Abstract
Aim
This study evaluated the prognostic implications of aVR ST elevation during ST elevation acute myocardial infarction (AMI).
Methods and results
The Hirulog and Early Reperfusion/Occlusion-2 study randomized 17 073 patients with acute ST elevation AMI within 6 h of symptom onset to receive either bivalirudin or heparin, in addition to streptokinase and aspirin. The treatments had no effect on the primary endpoint of 30-day mortality. Electrocardiographic recordings were performed at randomization and at 60 min after commencing streptokinase. aVR ST elevation ≥1 mm was associated with higher 30-day mortality in 15 315 patients with normal intraventricular conduction regardless of AMI location (14.7% vs. 11.2% for anterior AMI, P = 0.0045 and 16.0% vs. 6.4% for inferior AMI, P < 0.0001). After adjusting for summed ST elevation and ST depression in other leads, associations with higher mortality were found with aVR ST elevation of ≥1.5 mm for anterior [odds ratio 1.69 (95% CI 1.16 to 2.45)] and of ≥1 mm for inferior AMI [odds ratio 2.41 (95% CI 1.76 to 3.30)]. There was a significant interaction between aVR ST elevation and infarct location. Thirty-day mortality was similar with anterior and inferior AMI when aVR ST elevation was present (11.5% vs. 13.2%, respectively, P = 0.51 with 1 mm and 23.5% vs. 22.5% respectively, P = 0.84 with ≥ 1.5 mm ST elevation). After fibrinolytic therapy, resolution of ST elevation in aVR to <1 mm was associated with lower mortality, while new ST elevation ≥1 mm was associated with higher mortality.
Conclusion
aVR ST elevation is an important adverse prognostic sign in AMI.
Keywords: Electrocardiography, Mortality, Myocardial infarction
Introduction
Lead aVR is often neglected in routine clinical practice1 partly because the lead is non-adjacent to any other electrocardiographic (ECG) lead. Lead aVR has a frontal plane vector of −150° directly facing the thinner wall of the right ventricular outflow area and through it the basal aspect of the interventricular septum below the aortic and pulmonary valves. Lead aVR faces through the left ventricular cavity the inner side of the apex and lateral wall and is directionally opposite to standard leads I and II and chest leads V5 and V6. The basal septum receives blood supply usually from very proximal septal branches of the left anterior descending artery. Transmural infarction of this important area usually causes lead aVR ST elevation. Angiographic studies have demonstrated in small cohorts of patients with an ST elevation acute myocardial infarction (AMI) the association between aVR ST elevation and proximal left coronary occlusion before the first septal artery.2,3 While this implies that patients with aVR elevation are likely to be at high mortality risk, data from large scale trials are not available. Also, little is known about serial ST changes in lead aVR.4–8
In ST elevation AMI, the ST changes on a standard 12-lead ECG form the signature for diagnosis. These ST parameters better reflect the pathological AMI process than any readily available clinical parameter. If ST changes in lead aVR, the isolated lead with unique directional orientation adds to the other 11 leads, the additional clinical information will help clinical understanding and may help with management of patients with ST elevation AMI.
The Hirulog and Early Reperfusion/Occlusion (HERO)-2 study randomized 17,073 patients with acute ST elevation AMI within 6 h of symptom onset to receive either bivalirudin or heparin, in addition to streptokinase and aspirin. The randomized treatments had no effect on the primary endpoint of 30-day mortality.9 The trial protocol specified ECG recordings to be performed both at randomization and at 60 min after commencing streptokinase.10–14 In this study, we examined the influence of ST segment elevation in lead aVR on 30-day mortality.
Methods
The protocol and results of the HERO-2 trial have been previously reported.9 Patients who presented with >30 min of ischaemic chest pain and either ST segment elevation or presumed new left bundle branch block (LBBB) within 6 h of symptom onset were randomized to receive either bivalirudin or unfractionated heparin in addition to streptokinase and aspirin. The primary endpoint, 30-day mortality, was not different between the two groups.
The protocol pre-specified that a 12-lead ECG was to be performed at randomization and at 60 min after commencing fibrinolytic therapy.10–14 All ECGs were sent to the core laboratory at Green Lane Hospital for analysis by eight ECG technicians10–14 who were blinded to treatment assignment and patient outcomes.
Lead aVR ST level analysis
Analysis was performed for patients showing normal intraventricular conduction on both randomization and 60 min ECGs and for patients showing right bundle branch block (RBBB) on both ECGs.
The amount of ST segment deviation was measured to the nearest 0.5 mm at 60 ms after the J point (or at the J point for patients with RBBB) on all 12 leads including aVR if there was normal intraventricular conduction. For patients with RBBB, ST segment levels were measured at the J point by a cardiologist (C.K.W).11,14 The J point was chosen in RBBB patients for two reasons. First with RBBB the QRS duration is prolonged. Second these patients often have higher heart rates. Thus, these patients often have relatively shortened ST segments and J point measurements are easier than measurements (such as 60 ms) after the J point. Also the J point measurements in the RBBB subgroup have been used in our previous reports on the RBBB patients. In the other 11 leads (excluding lead aVR) on the baseline ECG, the magnitudes of ST elevation were summed; the same was done for the magnitudes of ST depression.
Statistical analysis
Discrete variables were reported as percentages, and continuous variables as the median and the 25th and 75th percentiles. Comparison of continuous variables was done by the Mann–Whitney U-test or the Kruskal–Wallis test where appropriate; whereas the χ2 test was used for categorical variables.
The primary analysis examined the relationship between the presence or absence of aVR ST elevation on the randomization ECG and 30-day mortality. Different cut-points (1 mm or 1.5 mm) of aVR ST elevation were assessed. The Cochran–Armitage test for trend was used to test the association between aVR ST elevation groups (≤0, 0.5, 1, and ≥1.5 mm) and 30-day mortality. Analysis of the interaction between aVR ST elevation and infarct location in association with 30-day mortality was performed using logistic regression.
Electrocardiographic and clinical risk factors for 30-day mortality in the HERO-2 cohort9–14 were entered into a logistic regression model for multivariable analysis. We first adjusted for summed baseline ST segment elevation present in the other 11 ECG leads. We then adjusted for summed baseline ST depression present in the other 11 ECG leads. A separate analysis was also performed adjusting for the summed absolute magnitudes of ST elevation and ST depression in the 11 ECG leads. After adjusting for the above ECG factors, we adjusted for age, prior AMI, and other clinical factors including sex, systolic blood pressure, Killip class, heart rate, diabetes, hypertension, prior angina, time from symptom onset to randomization, and geographic region of patient recruitment.
Further analysis was performed to ascertain if aVR ST level changes after fibrinolytic therapy were related to 30-day mortality.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) and a significance level of two-sided α = 0.05 was used to determine statistical significance for all comparisons.
Results
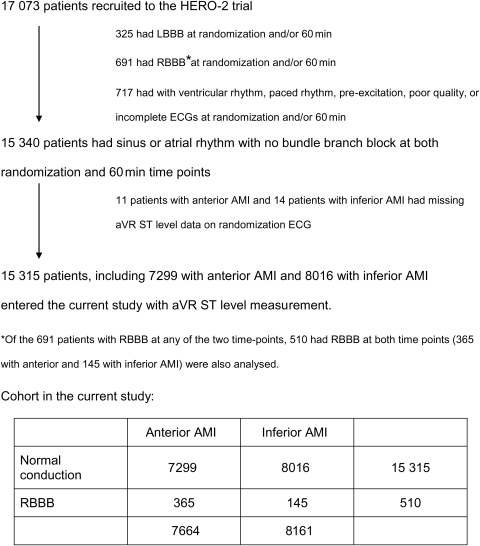
Of the 17 073 patients in HERO-2, 15 825 were studied. Among them 15 315 patients had sinus or atrial rhythm with normal intraventricular conduction on both the randomization ECG and the 60 min ECG (7299 with anterior and 8016 with inferior AMI). Another 510 patients had RBBB on both ECGs, of whom 365 had anterior and 145 inferior AMI (Figure 1).
Figure 1.
Patient flow chart. AMI, acute myocardial infarction; LBBB, left bundle branch block; RBBB, right bundle branch block.
Baseline characteristics and 30-day mortality with aVR ST elevation ≥1 mm
Patients with aVR ST elevation on their randomization ECG were older and more often had prior AMI compared with patients without aVR ST elevation. They had higher heart rates and worse Killip class. They also had lower summed total ST elevation but higher summed total ST depression on the other 11 leads. All aforementioned findings were true for both anterior and inferior AMI (Table 1). Among those with ≥1.5 mm aVR ST elevation, 19 of 213 anterior AMI patients had ST elevation in I and/or aVL of ≥1 mm; and 13 of 112 inferior AMI patients had ST elevation in V5 and/or V6 of ≥1 mm.
Table 1.
Baseline clinical and electrocardiography characteristics and 30-day mortality in patients with different aVR ST levels
| Lead aVR |
P-value | ||
|---|---|---|---|
| ST↑ ≥1 mm | No ST ↑ ≥1 mm | ||
| Anterior AMI group (n = 7664) | n = 779 | n = 6885 | |
| Demographics | |||
| Age (years) (IQR) | 64 (54–72) | 61 (51–70) | <0.0001 |
| Women (%) | 257 (33.0) | 1928 (28.0) | 0.0035 |
| History of cardiac disease | |||
| Angina (%) | 476 (61.1) | 3358 (48.8) | <0.0001 |
| Myocardial infarction (%) | 164 (21.1) | 1049 (15.2) | <0.0001 |
| Risk factors | |||
| Hypertension (%) | 461 (59.2) | 3580 (52.0) | <0.0001 |
| Diabetes (%) | 100 (12.8) | 941 (13.7) | 0.5214 |
| Smoking history | |||
| Never smoked (%) | 353 (45.3) | 2802 (40.7) | 0.0302 |
| Past smoker (%) | 130 (16.7) | 1157 (16.8) | |
| Current smoker (%) | 296 (38.0) | 2926 (42.5) | |
| Time from symptom onset to randomization | |||
| ≤2 h (%) | 167 (21.4) | 1323 (19.2) | 0.0803 |
| >2 but ≤4 h (%) | 404 (51.9) | 3475 (50.5) | |
| >4 h (%) | 208 (26.7) | 2085 (30.3) | |
| Haemodynamics | |||
| Systolic blood pressure (IQR) (mmHg) | 140 (120–150) | 139 (120–150) | 0.9033 |
| Diastolic blood pressure (IQR) (mmHg) | 80 (74–90) | 80 (75–90) | 0.8419 |
| Heart rate (IQR) (b.p.m.) | 82 (72–94) | 80 (69–90) | <0.0001 |
| Killip class | |||
| I (%) | 540 (69.3) | 5262 (76.4) | <0.0001 |
| II (%) | 185 (23.8) | 1360 (19.8) | |
| III (%) | 42 (5.4) | 185 (2.7) | |
| IV (%) | 12 (1.5) | 78 (1.1) | |
| Summed ST elevationa (IQR) (mm) | 12 (8–12) | 15.5 (10–23.5) | <0.0001 |
| Summed ST depressiona (IQR) (mm) | 6 (3.5–8.5) | 1.5 (0.5–3) | <0.0001 |
| 30-day mortality | 121 (15.5%) | 837 (12.2%) | 0.0069 |
| Without RBBB (n = 7299) (%) | 110 (14.7) | 735 (11.2) | 0.0045 |
| With RBBB (n = 365) (%) | 11 (34.4) | 102 (30.6) | 0.6617 |
| Inferior AMI group (n = 8161) | n = 365 | n = 7796 | |
| Demographics | |||
| Age (years) (IQR) | 66 (57–73) | 60 (51–69) | <0.0001 |
| Women (%) | 179 (49.0) | 2086 (26.8) | <0.0001 |
| History of cardiac disease | |||
| Angina (%) | 243 (66.6) | 3304 (42.4) | <0.0001 |
| Myocardial infarction (%) | 117 (32.1) | 966 (12.8) | <0.0001 |
| Risk factors | |||
| Hypertension (%) | 250 (68.5) | 3842 (49.3) | <0.0001 |
| Diabetes (%) | 81 (22.2) | 1057 (13.6) | <0.0001 |
| Smoking history | |||
| Never smoked (%) | 200 (54.8) | 2723 (34.9) | <0.0001 |
| Past smoker (%) | 56 (15.3) | 1366 (17.5) | |
| Current smoker (%) | 109 (29.9) | 3707 (47.6) | |
| Time from symptom onset to randomization | |||
| ≤2 h (%) | 51 (14.0) | 1777 (22.8) | <0.0001 |
| >2 but ≤4 h (%) | 187 (51.2) | 3921 (50.3) | |
| >4 h (%) | 127 (34.8) | 2093 (26.9) | |
| Haemodynamics | |||
| Systolic blood pressure (IQR) (mmHg) | 140 (120–150) | 130 (120–150) | 0.0010 |
| Diastolic blood pressure (IQR) (mmHg) | 80 (70–90) | 80 (70–90) | 0.0605 |
| Heart rate (IQR) (b.p.m.) | 80 (70–97) | 72 (62–84) | <0.0001 |
| Killip class | |||
| I (%) | 246 (67.4) | 6565 (84.2) | <0.0001 |
| II (%) | 84 (23.0) | 1061 (13.6) | |
| III (%) | 25 (6.9) | 87 (1.1) | |
| IV (%) | 10 (2.7) | 83 (1.1) | |
| Summed ST elevationa (IQR) (mm) | 5 (3–7.5) | 8 (5–12) | <0.0001 |
| Summed ST depressionb (IQR) (mm) | 12 (7.5–17.5) | 6.5 (3.5–11.5) | <0.0001 |
| 30-day mortality | 58 (15.9%) | 509 (6.5%) | <0.0001 |
| Without RBBB (n = 8016) (%) | 58 (16.0) | 492 (6.4) | <0.0001 |
| With RBBB (n = 145) (%) | 0 | 17 (12.0) | 1.0000 |
aSummed ST elevation refers to ST elevation.
bSummed ST depression refers to ST depression in the other 11 ECG leads other than lead aVR.
Mortality at 30 days was higher among patients with aVR ST elevation (15.5% vs. 12.2%, P = 0.0069 for anterior AMI; 15.9% vs. 6.5%, P < 0.0001 for inferior AMI; P < 0.0001 for the interaction term between aVR ST elevation and infarct location). Table 2 shows 30-day mortality rates according to the amount of aVR ST elevation, infarct location, and the presence of RBBB.
Table 2.
Thirty-day mortality according to aVR ST level, infarct location, and the presence of right bundle branch block
| Lead aVR ST level |
Trend P-value | ||||
|---|---|---|---|---|---|
| ≤0 mm | 0.5 mm | 1 mm | ≥1.5 mm | ||
| All patients | |||||
| Anterior AMI (n = 7664) | 12.4% | 10.6% | 12.5% | 23.5% | 0.0188 |
| Number of patients | 5795 | 1090 | 566 | 213 | |
| Inferior AMI (n = 8161) | 6.3% | 8.9% | 13.0% | 22.3% | <0.0001 |
| Number of patients | 7201 | 595 | 253 | 112 | |
| Normal conduction (without RBBB) | |||||
| Anterior AMI (n = 7299) | 11.4% | 10.3% | 11.5% | 23.5% | 0.0046 |
| number of patients | 5485 | 1067 | 547 | 200 | |
| Inferior AMI (n = 8016) | 6.3% | 8.3% | 13.2% | 22.5% | <0.0001 |
| Number of patients | 7075 | 579 | 251 | 111 | |
| With RBBB | |||||
| Anterior AMI (n = 365)a | 31.0% | 26.1% | (34.4%) | 0.8399 | |
| Number of patients | 310 | 23 | 19 | 13 | |
| Inferior AMI (n = 145)a | 9.5% | (26.3%) | 0.0339 | ||
| Number of patients | 126 | 16 | 2 | 1 | |
AMI, acute myocardial infarction.
aPercentages in brackets are those for the combined groups when patient numbers were small.
Among the 15 315 patients with normal intraventricular conduction, aVR ST level 1 mm was present in 7.5% of patients with anterior AMI and 3.1% of patients with inferior AMI; aVR ST level ≥1.5 mm was present in 2.7% of patients with anterior AMI and 1.4% of patients with inferior AMI. The 30-day mortality rates for anterior AMI vs. inferior AMI were, respectively, 11.5% vs. 13.2% (P = 0.51) with 1 mm of aVR ST elevation; and 23.5% vs. 22.5% (P = 0.84) with ≥1.5 mm aVR ST elevation.
Mortality at 30 days was 16% for the 300 patients with LBBB at randomization and 32% in the 25 patients who developed new LBBB (from normal intraventricular conduction at baseline) at 60 min after fibrinolysis, as we previously reported.13 Mortality in the 717 patients with other reasons for exclusion was 29.0%.
Univariable relationship between aVR ST elevation and 30-day mortality
In the whole cohort of 15 825 patients, aVR ST elevation ≥1 mm was associated with higher mortality among those with normal intraventricular conduction (14.7% vs. 11.2%, P = 0.0045 for anterior AMI and 16% vs. 6.4% P < 0.0001 for inferior AMI; P < 0.0001 for the interaction term between aVR ST elevation and infarct location) but not among those with RBBB (Table 1). Mortality was high with anterior AMI accompanied by RBBB regardless of the presence or absence of aVR ST elevation (34.4% vs. 30.6%, P = 0.66). aVR ST elevation ≥1 mm was associated with higher mortality for patients with either anterior or inferior AMI within the whole cohort or in patients with normal intraventricular conduction, but the magnitude of the odds ratio was higher for patients with inferior AMI than those with anterior AMI. The trend was similar using aVR ST elevation ≥1.5 mm as the cut-off value (P-values for the interaction between aVR ST level and infarction location was 0.0365 and 0.0676, respectively for the whole cohort and for patients with normal intraventricular conduction) (Table 3).
Table 3.
Univariable odds ratio and 95% confidence interval for 30-day mortality with different aVR ST levels
| Whole cohort n = 15825 |
Normal intraventricular conduction n = 15315 |
RBBB n = 510 |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Lead aVR ST level | ||||||
| ST↑ of ≥1 mm vs. ST↑ of <1 mm | ||||||
| Anterior AMI | 1.33 | (1.08, 1.64) | 1.37 | (1.10, 1.70) | 1.19 | (0.55, 2.55) |
| Inferior AMI | 2.70 | (2.01, 3.63) | 2.78 | (2.07, 3.73) | 1.02a | (0.05, 20.68) |
| P-value for interaction between aVR ST level and infarct location | <0.0001 | <0.0001 | 0.4897 | |||
| ST↑ of ≥1.5 mm vs. ST↑ of <1.5 mm | ||||||
| Anterior AMI | 2.21 | (1.60, 3.06) | 2.43 | (1.74, 3.39) | 0.66 | (0.18, 2.45) |
| Inferior AMI | 3.98 | (2.53, 6.26) | 4.09 | (2.60, 6.43) | 2.43a | (0.10, 61.97) |
| P-value for interaction between aVR ST level and infarct location | 0.0365 | 0.0676 | 0.7664 | |||
aThese logit estimators use a correction of 0.5 in every cell of those tables that contain a zero.
Multivariable relationship between aVR ST elevation and 30-day mortality in patients with normal intraventricular conduction
This analysis included the 15 315 patients with normal conduction and excluded the 510 with RBBB. Since the analyses above suggested interaction between aVR ST level and infarct location, multivariable analyses were stratified by infarct location. The risk for 30-day mortality associated with aVR ST elevation persisted after adjusting for summed ST elevation in the other 11 ECG leads (Table 4). After adjusting further for summed ST depression, the odds ratio was lowered. However, significant associations with higher 30-day mortality were still found with aVR ST elevation of ≥1 mm for inferior AMI [odds ratio 2.41 (95% CI 1.76 to 3.30)] and with aVR ST elevation ≥1.5 mm for anterior AMI [odds ratio 1.69 (95% CI 1.16–2.45)]. Further adjustment for age showed similar but weaker associations. When adjustment was made in addition for all clinical factors, the association between aVR ST elevation and 30-day mortality was not significant. Findings were similar when summed absolute magnitudes of ST elevation and ST depression in the 11 ECG leads were used for adjustments in the analysis (Table 4).
Table 4.
Multivariable analysis among patients with normal intraventricular conduction
| Lead aVR ST level | Unadjusted |
Adjusted for summed ST elevation in other ECG leads |
Adjusted for summed ST elevation and for summed ST depression in other ECG leads |
Adjusted further for age |
Adjusted further for age and prior AMI |
Adjusted further for all clinical factorsa |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| ST↑ of ≥1 mm vs. ST↑ of <1 mm | ||||||||||||
| Anterior AMI | 1.37 | 1.10–1.70 | 1.46 | 1.17–1.81 | 1.08 | 0.85–1.38 | 0.97 | 0.75–1.24 | 0.95 | 0.74–1.22 | 0.90 | 0.69–1.18 |
| Inferior AMI | 2.78 | 2.07–3.73 | 3.12 | 2.31–4.21 | 2.41 | 1.76–3.30 | 1.88 | 1.35–2.60 | 1.74 | 1.25–2.43 | 1.29 | 0.90–1.85 |
| ST↑ of ≥1.5 mm vs. ST↑ of <1.5 mm | ||||||||||||
| Anterior AMI | 2.43 | 1.74–3.39 | 2.58 | 1.84–3.61 | 1.69 | 1.16–2.45 | 1.47 | 0.99–2.18 | 1.42 | 0.96–2.12 | 1.39 | 0.85–1.99 |
| Inferior AMI | 4.09 | 2.6–6.43 | 4.49 | 2.84–7.09 | 2.92 | 1.80–4.74 | 2.16 | 1.31–3.56 | 1.92 | 1.16–3.19 | 1.26 | 0.73–2.18 |
The analysis adjusting for the summed absolute magnitudes of ST elevation and ST depression in the 11 ECG leads in lieu of serially adjusting for ST elevation and then ST depression yielded similar results. The adjusted OR for ST↑ of ≥1 mm vs. ST↑ of <1 mm was 1.34 (95% CI 1.08–1.67) for anterior AMI and 2.69 (95% CI 2.20–3.62) for inferior AMI. The corresponding final OR adjusting for all factors was 1.02 (95% CI 0.80–1.30) for anterior AMI and 1.29 (95% CI 0.91–1.82) for inferior AMI. The adjusted OR for ST↑ of ≥1.5 mm vs. ST↑ of <1.5 mm was 2.24 (95% CI 1.60–3.13) for anterior AMI and 3.51 (95% CI 2.21–5.55) for inferior AMI. The corresponding final OR adjusting for all factors was 1.46 (95% CI 0.99–2.15) for anterior AMI and 1.28 (0.75–2.17) for inferior AMI.
aThese factors included sex, systolic blood pressure, Killip class, heart rate, diabetes, hypertension, prior angina, time from symptom onset to randomization and, geographic region of patient recruitment.
Analysis using aVR ST level of 0 or 0.5 mm as reference in patients with normal intraventricular conduction
When aVR ST levels were analysed as three-step increments [0–0.5, 1–1.5, >2 mm; (Table 5)], each step increase of aVR ST elevation was associated with higher 30-day mortality for both anterior and inferior AMI, both before and after adjusting for summed ST elevation in the other 11 ECG leads. After further adjusting for summed ST depression in the other 11 leads, the association was only present for inferior AMI (odds ratio 1.59, 95% CI 1.21–2.10) and not anterior AMI (odds ratio 1.10, 95% CI 0.87–1.39). When adjustment was made in addition for all clinical factors, the association became non-significant. Findings were similar when summed absolute magnitudes of ST elevation and ST depression in the 11 ECG leads were used for adjustments in the analysis (Table 5).
Table 5.
Multivariable analysis among patients with normal intraventricular conduction and aVR ST level ≥0 mm
| Lead aVR ST level analysed as a three-step variable | Unadjusted |
Adjusted for summed ST elevation in other ECG leads |
Adjusted for summed ST elevation and for summed ST depression in other ECG leads |
Adjusted further for age |
Adjusted further for age and prior AMI |
Adjusted further for all clinical factorsa |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Anterior AMI (n = 4879) | 1.59 | 1.31–1.93 | 1.61 | 1.33–1.95 | 1.10 | 0.87–1.39 | 1.05 | 0.82–1.34 | 1.04 | 0.82–1.33 | 0.94 | 0.73–1.23 |
| Inferior AMI (n = 3554) | 2.32 | 1.81–2.97 | 2.33 | 1.81–2.98 | 1.59 | 1.21–2.10 | 1.38 | 1.03–1.84 | 1.31 | 0.98–1.75 | 1.11 | 0.81–1.52 |
The OR and 95% CI reported were per step increase of aVR ST elevation, analysing three steps of aVR ST↑ at baseline: 0–0.5 mm; 1–1.5 mm; ≥2 mm. The analysis adjusting for the summed absolute magnitudes of ST elevation and ST depression in the 11 ECG leads in lieu of serially adjusting for ST elevation and then ST depression yielded similar results. The adjusted OR was 1.44 (95% CI 1.19–1.75) for anterior AMI and 1.69 (95% CI 1.30–2.20) for inferior AMI. The corresponding final OR adjusting for all factors was 1.15 (95% CI 0.92–1.43) for anterior AMI and 1.12 (95% CI 0.83–1.52) for inferior AMI.
aThese factors included sex, systolic blood pressure, Killip class, heart rate, diabetes, hypertension, prior angina, time from symptom onset to randomization, and geographic region of patient recruitment.
ST changes in lead aVR between randomization electrocardiogram and 60 min electrocardiogram in patients with normal intraventricular conduction
Resolution of ST elevation to <1 mm was common among those with ST elevation ≥1 mm at randomization and was associated with lower 30-day mortality compared with those patients without ST resolution (11.0% vs. 20.6% for anterior AMI, P = 0.0003; and 11.1% vs. 21.5% for inferior AMI, P = 0.0067). Development of new ST elevation ≥1 mm was rare among those without ST elevation at randomization but was associated with higher 30-day mortality (18.5% vs. 10.4% for anterior AMI, P < 0.0001; and 12.6% vs. 6.0% for inferior AMI, P = 0.0006) (Table 6).
Table 6.
Serial ST changes in lead aVR among patients with normal intraventricular conduction
| Normal baseline ST level (−0.5 to 0.5 mm) |
Baseline aVR ST elevation ≥ 1 mm |
|||||
|---|---|---|---|---|---|---|
| aVR ST level | No new ST↑ | new ST↑ ≥1 mm | P-value | Persistent ST↑ ≥1 mm | Resolution of ST↑ <1 mm | P-value |
| Anterior AMI | ||||||
| Number of patients | 5288 | 184 | 292 | 454 | ||
| 30-day mortality, % | 10.4% | 18.5% | <0.0001 | 20.6% | 11.0% | 0.0003 |
| Inferior AMI | ||||||
| Number of patients | 4935 | 167 | 172 | 190 | ||
| 30-day mortality, % | 6.0% | 12.6% | 0.0006 | 21.5% | 11.1% | 0.0067 |
Discussion
Interpretation of the ECG is a cornerstone of the management of patients with AMI, with worse prognostic connotations for anterior AMI than for inferior AMI. However, lead aVR is rarely considered.1 This study evaluated the utility of aVR ST elevation in a large cohort of patients with ST elevation AMI treated by fibrinolytic therapy. Among all patients with normal intraventricular conduction, aVR ST elevation was associated with higher 30-day mortality independent of concomitant ST segment changes in the other ECG leads. This association was strong for patients with anterior AMI with an ST level cut point of ≥1.5 mm, and for patients with an inferior AMI with an ST level cut-point of ≥1 mm. It is noteworthy that above these ST level cut-points there was an approximately 2.5-fold increase in 30-day mortality (Table 3). When aVR ST elevation was present, 30-day mortality was high and similar for patients with anterior AMI and patients with inferior AMI.
Patients with aVR ST elevation had lower summed ST elevation and higher summed ST depression in the 11 other ECG leads compared with patients without ST elevation in aVR. This is not unexpected as the direction of the electrical vector of lead aVR is approximately opposite to the standard leads I and II and chest leads V5 and V6.
As the directional orientation of lead aVR is non-adjacent to other leads, aVR ST elevation provides information about ischemia additional to ST elevation in the other leads. The increased mortality risk associated with aVR ST elevation did not change after adjusting for summed ST elevation in the other 11 leads, both for anterior AMI and for inferior AMI (Tables 4 and 5).
Patients with aVR ST elevation may have severe proximal left coronary artery disease as the basal septum (which the lead aVR faces) receives blood supply either from the proximal septal branches of the left anterior descending artery or from the posterior descending branch of the right coronary artery in those with prior proximal left coronary artery occlusions. Of interest, a recent report concerning patients with proximal left anterior descending occlusions from a percutaneous coronary intervention (PCI) database described tall precordial T waves without the signature anterior ST elevation, but aVR ST elevation was usually present.15
In patients with inferior AMI, infarction of the basal septum may explain only a small group of patients with aVR ST elevation and poor outcomes. Given that patients with aVR ST elevation were older (66 vs. 60 years) and had a higher incidence of prior angina (66.6% vs. 42.4%) and prior MI (32.1% vs. 12.8%), aVR ST elevation may also mechanistically reflect diffuse non-transmural ischemia of the apex and lateral wall from multivessel involvement since lead aVR faces the inner walls of these areas through the left ventricular cavity. Some patients may also have ST depression in leads V5 and V6, the leads which are almost reciprocal to aVR. Indeed among patients with inferior AMI in the GUSTO-1 angiographic study, ST depression in V4–6 indicated a greater likelihood of multivessel disease16 (incidence of three-vessel disease 26% with V4–6 ST depression, 15.7% with V1–3 ST depression, and 13.5% with no precordial ST depression, P = 0.002).
The additional analysis using aVR ST level of 0 or 0.5 mm as a reference found similar results for the prognostic meaning of aVR ST elevation analysed as three-step variables (Table 5). This strengthens the primary analysis which tested dichotomous aVR ST elevation cut-points. The increased odds ratio associated with aVR ST elevation was reduced after adjusting for summed ST depression in the 11 other ECG leads. The odds ratio after this adjustment remained significantly increased with inferior AMI but not with anterior AMI.
Lead aVR has been neglected in clinical practice1 perhaps because it is directionally non-adjacent to any other ECG lead. This ‘relative isolation’ however is also the very reason why aVR changes could be particularly important. The current study using dichotomous cut points (1 or 1.5 mm) of aVR ST segment elevation establishes the prognostic value of ST segment changes in lead aVR in patients with ST elevation AMI. The importance of aVR ST elevation is also supported by the association of mortality with serial changes over 60 min. As with ST segment resolution in other leads8 aVR ST segment resolution was associated with lower mortality, and new aVR ST segment elevation was associated with higher mortality.
Limitations
Infarction of the basal septum may involve the right bundle branch and the presence of RBBB is associated with proximal left anterior descending artery occlusion.2 In the HERO-2 cohort, RBBB portended a worse outcome in patients with anterior AMI.11,13 However, in the current analysis the number of patients with RBBB and aVR ST elevation was small and precluded meaningful subgroup analysis. Unfortunately, we did not routinely collect ECGs with right-sided leads. Those data would have been valuable in their own right and be complementary to the current study.
Patients selected for participation in a clinical trial may differ from usual clinical practice. However, this potential bias is unlikely to explain the difference in outcome by aVR ST elevation. The international HERO-2 trial was a trial of thrombolytic therapy with major recruitment in non-western countries.9 This may explain the higher mortality compared with more contemporary trials of primary PCI. It is possible but not known whether earlier reperfusion or primary PCI may reduce the excess mortality associated with aVR ST elevation. We did not correlate our findings with the coronary anatomy as HERO-2 did not require systematic angiography, and angiographic data were not available.
The association between aVR ST elevation (of ≥1 mm in inferior AMI and ≥1.5 mm in anterior AMI) and 30-day mortality was independent of ST changes in the other leads. When age and prior AMI were put into the model, the odds ratios became smaller and association lost significance after adjusting further for all relevant clinical factors, reflecting that haemodynamics and the Killip class are very powerful prognostic factors. However, the ECG finding of aVR ST elevation has special significance because of its simplicity, and is relevant in acute situations when risk models involving several factors may not be practical to apply because of time constraints and missing clinical information. This will become increasingly more relevant with the use of pre-hospital ECG recordings,17 which enable shorter times to beginning fibrinolysis and shorter times to balloon inflation for primary angioplasty, and with the use of pocket-size ECG machines capable of networking through cell phones. Recent joint efforts from various professional bodies have made recommendations about the recording and interpretation of ST segment change during AMI.18
In addition to conventional risk stratification of patients with AMI by infarct location, the presence of aVR ST elevation identifies patients who are at higher risk for 30-day mortality. Notably its presence during an inferior AMI increases mortality to that of patients with aVR elevation and anterior AMI.
Funding
The HERO-2 trial was funded by The Medicines Company.
Conflict of interest: none declared. Phillip E.G.A. received a research grant from The Medicines Company. He also received payment for Speaker's Bureau, Consultancy fees and Honoraria from Commonwealth Serum Laboratories (CSL) Limited. H.D.W. received research grants from Alexion, Sanofi-Aventis, Eli Lilly, Merck Sharpe & Dohme, The Medicines Company, NIH, Neuren, Glaxo Smith Kline, Pfizer, Roche, Fournier Janssen Cilag, Johnson & Johnson, Proctor & Gamble, and Schering Plough. He also received honoraria from Sanofi Aventis and consultancy fees from CSL.
Acknowledgements
We would like to thank Charlene Nell, Team Support Administrator, Green Lane Cardiovascular Research Unit, for excellent secretarial assistance.
References
- 1.Gorgels AP, Engelen DJ, Wellens HJ. Lead aVR, a mostly ignored but very valuable lead in clinical electrocardiography. J Am Coll Cardiol. 2001;38:1355–1356. doi: 10.1016/s0735-1097(01)01564-9. doi:10.1016/S0735-1097(01)01564-9. [DOI] [PubMed] [Google Scholar]
- 2.Engelen DJ, Gorgels AP, Cheriex EC, de Muinck ED, Ophuis AJ, Dassen WR, Vainer J, van Ommen VG, Wellens HJ. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol. 1999;34:389–395. doi: 10.1016/s0735-1097(99)00197-7. doi:10.1016/S0735-1097(99)00197-7. [DOI] [PubMed] [Google Scholar]
- 3.Yamaji H, Iwasaki K, Kusachi S, Murakami T, Hirami R, Hamamoto H, Hina K, Kita T, Sakakibara N, Tsuji T. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1) J Am Coll Cardiol. 2001;38:1348–1354. doi: 10.1016/s0735-1097(01)01563-7. doi:10.1016/S0735-1097(01)01563-7. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RD, White HD, Ohman EM, Wagner GS, Krucoff MW, Armstrong PW, Weaver WD, Gibler WB, Stebbins AL, Califf RM, Topol EJ. Predicting outcome after thrombolysis in acute myocardial infarction according to ST-segment resolution at 90 minutes: a substudy of the GUSTO-III trial. Am Heart J. 2002;144:81–88. doi: 10.1067/mhj.2002.123319. doi:10.1067/mhj.2002.123319. [DOI] [PubMed] [Google Scholar]
- 5.Cura FA, Roffi M, Pasca N, Wolski KE, Lincoff AM, Topol EJ, Lauer MS. ST-segment resolution 60 minutes after combination treatment of abciximab with reteplase or reteplase alone for acute myocardial infarction (30-day mortality results from the resolution of ST-segment after reperfusion therapy substudy) Am J Cardiol. 2004;94:859–863. doi: 10.1016/j.amjcard.2004.06.018. doi:10.1016/j.amjcard.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 6.de Lemos JA, Antman EM, Giugliano RP, Morrow DA, McCabe CH, Cutler SS, Charlesworth A, Schroder R, Braunwald E for the InTIME-II Investigators. Comparison of a 60- versus 90 min determination of ST-segment resolution after thrombolytic therapy for acute myocardial infarction. Am J Cardiol. 2000;86:1235–1237. doi: 10.1016/s0002-9149(00)01207-8. doi:10.1016/S0002-9149(00)01207-8. [DOI] [PubMed] [Google Scholar]
- 7.Schroder K, Wegscheider K, Zeymer U, Tebbe U, Schroder R. Extent of ST-segment deviation in a single electrocardiogram lead 90 min after thrombolysis as a predictor of medium-term mortality in acute myocardial infarction. Lancet. 2001;358:1479–1486. doi: 10.1016/S0140-6736(01)06577-1. doi:10.1016/S0140-6736(01)06577-1. [DOI] [PubMed] [Google Scholar]
- 8.Schroder R, Wegscheider K, Schroder K, Dissmann R, Meyer-Sabellek W. Extent of early ST segment elevation resolution: a strong predictor of outcome in patients with acute myocardial infarction and a sensitive measure to compare thrombolytic regimens: a substudy of the International Joint Efficacy Comparison of Thrombolytics (INJECT) trial. J Am Coll Cardiol. 1995;26:1657–1664. doi: 10.1016/0735-1097(95)00372-x. doi:10.1016/0735-1097(95)00372-X. [DOI] [PubMed] [Google Scholar]
- 9.The Hirulog and Early Reperfusion or Occlusion (HERO)-2 Trial Investigators. Thrombin-specific anticoagulation with bivalirudin versus heparin in patients receiving fibrinolytic therapy for acute myocardial infarction: the HERO-2 randomised trial. Lancet. 2001;358:1855–1863. doi: 10.1016/s0140-6736(01)06887-8. doi:10.1016/S0140-6736(01)06887-8. [DOI] [PubMed] [Google Scholar]
- 10.Wong CK, French JK, Aylward PE, Stewart RA, Gao W, Armstrong PW, Van de Werf FJ, Simes RJ, Raffel OC, Granger CB, Califf RM, White HD. Patients with prolonged ischemic chest pain and presumed-new left bundle branch block have heterogeneous outcomes depending on the presence of ST-segment changes. J Am Coll Cardiol. 2005;46:29–38. doi: 10.1016/j.jacc.2005.02.084. doi:10.1016/j.jacc.2005.02.084. [DOI] [PubMed] [Google Scholar]
- 11.Wong CK, Gao W, Stewart RA, Van Pelt N, French JK, Aylward PE, White HD. Risk stratification of patients with acute anterior myocardial infarction and right bundle-branch block: importance of QRS duration and early ST-segment resolution after fibrinolytic therapy. Circulation. 2006;114:783–789. doi: 10.1161/CIRCULATIONAHA.106.639039. doi:10.1161/CIRCULATIONAHA.106.639039. [DOI] [PubMed] [Google Scholar]
- 12.Wong CK, Gao W, Raffel OC, French JK, Stewart RA, White HD. Initial Q waves accompanying ST-segment elevation at presentation of acute myocardial infarction and 30-day mortality in patients given streptokinase therapy: an analysis from HERO-2. Lancet. 2006;367:2061–2067. doi: 10.1016/S0140-6736(06)68929-0. doi:10.1016/S0140-6736(06)68929-0. [DOI] [PubMed] [Google Scholar]
- 13.Wong CK, Stewart RAH, Gao W, French JK, Raffel OC, White HD for the Hirulog, Early Reperfusion or Occlusion (HERO)-2 Trial Investigators. Prognostic differences between different types of bundle branch block during the early phase of acute myocardial infarction: insights from the Hirulog and Early Reperfusion or Occlusion (HERO)-2 trial. Eur Heart J. 2006;27:21–28. doi: 10.1093/eurheartj/ehi622. doi:10.1093/eurheartj/ehi622. [DOI] [PubMed] [Google Scholar]
- 14.Wong CK, Gao W, Stewart RAH, French JK, Aylward PEG, White HD. Relationship of QRS duration at baseline and changes over 60 minutes after fibrinolysis to 30-day mortality with different locations of ST elevation myocardial infarction: results from the HERO-2 trial. Heart. 2009;95:276–282. doi: 10.1136/hrt.2008.146365. doi:10.1136/hrt.2008.146365. [DOI] [PubMed] [Google Scholar]
- 15.de Winter RJ, Verouden NJW, Wellens HJJ, Wilde AAM. A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008;359:2071–2073. doi: 10.1056/NEJMc0804737. doi:10.1056/NEJMc0804737. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum Y, Wagner GS, Barbash GI, Gates K, Criger DA, Sclarovsky S, Siegel RJ, Granger CB, Reiner JS, Ross AM. Correlation of angiographic findings and right (V1 to V3) versus left (V4 to V6) precordial ST-segment depression in inferior wall acute myocardial infarction. Am J Cardiol. 1999;83:143–148. doi: 10.1016/s0002-9149(98)00814-5. doi:10.1016/S0002-9149(98)00814-5. [DOI] [PubMed] [Google Scholar]
- 17.Ting HH, Krumholz HM, Bradley EH, Cone DC, Curtis JP, Drew BJ, Field JM, French WJ, Gibler WB, Goff DC, Jacobs AK, Nallamothu BK, O'Connor RE, Schuur JD. Implementation and integration of prehospital ECGs into systems of care for acute coronary syndrome: a scientific statement from the American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research, Emergency Cardiovascular Care Committee, Council on Cardiovascular Nursing, and Council on Clinical Cardiology. Circulation. 2008;118:1066–1079. doi: 10.1161/CIRCULATIONAHA.108.190402. doi:10.1161/CIRCULATIONAHA.108.190402. [DOI] [PubMed] [Google Scholar]
- 18.Wagner GS, Macfarlane P, Wellens H, Josephson M, Gorgels A, Mirvis DM, Pahlm O, Surawicz B, Kligfield P, Childers R, Gettes LS, Bailey JJ, Deal BJ, Hancock EW, Kors JA, Mason JW, Okin P, Rautaharju PM, van Herpen G. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part VI: acute ischemia/infarction: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:1003–1011. doi: 10.1016/j.jacc.2008.12.016. doi:10.1016/j.jacc.2008.12.016. [DOI] [PubMed] [Google Scholar]