Abstract
Multiple system atrophy (MSA) is a rare neurodegenerative disease of undetermined cause manifesting with progressive autonomic failure (AF), cerebellar ataxia and parkinsonism due to neuronal loss in multiple brain areas associated with (oligodendro)glial cytoplasmic α-synuclein (αSYN) inclusions (GCIs). Using proteolipid protein (PLP)-α-synuclein (αSYN) transgenic mice we have previously reported parkinsonian motor deficits triggered by MSA-like αSYN inclusions. We now extend these observations by demonstrating degeneration of brain areas that are closely linked to progressive AF and other non-motor symptoms in MSA, in (PLP)-αSYN transgenic mice as compared to age-matched non-transgenic controls. We show delayed loss of cholinergic neurons in nucleus ambiguus at 12 months of age as well as early neuronal loss in laterodorsal tegmental nucleus, pedunculopontine tegmental nucleus and Onuf's nucleus at 2 months of age associated with αSYN oligodendroglial overexpression. We also report that neuronal loss triggered by MSA-like αSYN inclusions is absent up to 12 months of age in the thoracic intermediolateral cell column suggesting a differential dynamic modulation of αSYN toxicity within the murine autonomic nervous system. Although the spatial and temporal evolution of central autonomic pathology in MSA is unknown our findings corroborate the utility of the (PLP)-αSYN transgenic mouse model as a testbed for the study of oligodendroglial αSYN mediated neurodegeneration replicating both motor and non-motor aspects of MSA.
Keywords: Alpha-synuclein, Transgenic mouse, Neurodegenerative disorders, Oligodendrocytes
Introduction
Progressive autonomic failure (AF) including orthostatic hypotension, urogenital and sudomotor failure frequently accompanies or precedes the motor features of multiple system atrophy (MSA) (Stefanova et al., 2009a; Wenning and Stefanova, 2009). The presence of AF is therefore obligatory for a diagnosis of clinically probable MSA. (Gilman et al., 2008; Stefanova et al., 2009a; Wenning and Stefanova, 2009). The underlying neuropathological substrate of MSA involves a system-bound neurodegeneration that is associated with α-synuclein (αSYN)-positive (oligodendro)glial cytoplasmic inclusions (GCIs) (Papp and Lantos, 1994; Jellinger, 2003). Previous work has demonstrated that AF in MSA is associated with neuronal loss in selected regions of the autonomic nervous system (ANS) (Table 1). Clinicopathological studies have linked orthostatic hypotension with degeneration of preganglionic sympathetic neurons in the intermediolateral columns (IML) of the spinal cord (Oppenheimer, 1980; Wenning et al., 1997), with failure of supraspinal cardiovascular control due to degeneration of catecholaminergic neurons in the ventrolateral medulla and locus coeruleus as well as preganglionic cholinergic neurons of nucleus ambiguus (NAmb) (Benarroch et al., 2002, 2006; Benarroch, 2003). Additional non-motor features of MSA including REM sleep behaviour disorder, erectile dysfunction and bladder hyperactivity appear to be related to loss of neurons in pedunculopontine tegmental nucleus (PPT) and laterodorsal tegmental nucleus (LDT) (Schmeichel et al., 2008; Gaig et al., 2008; Salas et al., 2008; Koyama et al., 1999). Anal and urethral sphincter failure in MSA has been associated with loss of neurons in Onuf's nucleus of the spinal cord (Mannen, 2000; Yamamoto et al., 2005).
Table 1.
Suggested clinicopathological correlations in human MSA and the relevance of the (PLP)-αSYN mouse model. (Wenning et al., 1997; Yoshida, 2007; Benarroch, 2003; Schmeichel et al., 2008; Yamamoto et al., 2005; Stefanova et al., 2005a,b).
| Clinical presentation of MSA | Pathological findings in human MSA (including neuronal loss and GCIs) | Pathology in the (PLP)-αSYN mouse |
|
|---|---|---|---|
| Neuronal loss | GCIs | ||
| Parkinsonism | Substantia nigra | + | + |
| Striatum | − | + | |
| Pedunculopontine tegmental nucleus | + | + | |
| Ataxia | Olivopontocerebellar atrophy | − | + |
| Orthostatic hypotension | Intermediolateral columns | − | + |
| Ventrolateral medulla (C1 and A1) | Na | + | |
| Dorsal vagal nucleus | Na | + | |
| Locus coeruleus | + | + | |
| Nucleus ambiguus | + | + | |
| Urinary dysfunction | Suprapontine micturition centers | Na | + |
| Pontine micturition center | Na | + | |
| Intermediolateral columns | − | + | |
| Laterodorsal tegmental nucleus | + | + | |
| Onuf's nucleus | + | + | |
| Laryngeal stridor | Nucleus ambiguus | + | + |
| REM sleep behaviour disorder (RBD) | Laterodorsal tegmental nucleus | + | + |
+, reported; −, not reported; Na, not analysed.
Recently, transgenic mouse models, overexpressing human αSYN under specific oligodendroglial promotors have been developed to reproduce the specific GCI pathology of MSA (Kahle et al., 2002; Stefanova et al., 2005a; Yazawa et al., 2005; Shults et al., 2005). Abnormal motor behaviour may be striking in these models and appears to relate to neurodegeneration depending on the specific oligodendroglial promoter (Stefanova et al., 2005b). To our knowledge, brain regions associated with the non-motor symptoms of MSA have not been investigated in the αSYN MSA models so far. Here we report that NAmb, PPT, LDT and Onuf's nucleus undergo progressive degeneration in (PLP)-αSYN mice, thus directly and for the first time linking αSYN toxicity with vulnerability of central autonomic and related non-motor regions.
Materials and methods
Animals
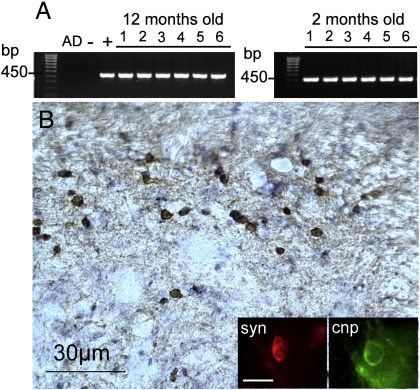
The generation and characterisation of (PLP)-α-SYN mice was previously described (Kahle et al., 2002). All experiments were performed in accordance with the Austrian law and permission for animal experiments of the Federal Ministry for Education, Science, and Research of Austria. (PLP)-α-SYN transgenic mice were bred at the Animal Facility of the Innsbruck Medical University and genotyped by tail clip PCR applying human αSYN specific primers which amplified a 450 bp fragment. In the present study we used homozygous (PLP)-α-SYN transgenic mice and non-transgenic control mice at 2 months of age and 12 months of age (for each experimental group n = 6, always 3 male and 3 female per group) (Fig. 1A). Animals were housed under a 12-h light/dark cycle with food and water available ad libitum. All efforts were made to minimise the number of animals used and their suffering.
Fig. 1.
(A) Tail clip PCR was applied to genotype (PLP)-α-SYN transgenic mice used in this study (i.e. six mice at 2 months of age and 6 mice at 12 months of age) by applying primers which amplified a 450 bp fragment of the human α-synuclein (AD, distilled water; −, non-transgenic negative control; +, positive control of the reaction for human αSYN). (B) Immunohistochemistry with 15G7 antibody against human α-synuclein demonstrated strong expression of the transgenic protein in the spinal cord of (PLP)-α-SYN transgenic mice. Inset: The colocalisation of 15G7 (red) and CNP (green) confirmed the expression of human α-synuclein in oligodendrocytes of (PLP)-α-SYN transgenic mice (bar, 10 µm).
Tissue processing
Animals were perfused transcardially with 4% paraformaldehyde under deep thiopental anaesthesia. Brains and spinal cords were removed, postfixed in the same fixative overnight at 4 °C, and cryoprotected with 20% sucrose. Brains and spinal cords were slowly frozen and kept at − 80 °C until further processing. Four series of 40 µm sections throughout the whole brain and 5 series of 50 µm sections throughout the thoracic and lumbosacral spinal cord were cut on a cryostat (Leica, Nussloch, Germany). We selected five nuclei (IML, LTD, PPT, NAmb, Onuf's nucleus) for morphometry based on the following criteria: 1) established role in human and rodent autonomic function, 2) evidence of αSYN pathology in human MSA. Thoracic series of spinal cord sections underwent cresyl violet (CV) and acetylcholine esterase (AchE) staining to define the borders and determine number of neurons in the IML. A series of brain sections (bregma + 4.04 - bregma + 7.50) underwent immunohistochemical staining for choline acetyl transferase (ChAT), to visualise cholinergic neurons of NAmb, LDT and PPT according to a standard protocol. Shortly, endogenous peroxidase activity was quenched in H2O2. After normal serum blocking sections were incubated with goat anti-ChAT antibody (Chemicon International) overnight at 4 °C, followed by incubation in biotinylated anti-goat IgG (Vector Laboratories, CA). After incubation in Vectastain ABC reagent (Vectastain ABC kit, Vector Laboratories, Burlingame, CA), the immunohistochemical reaction was developed with 3,3′-diaminobenzidine (DAB) and sections were mounted onto gelatine-coated slides, dehydrated and coverslipped with Entellan. Immunohistochemistry for human αSYN was performed using the rat monocloncal anti-human synuclein 15G7 antibody (Kahle et al., 2001) (generously provided by PJ Kahle, Tübingen) to confirm transgenic expression of the protein. Oligodendroglial expression of human αSYN was confirmed by double immunofluorescent staining with monoclonal rat 15G7 human αSYN antibody and monoclonal mouse 2',3'-cyclic nucleotide 3'-phosphohydrolase (CNP) antibody (Abcam, Cambridge, UK) followed by Cy3-conjugated rabbit anti-rat IgG (Jackson Immuno Research Laboratories, West Grove PA, USA) and Alexa-fluor 488-conjugated goat anti-mouse IgG (Molecular Probes, Leiden, the Netherlands) respectively.
Image analysis
All morphometric analyses were done by a blinded observer applying a computer-assisted image analysis system (Nikon E-800 microscope, Nikon digital camera DXM 1200; Stereo Investigator Software, MicroBrightField Europe e.K., Magdeburg, Germany). The optical fractionator (West et al., 1991) was used to estimate total number of neurons in the IML of thoracic spinal cord and number of cholinergic neurons in NAmb (Bregma −6.64 mm - −7.48 mm), LDT (Bregma −4.96 mm - −5.52 mm), and PPT (Bregma −4.16 mm - −4.96 mm) – borders determined as in the mouse brain atlas (Franklin and Paxinos, 1997). The mouse analogue of the human Onuf's nucleus was determined according to the mouse spinal cord atlas (Watson et al., 2008). Neurons in lamina 9 innervating the external urethral sphincter (ExU9) and the external anal sphincter (ExA9) were counted in sections at the level of L6.
Statistics
Statistical analysis was performed using the Graph Pad Prism Software. Data are presented as mean ± SEM. The data for the analogue of the Onuf's nucleus, IML, NAmb, LDT and PPT were compared by two way ANOVA for genotype and age effects followed by post hoc Bonferroni test to compare groups individually. P value of < 0.05 was considered significant.
Results
Human αSYN in oligodendrocytes was detected throughout the whole CNS of (PLP)-αSYN mice as determined by co-expression with the oligodendroglial marker CNP (Fig. 1B). In all cases non-transgenic animals had no human αSYN gene expression as determined by tail clip PCR and negative immunostaining for human αSYN (data not shown).
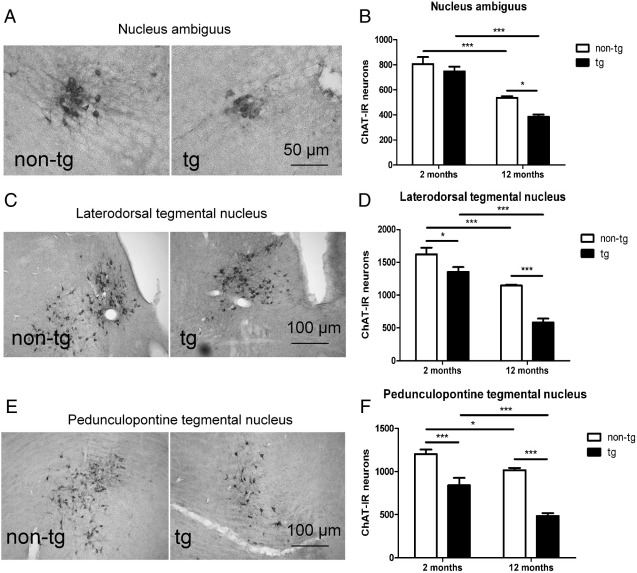
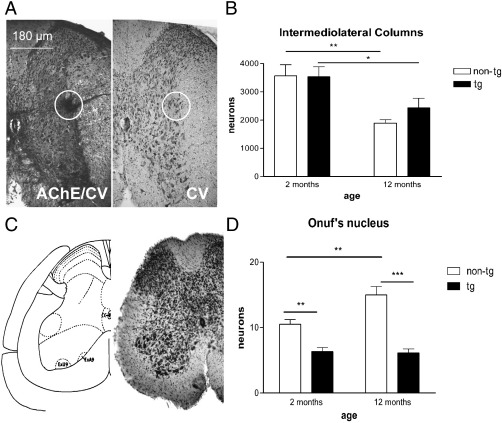
At 2 months of age no significant difference was observed between transgenic and non-transgenic mice in the number of cholinergic neurons in NAmb. However, (PLP)-αSYN transgenic mice at 2 months of age showed significant loss of cholinergic neurons in LDT and PPT compared to age-matched non-transgenic controls. At 12 months there was progressive brainstem pathology in the (PLP)-αSYN transgenic mice with neuronal loss affecting all cholinergic nuclei analysed (NAmb, LDT and PPT, Fig. 2). Preganglionic sympathetic neurons in the IML were reduced at 12 months in both transgenic and non-transgenic mice without any significant genotype effect (Fig. 3A and B). Further analysis of the lumbosacral region of the spinal cord showed an early loss of neurons in the mouse analogue of Onuf's nucleus that was associated with transgenic oligodendroglial αSYN overexpression and further augmented at 12 months.(Fig. 3C and D). Interestingly, non-transgenic mice showed an age-related increase in the number of motoneurons in Onuf's nucleus. Similar observation was previously reported in association with the breeding status of rodents (Seney et al., 2006) suggesting that both male and female breeders present with increased numbers of motoneurons in Onuf's nucleus. In this context, our results indicate that oligodendroglial αSYN overexpression in (PLP)-αSYN transgenic mice might result in disruption in the normal functional neurobiology of the analogue of Onuf's nucleus.
Fig. 2.
Immunolabelling for ChAT of the nucleus ambiguus (A), laterodorsal tegmental nucleus (C) and pedunculopontine tegmental nucleus (E) of non-transgenic (non-tg) and transgenic (tg) mice with oligodendroglial expression of human αSYN was performed. Morphometric analysis of ChAT-immunoreactive (ChAT-IR) neurons indicated age- and genotype-related loss of neurons in nucleus ambiguus (B), laterodorsal tegmental nucleus (D) and pedunculopontine tegmental nucleus (F). (*p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 3.
(A) Thoracic spinal cord was processed for acetylcholinesterase (AChE) and cresyl violet (CV) histochemistry to localise the intermediolateral column (IML, encircled). (B) Morphometric analysis indicated age-related loss of preganglionic sympathetic neurons in IML with no significant difference between non-transgenic (non-tg) and transgenic (tg) mice with oligodendroglial expression of human αSYN both at 2 and 12 months of age. (C) The mouse analogue of the human Onuf's nucleus (ExA9 and ExU9) in lamina 9 at the level of L6 (Jacob et al., 2005) was visualised in cresyl violet staining. (D) Significant loss of neurons in the Onuf's nucleus analogue was detected in transgenic versus non-transgenic mice both at 2 and 12 months of age. Age-related increase in the number of motoneurons was found in Onuf's nucleus of non-tg animals but not detected in tg mice with αSYN pathology (**p < 0.01; *** p < 0.001).
Discussion
In this study we investigated whether pathological αSYN accumulation induces degeneration in brain areas associated with autonomic and related non-motor features of MSA.
Parkinsonism and ataxia are generally considered cardinal motor features of MSA (Gilman et al., 2008; Stefanova et al., 2009a). However, MSA patients often develop AF and other non-motor features as well (Lipp et al., 2009). Although symptomatic therapies for cardiovascular autonomic and urogenital failure are available, health-related quality of life remains significantly impaired and deteriorates over time (Kollensperger et al., 2007). The lack of effective therapeutic options in MSA-associated AF generates a strong need for appropriate preclinical testbeds replicating MSA-like central ANS neuropathology resulting in quantifiable functional markers.
Transgenic overexpression of α1B-adrenergic receptors (α1B-AR) in mice generated wide-spread neurodegeneration linked to seizures (Zuscik et al., 2000; Kunieda et al., 2002), as well as cardiovascular autonomic dysfunction and motor impairment resembling MSA. This phenotype was associated with the formation of neuronal and oligodendroglial αSYN inclusions by yet unclear mechanisms (Papay et al., 2002; Zuscik et al., 2000, 2001). More recent evidence of increased MSA risk conferred by polymorphisms within the αSYN gene (Scholz et al., 2009; Al-Chalabi et al., 2009) corroborates the validity of the transgenic αSYN MSA models (Stefanova et al., 2005b). In our previous work on the (PLP)-αSYN mouse model we have focused on the motor phenotype and its pathogenesis (Stefanova et al., 2005a, 2007). Our data suggested a relationship between oligodendroglial αSYN overexpression, neuroinflammation and oxidative stress resulting in degeneration of striatonigral and olivopontocerebellar pathways that are typically affected in MSA. The model proved to be a powerful testbed for preclinical drug screening of candidate neuroprotective agents (Stefanova et al., 2008; Schapira, 2008). Further, transplantation of embryonic striatal tissue in the (PLP)-αSYN mouse model has recently identified evidence that αSYN aggregates may compromise graft maturation and integration (Stefanova et al., 2009b; Kordower and Brundin, 2009). Whether the ANS is affected in the transgenic αSYN MSA models has not been addressed so far.
The present findings indicate that αSYN overexpression in oligodendrocytes of (PLP)-αSYN mice augments age-dependent loss of neurons in cholinergic brainstem nuclei such as NAmb, LDT and PPT. Further, spinal αSYN pathology in (PLP)-αSYN mice leads to early progressive loss of neurons in Onuf's nucleus, whereas the age-related decline of IML neurons appeared unaffected by oligodendroglial αSYN pathology.
NAmb, LDT and PPT have been identified and extensively characterized in the mouse CNS in terms of structure and function. These murine nuclei show various degrees of selective vulnerability to different inductors of neurodegeneration. For example, injury of mesopontine cholinergic neurons in PPT and LDT can be induced by abnormal β-amyloid peptide expression in transgenic mice and appears to be associated with REM sleep perturbations relevant to AD (Zhang et al., 2005). Neuronal degeneration of NAmb associated with dysregulation of heart rate control may be observed in diabetic mice (Yan et al., 2009). Further, selective motoneuron pathology with preservation of the murine analogue of Onuf's nucleus was reported in a model of amyotrophic lateral sclerosis, intriguingly replicating the human disease (Hamson et al., 2002). Based on our current findings in the (PLP)-αSYN mice and the available knowledge on the functional correlate of degeneration of NAmb, LTD, PPT, and Onuf's nucleus, we predict cardiovascular, urogenital and REM sleep disturbances resulting from the oligodendroglial overexpression of human αSYN in the (PLP)-αSYN mice. These functional deficits will be characterized in a separate study using established methods (Zhang et al., 2005; Yan et al., 2009).
MSA is often associated with neuronal loss in IML, indeed this striking neuropathological feature has been highlighted by Shy and Drager (1960) in their landmark study. In thoracic IML of healthy persons, the number of preganglionic sympathetic neurons decreases with aging (Sakajiri et al., 1996) similar to our current observation in non-tg mouse IML. In the (PLP)-αSYN mouse oligodendroglial αSYN-overexpression induced neurodegeneration of the Onuf's nucleus analogue without significantly affecting age-related attrition of IML preganglionic sympathetic neurons. Remarkably, IML pathology in human MSA may be variable with little or no involvement in some cases and severe degeneration in others (Wenning et al., 1997; Kobayashi et al., 1998; Yoshida, 2007). The discordant effects of αSYN inclusion pathology on rates of neuronal loss seen in our study suggest that oligodendroglial αSYN overexpression may augment inherent age-related vulnerability patterns within the murine ANS. It is therefore conceivable that we might have observed aggravated IML pathology in older tg mice.
Our results corroborate the utility of the (PLP)-αSYN MSA model which replicates important non-motor and motor aspects of the human disease (Table 1). In contrast to other transgenic MSA models (Table 2), the (PLP)-αSYN model represents a unique phenotype expressing mild degrees of motor and autonomic pathology reminiscent of early-stage human MSA, thus providing a useful testbed for the screening of novel disease-modifying interventions (Stefanova et al., 2007). Further, αSYN mediated neurodegeneration can be strikingly exacerbated in (PLP)-αSYN mice by challenge with oxidative stress resulting in a model of end-stage MSA that features severe striatonigral degeneration and olivopontocerebellar pathology, the substrate of the MSA-associated motor disorder (Stefanova et al., 2005a). It is likely that central autonomic degeneration as demonstrated here in unchallenged (PLP)-αSYN can also be augmented and extended following oxidative stress, generating a murine analogue of severe MSA. An ongoing study in our laboratory aims to determine possible functional correlates of autonomic failure in (PLP)-αSYN mice including indices of cardiac, urinary and sleep disturbance. Although functional correlates of the autonomic degeneration pattern remain to be determined two major conclusions can be drawn from our current study: 1) In addition to our previous work demonstrating a mild but robust Parkinson-like phenotype with midbrain degeneration in (PLP)-αSYN mice we now show that abnormal αSYN expression in oligodendrocytes induces degeneration of MSA-linked autonomic and non-motor centers. These findings support the hypothesis of a primary αSYN oligodendrogliopathy in MSA resulting in selective neuronal multisystemdegeneration. They further highlight the utility of the current model as a preclinical testbed for novel therapeutic strategies targeting both autonomic and motor aspects of MSA. 2) The relative preservation of IML despite exposure to αSYN inclusion pathology suggests differential interactions of αSYN toxicity and subregional neuronal vulnerabilities within the ANS. In summary, the (PLP)-αSYN transgenic MSA model provides a powerful tool that can be further exploited to dissect pathogenetic mechanisms of glioneurodegeneration in MSA thereby identifying novel targets for interventional therapies.
Table 2.
Comparative overview of existing murine transgenic models of MSA summarizing salient behavioural and neuropathological features.
| Transgenic mouse model of MSA | Genetic design | Behaviour | Pathology | References |
|---|---|---|---|---|
| (PLP)-αSYN | Human αSYN overexpression under the PLP promoter | Shortened stride length Exacerbated phenotype with impaired rearing, pole test, hindlimb and truncal dystonia, bradikinesia |
GCIs Degeneration of SNc, LC, NAmb, LDT, PPT, and Onuf's nucleus Microglial activation 3NP exacerbated phenotype with SND and OPCA, astrogliosis |
(Kahle et al., 2002; Stefanova et al., 2005a, Stefanova et al., 2007) |
| (MBP)-αSYN | Human αSYN overexpression under the MBP promotor | Tremor Ataxia Impaired rotarod and pole test Seizures |
GCIs Astrogliosis Demyelination Axonal and dendritic degeneration in the cortex Reduced TH fiber density in striatum without loss of SNc dopaminergic neurons |
(Shults et al., 2005) |
| (CNP)-αSYN | Human αSYN overexpression under the CNP promotor | Impaired rotarod performance Impaired wire hanging |
GCIs Demyelination and secondary axonal degeneration Neuronal loss in hippocampus and cortical areas |
(Yazawa et al., 2005) |
| α1B-AR | α1B-AR overexpression under the mouse α1B-AR promoter | Decreased horizontal ambulation Decreased rearing (partly reversible by L-DOPA) Tremor Seizures Decreased heart rate Hypotension Increased in vivo spontaneous interictal epileptogenicity and EEG/behavioural seizures |
Granulovacular degeneration in all areas of the brain Neuronal and oligodendroglial αSYN aggregation Cardiac hypertrophy Reduced catecholamine plasma levels |
(Zuscik et al., 2000, 2001; Papay et al., 2002; Kunieda et al., 2002) |
αSYN, α-synuclein; α1B-AR, α1B adrenergic receptor; PLP, proteolipid protein; MBP, myelin basic protein; CNP, 2',3'-cyclic nucleotide 3'-phosphodiesterase; EEG, GCIs, glial cytoplasmic inclusions; SNc, substantia nigra pars compacta; LC, locus coeruleus; NAmb, nucleus ambiguus; LDT, laterodorsal tegmental nucleus; PPT, pedunculopontine tegmental nucleus; SND, striatonigral degeneration; OPCA, oligopontocerebellar atrophy; TH, tyrosine hydroxilase.
Acknowledgments
This study was supported by grants of the Tyrolian Research Funds (TWF UNI-0404/325) and the Austrian Research Foundation (FWF P-19989-B05 and DK-C34-B05). The authors are grateful to Ms. Monika Hainzer for the excellent technical assistance.
References
- Al-Chalabi A., Durr A., Wood N.W., Parkinson M.H., Camuzat A., Hulot J.S., Morrison K.E., Renton A., Sussmuth S.D., Landwehrmeyer B.G., Ludolph A., Agid Y., Brice A., Leigh P.N., Bensimon G. Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS ONE. 2009;4:e7114. doi: 10.1371/journal.pone.0007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. Brainstem in multiple system atrophy: clinicopathological correlations. Cell. Mol. Neurobiol. 2003;23:519–526. doi: 10.1023/A:1025067912199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E., Schmeichel A.M., Parisi J.E. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology. 2002;59:944–946. doi: 10.1212/wnl.59.6.944. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E., Schmeichel A.M., Sandroni P., Low P.A., Parisi J.E. Involvement of vagal autonomic nuclei in multiple system atrophy and Lewy body disease. Neurology. 2006;66:378–383. doi: 10.1212/01.wnl.0000196638.98781.bb. [DOI] [PubMed] [Google Scholar]
- Franklin K.B.J., Paxinos G. Academic Press; 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Gaig C., Iranzo A., Tolosa E., Vilaseca I., Rey M.J., Santamaria J. Pathological description of a non-motor variant of multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 2008;79:1399–1400. doi: 10.1136/jnnp.2008.145276. [DOI] [PubMed] [Google Scholar]
- Gilman S., Wenning G.K., Low P.A., Brooks D.J., Mathias C.J., Trojanowski J.Q., Wood N.W., Colosimo C., Durr A., Fowler C.J., Kaufmann H., Klockgether T., Lees A., Poewe W., Quinn N., Revesz T., Robertson D., Sandroni P., Seppi K., Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson D.K., Hu J.H., Krieger C., Watson N.V. Lumbar motoneuron fate in a mouse model of amyotrophic lateral sclerosis. NeuroReport. 2002;13:2291–2294. doi: 10.1097/00001756-200212030-00024. [DOI] [PubMed] [Google Scholar]
- Jacob D.A., Bengston C.L., Forger N.G. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J. Neurosci. 2005;25:5638–5644. doi: 10.1523/JNEUROSCI.1200-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K.A. Neuropathological spectrum of synucleinopathies. Mov. Disord. 2003;18(Suppl 6):S2–S12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- Kahle P.J., Neumann M., Ozmen L., Muller V., Odoy S., Okamoto N., Jacobsen H., Iwatsubo T., Trojanowski J.Q., Takahashi H., Wakabayashi K., Bogdanovic N., Riederer P., Kretzschmar H.A., Haass C. Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am. J. Pathol. 2001;159:2215–2225. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle P.J., Neumann M., Ozmen L., Muller V., Jacobsen H., Spooren W., Fuss B., Mallon B., Macklin W.B., Fujiwara H., Hasegawa M., Iwatsubo T., Kretzschmar H.A., Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Fukutani Y., Hayashi M., Miyazu K., Muramori F., Aoki T., Mukai M., Sasaki K., Isaki K., Koshino Y. Non-familial olivopontocerebellar atrophy combined with late onset Alzheimer's disease: a clinico-pathological case report. J. Neurol. Sci. 1998;154:106–112. doi: 10.1016/s0022-510x(97)00209-8. [DOI] [PubMed] [Google Scholar]
- Kollensperger M., Stampfer-Kountchev M., Seppi K., Geser F., Frick C., del Sorbo F., Albanese A., Gurevich T., Giladi N., Djaldetti R., Schrag A., Low P.A., Mathias C.J., Poewe W., Wenning G.K. Progression of dysautonomia in multiple system atrophy: a prospective study of self-perceived impairment. Eur. J. Neurol. 2007;14:66–72. doi: 10.1111/j.1468-1331.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Brundin P. Propagation of host disease to grafted neurons: accumulating evidence. Exp. Neurol. 2009;220:224–225. doi: 10.1016/j.expneurol.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Koyama Y., Imada N., Kawauchi A., Kayama Y. Firing of putative cholinergic neurons and micturition center neurons in the rat laterodorsal tegmentum during distention and contraction of urinary bladder. Brain Res. 1999;840:45–55. doi: 10.1016/s0006-8993(99)01770-9. [DOI] [PubMed] [Google Scholar]
- Kunieda T., Zuscik M.J., Boongird A., Perez D.M., Luders H.O., Najm I.M. Systemic overexpression of the alpha 1B-adrenergic receptor in mice: an animal model of epilepsy. Epilepsia. 2002;43:1324–1329. doi: 10.1046/j.1528-1157.2002.13202.x. [DOI] [PubMed] [Google Scholar]
- Lipp A., Sandroni P., Ahlskog J.E., Fealey R.D., Kimpinski K., Iodice V., Gehrking T.L., Weigand S.D., Sletten D.M., Gehrking J.A., Nickander K.K., Singer W., Maraganore D.M., Gilman S., Wenning G.K., Shults C.W., Low P.A. Prospective differentiation of multiple system atrophy from Parkinson disease, with and without autonomic failure. Arch. Neurol. 2009;66:742–750. doi: 10.1001/archneurol.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannen T. Neuropathological findings of Onuf's nucleus and its significance. Neuropathology. 2000;20(Suppl):S30–S33. doi: 10.1046/j.1440-1789.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer D.R. Lateral horn cells in progressive autonomic failure. J. Neurol. Sci. 1980;46:393–404. doi: 10.1016/0022-510x(80)90064-7. [DOI] [PubMed] [Google Scholar]
- Papay R., Zuscik M.J., Ross S.A., Yun J., McCune D.F., Gonzalez-Cabrera P., Gaivin R., Drazba J., Perez D.M. Mice expressing the alpha(1B)-adrenergic receptor induces a synucleinopathy with excessive tyrosine nitration but decreased phosphorylation. J. Neurochem. 2002;83:623–634. doi: 10.1046/j.1471-4159.2002.01170.x. [DOI] [PubMed] [Google Scholar]
- Papp M.I., Lantos P.L. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117(Pt 2):235–243. doi: 10.1093/brain/117.2.235. [DOI] [PubMed] [Google Scholar]
- Sakajiri K., Makifuchi T., Fukuhara N., Nakajima T. Quantitative study of intermediolateral column cell counts in Machado-Joseph disease. J. Neurol. Sci. 1996;144:156–159. doi: 10.1016/s0022-510x(96)00221-3. [DOI] [PubMed] [Google Scholar]
- Salas J.C., Iwasaki H., Jodo E., Schmidt M.H., Kawauchi A., Miki T., Kayama Y., Otsuki M., Koyama Y. Penile erection and micturition events triggered by electrical stimulation of the mesopontine tegmental area. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R102–R111. doi: 10.1152/ajpregu.00226.2007. [DOI] [PubMed] [Google Scholar]
- Schapira A.H. Rasagiline in neurodegeneration. Exp. Neurol. 2008;212:255–257. doi: 10.1016/j.expneurol.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Schmeichel A.M., Buchhalter L.C., Low P.A., Parisi J.E., Boeve B.W., Sandroni P., Benarroch E.E. Mesopontine cholinergic neuron involvement in Lewy body dementia and multiple system atrophy. Neurology. 2008;70:368–373. doi: 10.1212/01.wnl.0000298691.71637.96. [DOI] [PubMed] [Google Scholar]
- Scholz S.W., Houlden H., Schulte C., Sharma M., Li A., Berg D., Melchers A., Paudel R., Gibbs J.R., Simon-Sanchez J., Paisan-Ruiz C., Bras J., Ding J., Chen H., Traynor B.J., Arepalli S., Zonozi R.R., Revesz T., Holton J., Wood N., Lees A., Oertel W., Wullner U., Goldwurm S., Pellecchia M.T., Illig T., Riess O., Fernandez H.H., Rodriguez R.L., Okun M.S., Poewe W., Wenning G.K., Hardy J.A., Singleton A.B., Gasser T. SNCA variants are associated with increased risk for multiple system atrophy. Ann. Neurol. 2009;65:610–614. doi: 10.1002/ana.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney M., Goldman B.D., Forger N.G. Breeding status affects motoneuron number and muscle size in naked mole-rats: recruitment of perineal motoneurons? J. Neurobiol. 2006;66:1354–1364. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- Shults C.W., Rockenstein E., Crews L., Adame A., Mante M., Larrea G., Hashimoto M., Song D., Iwatsubo T., Tsuboi K., Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J. Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy G.M., Drager G.A. A neurological syndrome associated with orthostatic hypotension: a clinical-pathologic study. Arch. Neurol. 1960;2:511–527. doi: 10.1001/archneur.1960.03840110025004. [DOI] [PubMed] [Google Scholar]
- Stefanova N., Reindl M., Neumann M., Haass C., Poewe W., Kahle P.J., Wenning G.K. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am. J. Pathol. 2005;166:869–876. doi: 10.1016/s0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N., Tison F., Reindl M., Poewe W., Wenning G.K. Animal models of multiple system atrophy. Trends Neurosci. 2005;28:501–506. doi: 10.1016/j.tins.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Stefanova N., Reindl M., Neumann M., Kahle P.J., Poewe W., Wenning G.K. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov. Disord. 2007;22:2196–2203. doi: 10.1002/mds.21671. [DOI] [PubMed] [Google Scholar]
- Stefanova N., Poewe W., Wenning G.K. Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp. Neurol. 2008;210:421–427. doi: 10.1016/j.expneurol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Stefanova N., Bucke P., Duerr S., Wenning G.K. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- Stefanova N., Hainzer M., Stemberger S., Couillard-Despres S., Aigner L., Poewe W., Wenning G.K. Striatal transplantation for multiple system atrophy - Are grafts affected by alpha-synucleinopathy? Exp. Neurol. 2009;219:368–371. doi: 10.1016/j.expneurol.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Watson C., Paxinos G., Kayalioglu G. A Christopher and Dana Reeve Foundation Text and Atlas. Academic Press; 2008. The Spinal Cord. [Google Scholar]
- Wenning G.K., Stefanova N. Recent developments in multiple system atrophy. J. Neurol. 2009;256:1791–1808. doi: 10.1007/s00415-009-5173-8. [DOI] [PubMed] [Google Scholar]
- Wenning G.K., Tison F., Ben Shlomo Y., Daniel S.E., Quinn N.P. Multiple system atrophy: a review of 203 pathologically proven cases. Mov. Disord. 1997;12:133–147. doi: 10.1002/mds.870120203. [DOI] [PubMed] [Google Scholar]
- West M.J., Slomianka L., Gundersen H.J. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Sakakibara R., Uchiyama T., Liu Z., Ito T., Awa Y., Yamamoto K., Kinou M., Yamanishi T., Hattori T. When is Onuf's nucleus involved in multiple system atrophy? A sphincter electromyography study. J. Neurol. Neurosurg. Psychiatry. 2005;76:1645–1648. doi: 10.1136/jnnp.2004.061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Li L., Harden S.W., Epstein P.N., Wurster R.D., Cheng Z.J. Diabetes induces neural degeneration in nucleus ambiguus (NA) and attenuates heart rate control in OVE26 mice. Exp. Neurol. 2009;220:34–43. doi: 10.1016/j.expneurol.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Yazawa I., Giasson B.I., Sasaki R., Zhang B., Joyce S., Uryu K., Trojanowski J.Q., Lee V.M. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Zhang B., Veasey S.C., Wood M.A., Leng L.Z., Kaminski C., Leight S., Abel T., Lee V.M., Trojanowski J.Q. Impaired rapid eye movement sleep in the Tg2576 APP murine model of Alzheimer's disease with injury to pedunculopontine cholinergic neurons. Am. J. Pathol. 2005;167:1361–1369. doi: 10.1016/S0002-9440(10)61223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuscik M.J., Sands S., Ross S.A., Waugh D.J., Gaivin R.J., Morilak D., Perez D.M. Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy. Nat. Med. 2000;6:1388–1394. doi: 10.1038/82207. [DOI] [PubMed] [Google Scholar]
- Zuscik M.J., Chalothorn D., Hellard D., Deighan C., McGee A., Daly C.J., Waugh D.J., Ross S.A., Gaivin R.J., Morehead A.J., Thomas J.D., Plow E.F., McGrath J.C., Piascik M.T., Perez D.M. Hypotension, autonomic failure, and cardiac hypertrophy in transgenic mice overexpressing the alpha 1B-adrenergic receptor. J. Biol. Chem. 2001;276:13738–13743. doi: 10.1074/jbc.M008693200. [DOI] [PubMed] [Google Scholar]