtypes in aortic smooth muscle cell (ASMC). Quantitative PCR was used to measure relative levels of mRNA encoding the subtypes of P2Y receptors in either cultures of ASMC (A, passage 3–7) or in freshly isolated ASMC (B). Results, expressed as percentages of total mRNA encoding P2Y receptors, are means ± SEM from three experiments, each performed in duplicate.
Some of the P2Y receptor subtypes identified by PCR analysis are activated by UTP and/or UDP, rather than ATP (Figure 1). We therefore examined the effects of UTP and UDP on Ca2+ signals in ASMC. Responses to UTP were unaffected by treatment with a regenerating system (as described above) (Figure S2B), but responses to the highest concentrations of UDP (>10 µM) were reduced after the UDP was treated (1 h, 37°C) with hexokinase (50 U·mL−1, pH 7.0 in 0.1 M phosphate buffer with 6.5 mM MgCl2) and glucose (110 mM) (Figure 4A). This indicated that the UDP was contaminated with UTP (Harden et al., 1997; Kumari et al., 2003). We therefore treated UDP with glucose/hexokinase before all assays. Both UTP and UDP evoked Ca2+ release and Ca2+ entry (Figure 4B and C; Table 1). These responses, like those to ATP, were abolished by inhibition of phospholipase C with U73122 (not shown). The peak increases in [Ca2+]i were similar for ATP and UTP, but clearly smaller for UDP (Table 1). The results so far established that ATP, UTP and UDP, via P2Y receptors, evoked both Ca2+ release and Ca2+ entry via their ability to activate phospholipase C in rat cultured ASMC.
Figure 4.
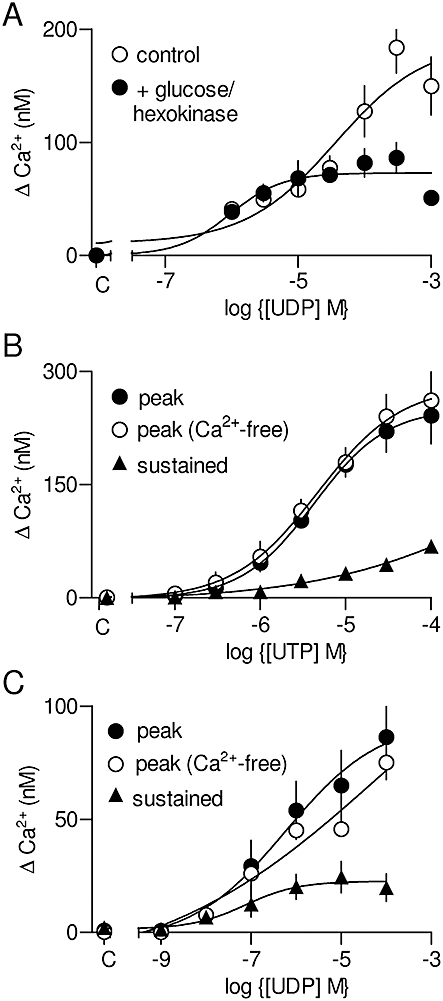
Ca2+ signals evoked by UTP and UDP. Concentration-dependent effects of UDP on the peak Ca2+ signal with or without prior treatment of the UDP with glucose and hexokinase (see text for details) (A). These results (A) are shown as means ± SEM from a single experiment with three replicates. Concentration-dependent effects of UTP (B) or glucose/hexokinase-treated UDP (C) on the peak increase in [Ca2+]i (cytosolic free Ca2+ concentration) in the presence or absence of extracellular Ca2+, and on the sustained Ca2+ entry signal. C denotes control.
Activation of P2Y1 receptors evokes Ca2+ signals
MRS2365 is a selective full agonist of P2Y1 receptors (Chhatriwala et al., 2004), while MRS2279 is a selective high-affinity (KD∼ 8 nM) competitive antagonist (Boyer et al., 2002; Waldo et al., 2002). MRS2365 evoked concentration-dependent increases in [Ca2+]i (pEC50 is shown in Table 1) arising from both release of Ca2+ from intracellular stores and Ca2+ entry (Figure 5A). Increasing concentrations of MRS2279 caused rightward parallel shifts in the concentration–effect relationships for the Ca2+ signals evoked by MRS2365 (Figure 5B). The pKD of MRS2279 for the receptor through which MRS2365 evokes Ca2+ signals was 8.16 ± 0.26 (Figure 5C). These results and the KD for MRS2279 are consistent with expression of functional P2Y1 receptors in rat cultured ASMC.
Figure 5.
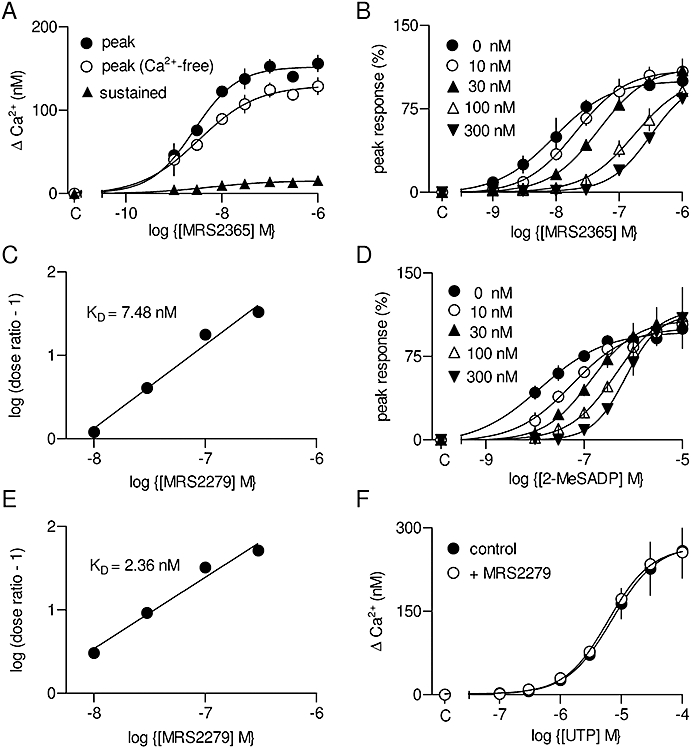
Ca2+ signals evoked by activation of P2Y1 receptors. Concentration-dependent effects of MRS2365 [(N)-methanocarba-2-MeSADP] on the peak Ca2+ signal in the presence or absence of extracellular Ca2+, and on the sustained Ca2+ entry (A). Concentration-dependent effects of MRS2365 (B) and 2-MeSADP (2-methylthio-adenosine-5′-diphosphate) (D) on the peak Ca2+ signal in the presence of 0–300 nM MRS2279 (2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosione-3′,5′-bisphosphate) (as indicated). Results are plotted as percentages of the maximal response in the absence of antagonist. The results from (B and D) are shown as Schild plots (C and E). Calculated affinities of the antagonist (KD) are shown. Concentration-dependent effects of UTP, alone or with MRS2279 (3 µM), on the peak Ca2+ signal (F). C denotes control (in A, B, D and F).
2-MeSADP is a full agonist for three of the P2Y receptor subtypes identified in ASMC by PCR (P2Y1, P2Y12 and P2Y13) (Figure 1). 2-MeSADP caused a concentration-dependent increase in [Ca2+]i (Figure 5D; Table 1). The response to 2-MeSADP was competitively antagonized by MRS2279 with an affinity (pKD= 8.64 ± 0.26; Figure 5D and E) not significantly different from that for its antagonism of responses to the P2Y1-selective ligand, MRS2365 (pKD = 8.16 ± 0.26). Because 2-MeSADP interacts with three P2Y receptor subtypes, but MRS2279 binds only to P2Y1 receptors (Boyer et al., 2002; Waldo et al., 2002; Marteau et al., 2003), these results established that the Ca2+ signals evoked by 2-MeSADP were entirely mediated by P2Y1 receptors. That conclusion was further substantiated by results with MRS2395 (30 µM) and MRS2211 (10 µM), selective antagonists of P2Y12 and P2Y13 receptors, respectively (Xu et al., 2002; Kim et al., 2005), neither of which affected the Ca2+ signals evoked by any concentration of 2-MeSADP (Figure S3). A concentration of MRS2279 (3 µM) sufficient to abolish responses to a maximally effective concentration of MRS2365 had no effect on the Ca2+ signals evoked by any concentration of UTP (Figure 5F) or ATP (Figure S4). This is consistent with ATP being only a weak partial agonist of P2Y1 receptors (Palmer et al., 1998) and with the lack of interaction between UTP and P2Y1 receptors (Waldo et al., 2002). We conclude that of the three P2Y receptors expressed in ASMC that were stimulated by 2-MeSADP (P2Y1, P2Y12 and P2Y13), only the P2Y1 receptors evoke Ca2+ signals. The peak increase in [Ca2+]i evoked by maximal activation of P2Y1 receptors was only 62 ± 2% of that evoked by ATP (Table 1).
ATP evokes Ca2+ signals by activating P2Y2 and P2Y4 receptors
The only remaining P2Y receptors expressed in cultured ASMC that are activated by ATP are P2Y2 and P2Y4 receptors (Figure 1). ATP and UTP are full agonists for both rat receptors, and each ligand has similar potency for each receptor (Wildman et al., 2003). The peak amplitudes of the Ca2+ signals evoked by ATP and UTP in ASMC were similar (Table 1), consistent with each agonist similarly activating the same population of receptors. UTP analogues have been used to differentiate between human P2Y2 and P2Y4 receptors. 2′-amino-UTP and 2-thio-UTP are more selective for human P2Y2 receptors, while 2′-azido-UTP is more selective for human P2Y4 receptors (Jacobson et al., 2006); each analogue is a full agonist for its respective receptor, but neither is entirely selective. There are no antagonists that discriminate between rat P2Y2 and P2Y4 receptors (Abbracchio et al., 2006). Each of the three UTP analogues evoked both Ca2+ release and Ca2+ entry in ASMC (Figures 6A–C; Table 1). The analogues likely to be selective for P2Y2 receptors (2′-amino-UTP and 2-thio-UTP) evoked substantially larger Ca2+ signals than did the P2Y4-selective agonist (2′-azido-UTP) (Figure 6D). We noted that the peak Ca2+ signal evoked by activation of both P2Y2 and P2Y4 receptors by UTP (273 ± 40 nM) was no larger than that evoked by activation of only the P2Y2 receptor by 2′-amino-UTP (301 ± 34 nM) (Table 1). The likely explanation is that maximal responses to full agonists may be limited by the finite size of the inositol 1,4,5-trisphosphate (IP3)-sensitive Ca2+ stores. Our results allow us to conclude that in cultured ASMC, both P2Y2 and P2Y4 receptors evoke Ca2+ signals, with the P2Y2 receptors contributing most to the responses evoked by ATP and UTP.
Figure 6.
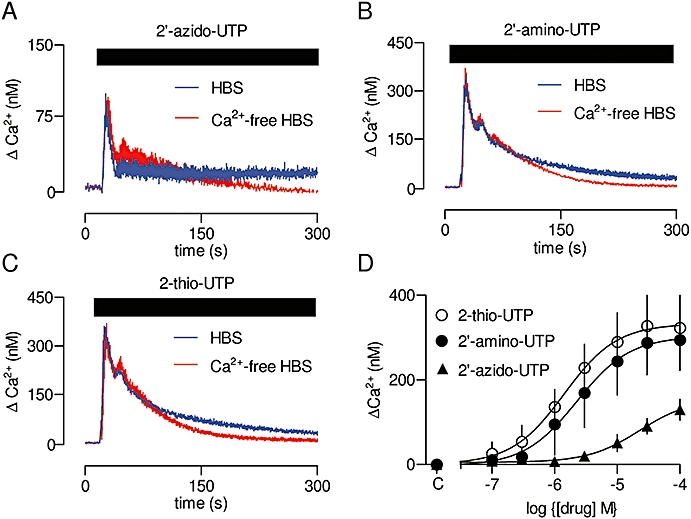
Ca2+ signals evoked by activation of P2Y2 and P2Y4 receptors. Typical results from populations of cultured ASMC stimulated with 2′-azido-UTP (100 µM, A), 2′-amino-UTP (100 µM, B) or 2-thio-UTP (100 µM, C). Results are means ± SEM from three wells on a single plate and are typical of results from three independent plates. Summary results showing concentration-dependent effects of the three analogues on the peak Ca2+ signal (D). C denotes control. 2′-amino-UTP, 2′-amino-2′-deoxyuridine-5′-triphosphate; 2′-azido-UTP, 2′-azido-2′-deoxyuridine-5′-triphosphate; 2-thio-UTP, 2-thio-uridine-5′-triphosphate; ASMC, aortic smooth muscle cell; HBS, HEPES-buffered saline.
P2Y6 receptors evoke Ca2+ signals
From the seven P2Y receptor subtypes expressed in cultured ASMC (Figure 3A), we have so far shown that P2Y1 (Figure 5), P2Y2 and P2Y4 receptors (Figure 6) evoke Ca2+ signals, while P2Y12 and P2Y13 receptors do not (Figure 5). The physiological ligands of the receptors are likely to be ADP for P2Y1 receptors, and ATP or UTP for P2Y2 and P2Y4 receptors (Abbracchio et al., 2006). The remaining subtypes of rat P2Y receptors (P2Y6 and P2Y14) are each activated by UDP (Nicholas et al., 1996; Fricks et al., 2008), which evokes smaller Ca2+ signals than either ATP or UTP in ASMC (Figure 4A). MRS2578 has been reported to inhibit rat P2Y6 receptors selectively and irreversibly (Mamedova et al., 2004), although it is both sparingly soluble and unstable in aqueous solution (Abbracchio et al., 2006). In our hands, MRS2578 (10 µM) had no effect on the Ca2+ signals evoked by any concentration of UDP in ASMC (not shown). There are no selective antagonists of P2Y14 receptors, although MRS2690 (Ko et al., 2007) and UDP-glucose (Chambers et al., 2000) are selective agonists. Neither MRS2690 (10 µM) nor UDP-glucose (10 µM) evoked Ca2+ signals in cultured ASMC (not shown).
We attempted further to resolve the roles of P2Y6 and P2Y14 receptors by exploiting their preferences for coupling to different G proteins (Abbracchio et al., 2006). P2Y14 receptors activate Gαi/o (Moore et al., 2003), whereas P2Y6 receptors are expected to couple to phospholipase C via Gq/11 (Chang et al., 1995; Robaye et al., 1997). We used Pertussis toxin to uncouple P2Y14 receptors from Gi/o and so resolve the relative roles of P2Y6 and P2Y14 receptors in initiating the Ca2+ signals evoked by UDP. ASMC were incubated with Pertussis toxin (100 ng·mL−1) for 24 h prior to examining the effects of nucleotides on [Ca2+]. This treatment significantly reduced the response to ATP: in paired comparisons, the maximal response was reduced by 24 ± 6% and the pEC50 shifted from 5.63 ± 0.09 to 5.30 ± 0.08 (Figure 7A). This is consistent with evidence that P2Y2 receptors (via which ATP exerts its major Ca2+-mobilizing effect in ASMC, Figure 6) stimulate phospholipase C via both Gi and Gq/11 (and/or Gα16) (Baltensperger and Porzig, 1997; Murthy and Makhlouf, 1998). However, under identical conditions responses to UDP were unaffected by Pertussis toxin (Figure 7B). These results, together with the lack of response to the P2Y14-selective agonists (MRS2690 and UDP-glucose), suggest that responses of ASMC to UDP are mediated by P2Y6 rather than P2Y14 receptors.
Figure 7.
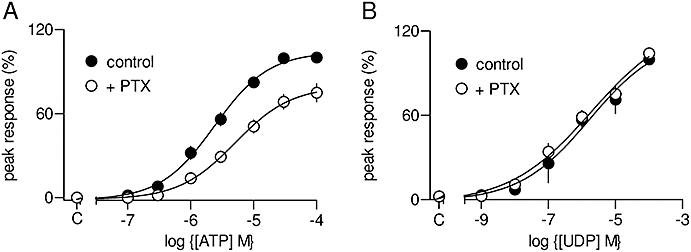
Ca2+ signals evoked by activation of P2Y6 receptors. Concentration-dependent effects of ATP (A) or UDP (B) on the peak Ca2+ signal with or without pretreatment with Pertussis toxin (PTX, 100 ng·mL−1, 24 h). Results are expressed as percentages of the peak Ca2+ signal evoked by a maximally effective concentration of the agonist alone. C denotes control.
Discussion
Rat (Erlinge et al., 1998) and human (Wang et al., 2002) vascular smooth muscle cells express several subtypes of P2Y receptors, but with the limited range of selective ligands available it has proven difficult to resolve fully the contributions of each to functional responses. We have shown that mRNA encoding each of the seven rat P2Y receptor subtypes is expressed in cultured ASMC, and a subset of these (P2Y2, P2Y12 and P2Y14) is expressed in freshly isolated ASMC (Figure 3). Our finding that P2Y6 predominates in cultured cells, whereas P2Y12 is the major subtype in fresh cells, is consistent with earlier evidence that P2Y6 receptors regulate proliferation (Hou et al., 2002).
In cultured ASMC, ATP, UTP and UDP evoke Ca2+ signals via activation of phospholipase C, and each stimulates both release of Ca2+ from intracellular stores and Ca2+ entry (Figures 2C–E and 3). We have not further investigated the identity of the Ca2+ entry pathway, although published evidence suggests that it may be via store-operated Ca2+ entry (Potier et al., 2009), a diacylglycerol-regulated TRP protein (Inoue et al., 2001) or by a Na+/Ca2+ exchanger (Lemos et al., 2007). By combining analyses of ligands that are either entirely or partially subtype-selective, we have established that four P2Y receptors evoke Ca2+ signals and the conditions that allow each to be activated selectively (Figure 8). P2Y1 receptors, for which ADP is the most likely physiological stimulus, evoke Ca2+ signals that appear to desensitize rapidly. P2Y2 receptors, which are likely to be activated endogenously by ATP and UTP, evoke the largest Ca2+ signals, and these are sustained by Ca2+ entry during prolonged stimulation. P2Y4 receptors are also likely to be activated by ATP and UTP, but they evoke considerably smaller Ca2+ signals than do P2Y2 receptors, although again the sustained phase of the response is maintained without obvious desensitization. The relative size of the Ca2+ signals evoked by selective activation of P2Y2 and P2Y4 receptors is consistent with an earlier suggestion that P2Y2 receptors mediate most of the response to UTP and ATP (Kumari et al., 2003). In the absence of better ligands, that conclusion derived from use of suramin as a competitive antagonist of P2Y2 receptors (Wildman et al., 2003), but suramin is now known to interact also with P2Y1 and P2Y6 receptors (Abbracchio et al., 2006), both of which evoke Ca2+ signals in cultured ASMC (Figure 8). Finally, P2Y6 receptors, for which the endogenous ligand is probably UDP, evoke Ca2+ signals with a large sustained Ca2+ entry phase (Figure 8). This sustained response is consistent with the lack of desensitization reported for human P2Y6 receptors (Robaye et al., 1997). We also detected mRNA encoding P2Y12, P2Y13 and P2Y14 receptors in cultured ASMC (Figure 3A). Of these, P2Y14 receptors have been shown to be capable of evoking Ca2+ signals (Fumagalli et al., 2003), but we found no evidence to suggest that any of these P2Y receptors evokes Ca2+ signals in cultured ASMC (Figures 5 and 7).
Figure 8.
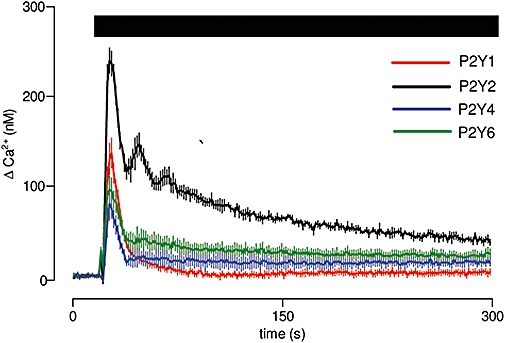
Different Ca2+ signals evoked by selective activation of different P2Y receptors. Typical results from populations of cultured ASMC stimulated in HBS with agonists that allow selective activation of P2Y1 (1 µM MRS2365), P2Y2 (100 µM 2′-amino-UTP), P2Y4 (100 µM 2′-azido-UTP) or P2Y6 receptors (100 µM UDP). Results are means ± SEM from three wells on a single plate and are typical of results from three independent plates. 2′-amino-UTP, 2′-amino-2′-deoxyuridine-5′-triphosphate; 2′-azido-UTP, 2′-azido-2′-deoxyuridine-5′-triphosphate; ASMC, aortic smooth muscle cell; MRS2365, (N)-methanocarba-2-MeSADP.
Acknowledgments
We thank Kendall Harden (University of North Carolina Chapel Hill) for his helpful advice. This work was supported by the Medical Research Council (G0700843) and the Wellcome Trust (085295).
Glossary
Abbreviations:
- [Ca2+]i
cytosolic free Ca2+ concentration
- 2′-amino-UTP
2′-amino-2′-deoxyuridine-5′-triphosphate
- 2′-azido-UTP
2′-azido-2′-deoxyuridine-5′-triphosphate
- 2-MeSADP
2-methylthio-adenosine-5′-diphosphate
- 2-thio-UTP
2-thio-uridine-5′-triphosphate
- αβ-meATP
αβ-methylene-adenosine-5′-triphosphate
- ASMC
aortic smooth muscle cell
- DMEM
Dulbecco's modified Eagle's medium
- HBSS
Hank's balanced salt solution
- MRS2211
2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosione-3′,5′-bisphosphate
- MRS2365
(N)-methanocarba-2-MeSADP
- MRS2395
2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester
- MRS2578
N,N″-1,4-butanediylbis [N′-(3-isothiocyanatophenyl)thio] urea
- MRS2690
diphosphoric acid 1-α-d-glucopyranosyl ester 2-[(4′-methylthio)uridin-5″-yl] ester
- PBS
phosphate-buffered saline
- U73122
1-[6-[[(17β)-3-methoxyestra-1,3,5 (10)-trien-17-yl] amino] hexyl]-1H-pyrrole-2,5-dione
- U73343
1-[6-[((17β)-3-methoxyestra-1,3,5 (10)-trien-17-yl)amino] hexyl]-2,5-pyrrolidinedione
- UDP-Glc
uridine-5′-diphospho-α-d-glucose
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Structures of the ligands used.
Figure S2 Effects of an ATP/UTP-regenerating system on responses to ATP and UTP. Concentration-dependent effects of ATP (A) and UTP (B) on [Ca2+]i (cytosolic free Ca2+ concentration) in aortic smooth muscle cell in HEPES-buffered saline alone or after pretreatment of cells and ligands with an ATP/UTP-regenerating system (as described in the text).
Figure S3 Effects of MRS2395 (2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester) and MRS2211 (2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde) on the Ca2+ signals evoked by 2-MeSADP (2-methylthio-adenosine-5′-diphosphate). Concentration-dependent effects of 2-MeSADP on [Ca2+]i (cytosolic free Ca2+ concentration) alone or in the presence of MRS2395 (30 μM, A) or MRS2211 (10 μM, B). Results are expressed as percentages of the maximal response to 2-MeSADP alone.
Figure S4 Effects of MRS2279 (2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosione-3′,5′-bisphosphate) on the Ca2+ signals evoked by ATP. Concentration-dependent effects of ATP on [Ca2+]i (cytosolic free Ca2+ concentration) alone or with MRS2279 (3 μM).
Table S1 Primers used for quantitative PCR
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharm Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltensperger K, Porzig H. The P2U purinoceptor obligatorily engages the heterotrimeric G protein G16 to mobilize intracellular Ca2+ in human erythroleukemia cells. J Biol Chem. 1997;272:10151–10159. doi: 10.1074/jbc.272.15.10151. [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, et al. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, et al. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen Pharmacol. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Hou M, Webb TE, Barnard EA, Moller S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- Fricks IP, Maddileti S, Carter RL, Lazarowski ER, Nicholas RA, Jacobson KA, et al. UDP is a competitive antagonist at the human P2Y14 receptor. J Pharmacol Exp Ther. 2008;325:588–594. doi: 10.1124/jpet.108.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D'Ambrosi N, Volonte C, Matteoli M, Verderio C, et al. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43:218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- Harden TK, Lazarowski ER, Boucher RC. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- Harper S, Webb TE, Charlton SJ, Ng LL, Boarder MR. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br J Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Hou M, Harden TK, Kuhn CM, Baldetorp B, Lazarowski E, Pendergast W, et al. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol. 2002;282:H784–H792. doi: 10.1152/ajpheart.00997.2000. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Ooue H, Hara Y, Shimizu S, Naitoh S, et al. The transient receptor potential homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73:615–621. doi: 10.1253/circj.cj-09-0059. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Costanzi S, Ivanov AA, Tchilibon S, Besada P, Gao ZG, et al. Structure activity and molecular modeling analyses of ribose- and base-modified uridine 5′-triphosphate analogues at the human P2Y2 and P2Y4 receptors. Biochem Pharmacol. 2006;71:540–549. doi: 10.1016/j.bcp.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee JS, Sak K, Marteau F, Mamedova L, Boeynaems JM, et al. Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol. 2005;70:266–274. doi: 10.1016/j.bcp.2005.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Fricks I, Ivanov AA, Harden TK, Jacobson KA. Structure-activity relationship of uridine 5′-diphosphoglucose analogues as agonists of the human P2Y14 receptor. J Med Chem. 2007;50:2030–2039. doi: 10.1021/jm061222w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Goh G, Ng LL, Boarder MR. ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br J Pharmacol. 2003;140:1169–1176. doi: 10.1038/sj.bjp.0705526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos VS, Poburko D, Liao CH, Cole WC, van Breemen C. Na+ entry via TRPC6 causes Ca2+ entry via NCX reversal in ATP stimulated smooth muscle cells. Biochem Biophys Res Commun. 2007;352:130–134. doi: 10.1016/j.bbrc.2006.10.160. [DOI] [PubMed] [Google Scholar]
- Mamedova LK, Joshi BV, Gao ZG, von Kugelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1170. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, et al. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, et al. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res. 2003;118:10–23. doi: 10.1016/s0169-328x(03)00330-9. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Coexpression of ligand-gated P2X and G protein-coupled P2Y receptors in smooth muscle. Preferential activation of P2Y receptors coupled to phospholipase C (PLC)-β1 via Gaq/11 and to PLC-β3 via Gβγi3. J Biol Chem. 1998;273:4695–4704. doi: 10.1074/jbc.273.8.4695. [DOI] [PubMed] [Google Scholar]
- Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Orallo F. Study of the in vivo and in vitro cardiovascular effects of a hydralazine-like vasodilator agent (HPS-10) in normotensive rats. Br J Pharmacol. 1997;121:1627–1636. doi: 10.1038/sj.bjp.0701314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Pediani JD, McGrath JC, Wilson SM. P2Y receptor-mediated Ca2+ signalling in cultured rat aortic smooth muscle cells. Br J Pharmacol. 1999;126:1660–1666. doi: 10.1038/sj.bjp.0702470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaye B, Boeynaems JM, Communi D. Slow desensitization of the human P2Y6 receptor. Eur J Pharmacol. 1997;329:231–236. [PubMed] [Google Scholar]
- Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986;58:427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. P2X1 receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, et al. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, et al. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem. 2002;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


