Abstract
The basic biology of the menstrual cycle is a complex, coordinated sequence of events involving the hypothalamus, anterior pituitary, ovary, and endometrium. The menstrual cycle with all its complexities can be easily perturbed by environmental factors such as stress, extreme exercise, eating disorders, and obesity. Furthermore, genetic influences such as fragile X premutations (Chapter X), X chromosome abnormalities (Chapter X), and galactose-1-phosphate uridyltransferase (GALT) point mutations (galactosemia) also contribute to perturbations of the menstrual cycle. Although not perfect, mouse model have helped to identify and confirm additional components and pathways in menstrual cycle function and dysfunction in humans.
Keywords: Biology, menstrual cycle, mouse models, premature ovarian insufficiency (POI), genetics, endometriosis, folliculogenesis
Overview of the Menstrual Cycle in Humans
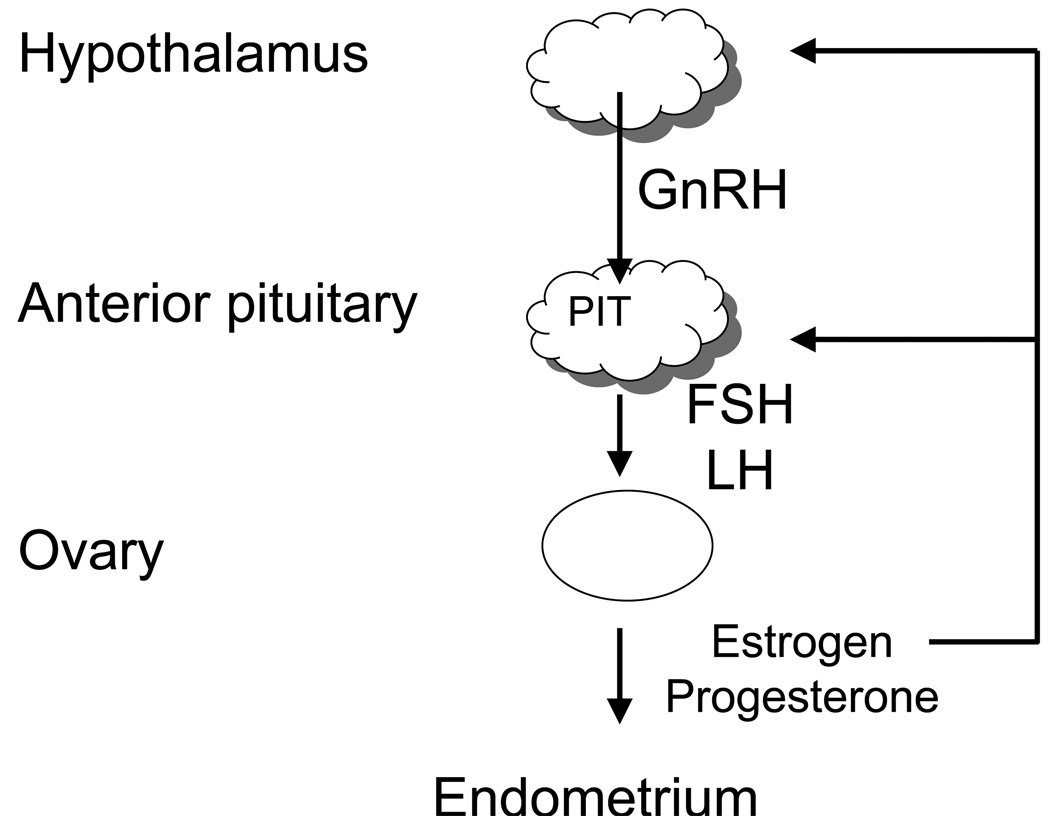
Figure 1 shows a general overview of the key regulatory factors in the menstrual cycle. The hypothalamus secretes gonadotropin releasing hormone (GnRH) which stimulates the anterior pituitary to secrete both follicle stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH are heterodimeric members of the glycoprotein hormone family and have an α:FSHβ and α:LHβ non-covalent structure, respectively. The α subunit is common to all glycoprotein hormone family members. The levels and timing of secretion of each gonadotropin is correlated by GnRH, feedback from sex steroid hormones, and other autocrine and paracrine factors such as inhibin and activin. The gonadotropins stimulate the ovary to produce the steroid hormones, estrogen or progesterone as well as several key autocrine, paracrine, and endocrine peptides. Similar to the pituitary, ovarian steroidogenesis is regulated by multiple factors. The ovarian steroid hormones in turn stimulate endometrial proliferation and affect many end organs. Although estrogen and progesterone have some feedback at the level of the hypothalamus, the more dynamic feedback occurs at the level of the anterior pituitary. Folliculogenesis, ovulation, luteinization, and endometrium growth and decline during the menstrual cycle depend on the above-mentioned autocrine, paracrine, and endocrine factors produced from this axis1.
Figure 1. General overview of the important factors in the menstrual cycle.
Regulation of the menstrual cycle begins with influences at the level of the hypothalamus. The hypothalamus stimulates the anterior pituitary which stimulates the ovaries. One of the end organs for the ovarian sex hormones is the endometrium. The menstrual cycle is regulated by feed back and cross talk between these different components.
Oocyte Development
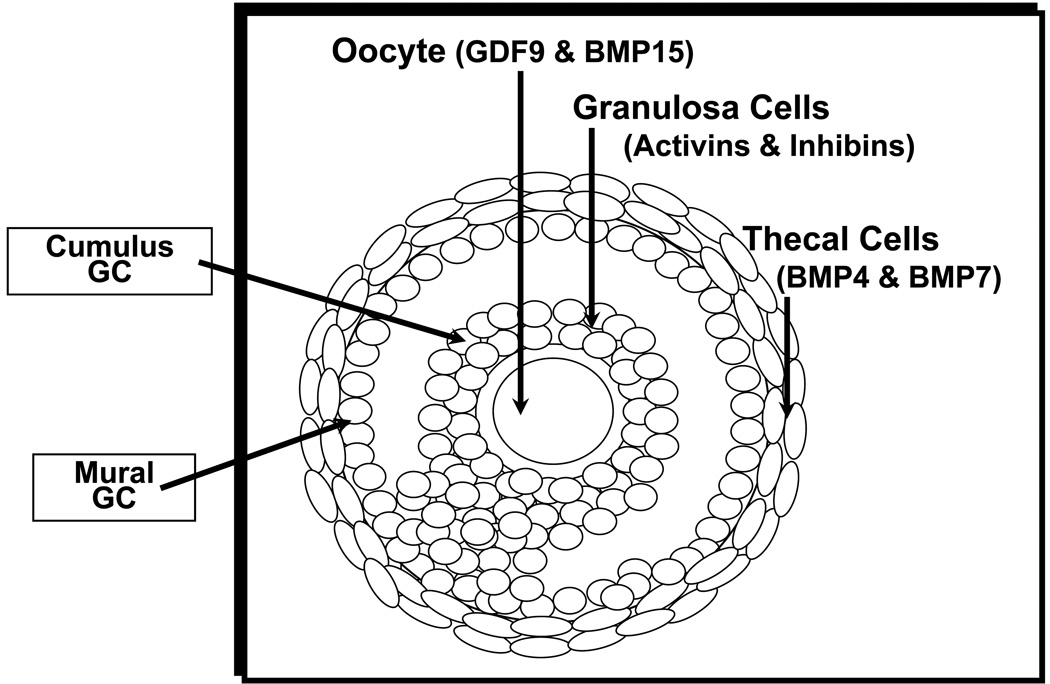
In humans, germ cells begin to develop at 5–6 weeks of gestation1, 2. These cells migrate to the genital ridge and multiply, giving a finite number of germ cells by the time of birth in females. These germ cells will be encapsulated by “pre-granulosa” cells to become oocytes at the primordial follicle stage. At this point, the oocyte will arrest at the diplotene stage of meiosis. Oocytes are surrounded by supporting cells called granulosa cells, while thecal cells surround the follicle after the primary follicle stage. The surrounding cells as well as the oocyte itself secrete factors which regulate folliculogenesis3. Folliculogenesis is the process of preparing a single oocyte from a primordial follicle for ovulation1, 4, 5. Figure 2 shows the anatomy of a follicle with representative factors.
Figure 2. Anatomy of an ovarian follicle.
This cartoon depicts the various cell types of an antral follicle and some of the factors secreted by each cell type. The oocyte is surrounded by cumulus granulosa cells, while the mural granulosa cells surround the antrum. The theca cells surround the entire follicle. Crosstalk between these cell types by the factors listed and others are important for ovarian folliculogenesis.
Overview of Ovarian Folliculogenesis
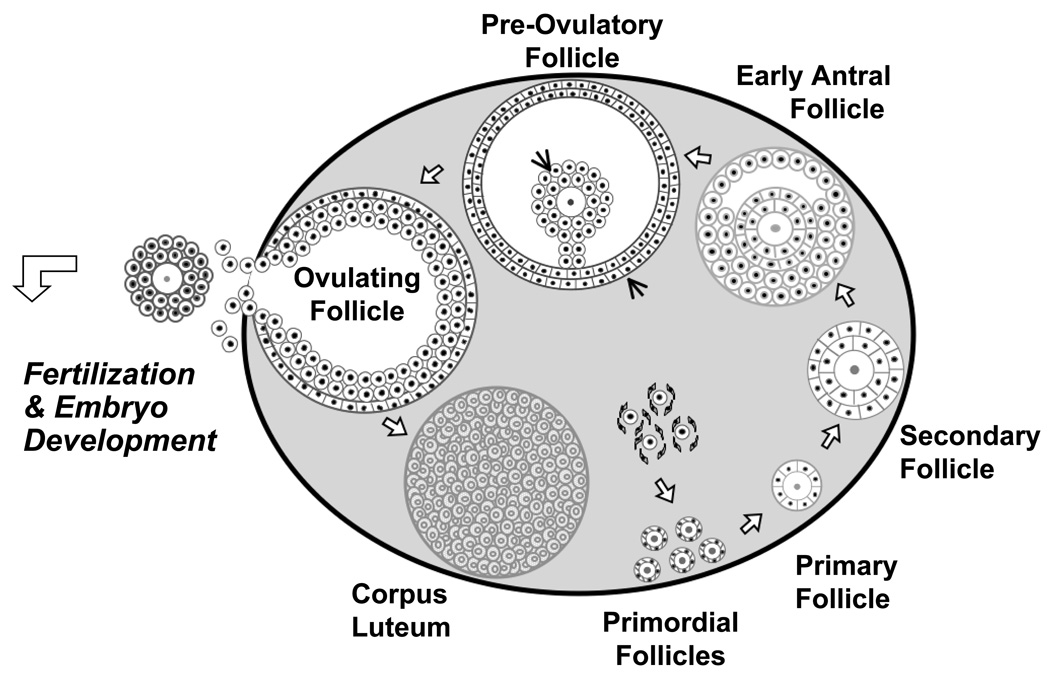
In the ovary, folliculogenesis can simply be divided into the follicular phase, prior to ovulation, and the luteal phase, after ovulation1. Figure 3 depicts the stages of ovarian folliculogenesis in humans. Ovarian folliculogenesis begins with the recruitment from a pool of growing primordial follicles1. Despite intense work in both mice and other species, the critical signals that initiate the recruitment of primordial follicles are still unknown1, 6–8. After recruitment, these follicles grow and become primary follicles. Once the multilayer (secondary) follicles express FSH receptors, they are then subject to endocrine regulation.
Figure 3. Stages of ovarian follicular development.
Folliculogenesis requires a coordinated progression of growth of ovarian follicles. The process begins with the germ cells which are recruited to a pool of primordial follicles. The primordial follicles progress to primary and then secondary follicles. At the secondary follicle stage, theca cells are present. The early antral follicle stage is defined by the presence of antrum. The peri-ovulatory follicle stage is also known as the dominant follicle and is ready for ovulation. this stage, cumulus and mural granulosa cells are present. Once the oocyte is ovulated, the remaining granulosa cells become the corpus luteum. This cycle of folliculogenesis occurs for every single oocyte ovulated.
In the presence of FSH, these follicles begin to grow even more and are competent to develop into an antral follicle. Without FSH, the follicles become atretic. The theca, a layer of cells surrounding the follicle, is formed first at the two-layer pre-antral follicle stage, and with exposure to low levels of LH, produces androgens in humans. Androgens are converted to estrogen via a member of the cytochrome P450 superfamily, CYP19 (aromatase) in the granulosa cells. FSH induces granulosa cell proliferation, induction of aromatase, and increased FSH receptors on the granulosa cells, thus leading to a very high estrogen microenvironment. With an increase in estrogen, the antral follicle develops further. At the pre-antral follicle stage, the follicle is a two cell (granulosa and thecal cells), two gonadotropin (FSH and LH) system. This cross talk between the granulosa and thecal cells results in high estrogen levels within the follicle. This high estrogen down regulates FSH from the anterior pituitary and begins the process of selecting for a single dominant follicle. Follicles that are not at the appropriate stage and are not able to maintain a high estrogen microenvironment without stimulation from FSH degenerate and become atretic follicles. This process selects for the dominant follicle1.
The very high estrogen levels feed back to the anterior pituitary to induce the LH surge, which ultimately leads to ovulation. During ovulation, the oocyte is expulsed from the follicle with cumulus granulosa cells surrounding it. The remaining follicular cells in the ovary become luteinized as part of the corpus luteum which secretes progesterone. During the luteal phase, the granulosa cells within the corpus luteum also produce inhibin A, an α:βA heterodimeric member of the transforming growth factor β (TGFβ) superfamily, which acts as an endocrine hormone to suppresses pituitary FSH, inhibiting growth of other ovarian follicles. With no fertilization or implantation of the embryo, the corpus luteum degenerates, possibly in response to activin, homodimers (βA:βA or βB:βB) or heterodimers (βA:βB) that share the β subunits with inhibin A and inhibin B (α:βB). When inhibin and progesterone levels fall with regression of the corpus luteum, suppression of FSH is released. At the luteal-follicular transition, FSH levels increase, and the next menstrual cycle begins1.
With fertilization and implantation, the corpus luteum is maintained by stimulation of human chorionic gonadotropin (hCG) by the placenta. HCG (α:CGβ) is also a member of the glycoprotein hormone family along with LH, FSH, and thyroid stimulating hormone (α:TSHβ; TSH), sharing the same common α subunit and binding to identical gonadal LH/CG receptors as LH. HCG will peak at approximately the end of the first trimester. Detection of hCG in the urine (approximately 10 days after fertilization and shortly after implantation when hCG can enter the woman’s bloodstream) is the major test of pregnancy. The rate of rise or fall of serum levels of hCG during the first trimester can be used to detect ectopic pregnancy, miscarriage, multiple pregnancies, and often-times, placental derived cancers such as hydatidform moles, gestational trophoblastic disease, and choriocarcinoma1.
Menstrual cycles continue for a woman until her finite population of oocytes is exhausted. Primary hypogonadism occurs in women with gonadal failure, low estrogen, with elevated FSH levels, also known as hypergonadotropic hypogonadism. If these clinical findings are observed in a woman under 40, it is known as premature ovarian insufficiency (POI).
Mouse Models with Folliculogenesis Defects
Folliculogenesis in the mouse is similar to human ovarian folliculogenesis. To date, approximately 100 factors have been demonstrated to affect folliculogenesis or female fertility in mice using knockout or transgenic technology [reviewed in5]. Consistent with mouse models, mutations in some of these factors in mice have also been identified in humans, particularly in women with POI. Here, we attempt to review some of the factors shown to cause defects in mouse folliculogenesis that have mutations found in humans with POI.
One of the earliest genes involved in postnatal folliculogenesis is NOBOX. NOBOX is an oocyte-specific homeobox gene and is thought to be specifically expressed in the germ cells, primordial follicles, and growing oocytes9. NOBOX null female mice are infertile and have no follicular development past the primordial follicle stage. Furthermore, as these mice age, they have a decreased number of oocytes, or very early POI10.
Moving up to the anterior pituitary, the two most important extra-gonadal factors in ovarian folliculogenesis are FSH and LH. As mentioned earlier, FSH and LH share a common α subunit, but each has a unique β subunit1. FSHβ null female mice are infertile with small ovaries. The FSHβ null mice have follicular arrest at the secondary follicle11. Similarly, LHβ null female mice are infertile. The follicles in the LHβ null animals arrest at the pre-ovulatory follicle and undergo degeneration. Corpora lutea are not observed in these mice12. Not surprisingly, the gonadotropins are essential for folliculogenesis in the mouse.
Although orchestrated stimulation of the follicle by gonadotropins is important, the cells within the follicle secrete factors important for their own regulation. This crosstalk between the oocyte and the granulosa and thecal cells additionally regulates folliculogenesis4, 13. The factors secreted from these cells include the transforming growth factor β (TGFβ) family of proteins. The expression of these factors is not only temporally regulated within the menstrual cycle, but also spatial regulated. The oocyte secretes growth and differentiation factor 9 (GDF9), bone morphogenic protein 15 (BMP15), and BMP6 while the granulosa cells secrete activins and inhibins. The thecal cells secrete TGFβ1, TGFβ2, BMP4, and BMP7. All of these factors act as autocrine and paracrine factors and influence folliculogenesis [reviewed in3].
GDF9, the first oocyte factor discovered, is a member of the TGFβ superfamily. GDF9 is expressed in the oocyte from early folliculogenesis through ovulation and controls the function of ovarian follicles. GDF9 null female mice are infertile and have small ovaries. Histological analysis of GDF9 null mice ovaries shows a block in folliculogenesis at the primary follicle. Thus, GDF9 is important in early folliculogenesis, essential for the primary and secondary follicle transition7.
Additionally, GDF9 is important in later stages of folliculogenesis. Specifically, it is important for cumulus cell expansion. The cumulus is the layer of granulosa cells directly surrounding the oocytes along with its rich hyaluronic acid matrix. The mural granulosa cells line the follicle wall (Figure 2). During the peri-ovulatory period, the cumulus granulosa cells undergo expansion in preparation for ovulation. The cumulus protects the oocyte from the harsh environment, helps with extrusion of the cumulus oocyte complex, and permits capture of the freshly ovulated oocyte by the fibria. Importantly, it also enhances the ability of the sperm to fertilize the ovary in vivo13–21. In vitro, GDF9 exposure results in the expansion of mouse cumulus cells, suggesting its critical role in the function of this complex22–24.
BMP15 is another TGFβ family member that is homologous to GDF9 and is also important for folliculogenesis in the mouse. BMP15 null female animals are subfertile but not infertile. Thus, BMP15 is important but not as essential as GDF9 in the mouse. When the BMP15 null mice were bred to the GDF9 mice, the BMP15 null, GDF9 heterozygote female mice were even more subfertile than the BMP15 null mutation alone. These double mutant mice had late folliculogenesis defects noted on ovarian histology, having decreased numbers of late stage follicles. BMP15 and GDF9 may play synergistic roles in folliculogenesis as suggested by the more severe fertility defects in the heterozygous GDF9 and BMP15 knockout mice. Furthermore, the ratio of the number of oocytes ovulated to embryos created was extremely low. Of significant note, these double mutant animals lacked cumulus cell expansion. Thus, BMP15 and GDF9 play important complementary roles in cumulus cell expansion25.
Four major genes downstream of GDF9 in cumulus expansion are cyclooxygenase 2 (COX2), hyaluronase synthase 2, pentraxin 3 (PTX3), and tumor necrosis factor α induced protein 6 (TNFAIP6). COX2, PTX3, and TNFAIP6 mutant mice have also been produced and show cumulus expansion and female fertility defects26–28. Thus, these studies confirm the importance of these factors downstream of GDF9/BMP pathway in cumulus cell expansion and mouse fertility.
These factors are only the beginning of the list of autocrine, paracrine, and endocrine factors involved in female fertility. Many more mouse models that display reproductive phenotypes have been created to understand the menstrual cycle, female infertility, and premature ovarian insufficiency [reviewed in5]. The studies below in humans will go into the genetic defects and the translational aspect of this work in the clinic.
Human models of ovarian dysfunction
Although the above members of the TGFβ superfamily play important roles in folliculogenesis in mice, the correlation to human premature ovarian insufficiency (POI) is not so simple. Many of the factors described above as important in mouse folliculogenesis have been directly sequenced in patients with POI. However, mutations in these genes seem to be uncommon factors in the pathophysiology of POI.
Multiple studies over many years have attempted to discover gene mutations involved in POI. Early studies focused on gonadotropin gene and gonadotropin receptor defects [reviewed in29]. Mutations in the α subunit of the glycoprotein hormone family have not been found in women with POI. Until recently, mutations in LHβ had not been found in women with POI. Recently, one woman with secondary amenorrhea was found to have a point mutation in exon 2 of the LHβ gene. This mutation caused a frame shift of exon 3 and LH deficiency, leading to her secondary amenorrhea. However, she did not have elevated levels of FSH30. Mutations in the FSHβ gene have been found in women with primary amenorrhea and infertility. This mutation is a 2 base pair deletion, resulting in a stop codon, and early ovarian failure. Females with mutations in the LH receptor (LHR) have primary amenorrhea with elevated FSH levels, POI. Females with FSH receptor (FSHR) mutations also have POI, demonstrating ovarian dysgenesis and lack of ovarian follicle development29. Overall, these studies reveal that mutations in gonadotropins or their receptors are involved in human folliculogenesis and some isolated cases of POI, but are not a common cause of clinical POI.
Over the years, more sophisticated gene chip and computational experiments have discovered additional candidate genes for POI. However, mutations in these genes in humans with POI are still uncommon. For example, in humans with POI, NOBOX gene mutations are present in <1% of analyzed population31. Additionally, mutations in GDF9 or BMP15 are found in few patients with POI32–38. In one of the largest studies of women with POI, 6 of 166 women with POI had missense substitutions in BMP15, but 0 out of 392 controls had this variation in BMP15. Additional variations were found in BMP15 in both the POI and control population39. Although statistically significant, the functional significance of many of these mutations (except the BMP15 mutation in two sisters with infertility40) have not been demonstrated. Overall, NOBOX, GDF9, and BMP15 mutations do not appear to be common causes of POI, but other factors within the TGFβ signaling pathway may be important.
Overview of the Cyclic Endometrium
The endometrium is one of the most sensitive organs to ovarian steroid hormones. The endometrium is composed of two layers. The most luminal layer is the functionalis which is thickened and sloughed in response to ovarian hormones. The basalis is closest to the myometrium and remains throughout the menstrual cycle1.
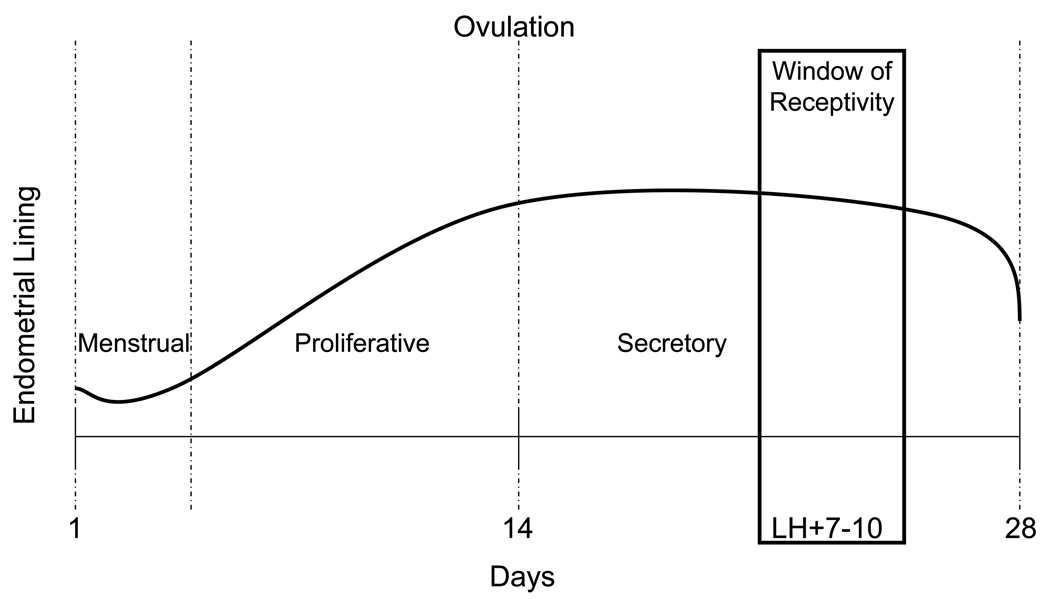
The endometrium can simply be divided into the proliferative phase, corresponding to the follicular phase in the ovary, and the secretory phase, corresponding to the luteal phase in the ovary. Figure 4 shows a representation of the endometrium throughout the menstrual cycle. In menstrual cycle dating, the first day of the menstrual bleed is considered day 1. During the menstrual phase, the endometrium undergoes changes and is sloughed in women, due to low estrogen levels. The proliferative phase is defined as the period of time from the menstrual phase to ovulation. As estrogen levels begin to rise, the endometrial lining thickens, giving a proliferative pattern. Estrogen leads to a proliferation of stroma and glands and elongation of the spiral arteries. The secretory phase is from ovulation until menstruation. After ovulation, progesterone levels begin to rise in the early secretory phase. This leads to secretion of glycogen and mucus. In the mid-secretory phase, the endometrium becomes decidualized and receptive to a fertilized embryo. In the late secretory phase, in the absence of pregnancy, and with the accompanying drop in both estrogen and progesterone, the spiral arteries vasoconstrict, leading to involution of the endometrium. The cycle then repeats1.
Figure 4. Endometrial lining throughout the menstrual cycle.
The endometrial lining thickens in response to estrogen in the proliferative phase. After ovulation, the endometrium becomes decidualized in the secretory phase. The window of receptivity is 7–10 days after the LH surge, near the time of ovulation. After the cycle is finished, menstruation occurs and the cycle begins again.
Mouse Models with Endometrial Dysfunction
Numerous factors have been demonstrated to play a role in implantation, decidualization, or embryo spacing in knockout mouse models. These factors include cytokines, transcription factors, ovarian hormones, and other autocrine/paracrine factors [reviewed in41–44]. Additionally, a mouse model of endometriosis has been created45.
Estrogen (E2) and progesterone (P4) are obviously important in the development of the endometrium. E2 signals through either estrogen receptor alpha (ERα) or beta (ERβ) to activate a number of estrogen responsive genes. P4 signals through progesterone receptor A (PRA) and B (PRB) to activate a number of progesterone responsive genes in the mouse42.
ERα null mice are infertile, have abnormalities of the female reproductive tract, and cannot support implantation. However, ERβ null mice support implantation. Leukemia inhibitory factor is a member of the IL-6 family and is a down stream target for estrogen. LIF null mice do not support implantation, although a similar role of LIF in humans has not been observed. Thus, ERα and cytokines, perhaps acting downstream of ERα, are important to maintain endometrial receptivity42.
Progesterone receptor null mice (lacking both PRA and PRB) also have reproductive tract anomalies and lack of decidualization. However, PRB null mice have normal reproductive features suggesting that PRA is more important to reproduction and possibly endometrial function in the mouse. Indian hedge hog (IHH) is a progesterone responsive gene. IHH null mice with conditional deletion in the uterus are infertile due to lack of a decidual response42. Thus, PRA and progesterone responsive genes are important for the decidual response during endometrial receptivity.
For successful pregnancy to occur, the endometrium must be receptive, the blastocyst must come into contact with the endometrium, and the blastocyst must penetrate the decidua to access a blood supply. The homeobox transcription factors, HOXA10 and HOXA11, are expressed during the window of receptivity in both mice and humans and are important for decidualization. HOXA10 null mice are subfertile. They demonstrate a failure of embryos to implant and the uterus to decidualize, most likely due to a lack of stromal proliferation. HOXA11 null mice have a more severe phenotype with hypoplastic uteri and failure of implantation. FKBP52 null mice have defects in luminal closure of the uterus during implantation and thus defects in apposition of the blastocysts to the endometrium. Prostaglandins are important for increased vascular permeability at the time of implantation. PTGS2 (COX2) null animals are deficient in the enzyme which mediates prostaglandin synthesis and are infertile41.
Most mouse models of endometriosis use autologous endometrial tissue transplanted into the abdominal cavity to reproduce the phenotypic endometriotic peritoneal implants. However, the single mutant K-ras oncogene mouse developed peritoneal endometriotic implants by 8 months of age. Furthermore, when the K-ras oncogene mouse was crossed with a Pten conditional mouse, the mice developed endometrioid ovarian cancers which are more common in women with endometriosis. Currently, this is the only genetic mouse model for endometriosis45.
Human Models with Endometrial Dysfunction
In the human, a coordinated response to estrogen and progesterone leads to the cyclic changes in the endometrium. With inappropriate thickening or decidualization of the endometrium, clinical problems such as break through bleeding, metrorhagia, or cancer occur. Furthermore, endometrial tissue located outside the uterine cavity as in the case of endometriosis is still hormonally sensitive, potentially leading to cyclic pain. Lastly, an endometrium that is not receptive to a blastocyst will not support a normal pregnancy, and thus defects in receptivity of the endometrium lead to infertility or recurrent pregnancy loss1.
Since the endometrium is a hormonally-responsive organ, the gene expression profile changes dependent on the phase of the cycle. Gene expression projects over the years have attempted to create a database of gene expression patterns based on timing of the cycle for normal women. Some of this data can be found in the Gene Expression Omniobus http://www.ncbi.nlm.nih.gov/geo/.
In humans, the receptive phase is 7–10 days into the secretory phase, designated as 7–10 days past the LH surge (LH+7–10) (Figure 4). Prior to this, the endometrium is not supportive of a blastocyst. After this receptive phase, the endometrium is hostile to the blastocyst1. Multiple translational studies during the receptive phase have searched for factors responsible for receptivity defects, but no good candidates have been identified. Additionally, multiple gene expression studies have attempted to identify dysregulated genes at the receptive time point of the endometrium in women with infertility [reviewed in46]. Likewise, important factors for these receptivity defects have not yet been identified in humans.
Similar gene expression studies have attempted to determine dysregulated genes involved in endometriosis, but to date no good gene candidates have been discovered47–49. Recently, endometrium from patients with severe endometriosis at different times within the menstrual cycle was compared to endometrium from normal women using robust gene expression arrays. Although the expression of many genes was different, the progesterone responsive genes showed the most significant dysregulation. This confirms the progesterone resistance found with endometriosis. Furthermore, additional analysis revealed that the gene expression pattern did not fit the timing of the cycle, showing some delay in expression of early secretory genes. Thus, endometriosis and the resulting infertility may result from a combination of progesterone resistance and a menstrual cycle timing defect50. Additionally, mutation screening studies of women with endometriosis did not reveal any mutations in K-ras or Pten51, 52.
Conclusions
The basic biology of the menstrual cycle is not so basic. However, mouse models have improved our understanding of folliculogenesis, implantation, and endometriosis in mammals. Even though the factors important in mouse folliculogenesis do not play a large role in POI, the concepts open avenues for further study and may lead to an understanding of human POI and eventually treatment. Furthermore, a better understanding of implantation and decidualization defects in mice may lead to treatment for recurrent pregnancy loss, infertility, and possibly endometriosis.
Acknowledgements
Work on fertility and the menstrual cycle has been supported by American Society of Reproductive Medicine-National Institute of Child Health and Disease Reproductive Scientist Development Program HD000849-19 to SMH, and the Specialized Cooperative Centers in Reproductive and Infertility Research (HD07495) and grants CA60651, HD32067, HD33438, HD 42500 to MMM.
References
- 1.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 2.Byskov AG, Hoyer PE. Embryology of mammalian gonads. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press, Ltd.; 1994. pp. 487–540. [Google Scholar]
- 3.Pangas SA, Matzuk MM. Genetic models for transforming growth factor beta superfamily signaling in ovarian follicle development. Mol Cell Endocrinol. 2004;225:83–91. doi: 10.1016/j.mce.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Matzuk MM, et al. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 5.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Med. 2002;8(S1):S41–S49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 6.Anderson LD, Hirshfield AN. An overview of follicular development in the ovary: From embryo to the fertilized ovum in vitro. Maryland Medical Journal. 1992;41:614–620. [PubMed] [Google Scholar]
- 7.Dong J, et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 8.Hirshfield AN. Development of follicles in the mammalian ovary. International Review of Cytology. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 9.Suzumori N, et al. Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech Dev. 2002;111:137–141. doi: 10.1016/s0925-4773(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 10.Rajkovic A, et al. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 11.Kumar TR, et al. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nature Genetics. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, et al. Targeted disruption of luteinizing hormone β subunit leads to hypogonadism, defects in gonadal steroidogenesis and infertility. Proc Natl Acad Sci USA. 2004;101:17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–938. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 14.Diaz FJ, et al. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Eppig JJ. Oocyte-somatic cell communication in the ovarian follicles of mammals. Seminars in Developmental Biology. 1994;5:51–59. [Google Scholar]
- 16.Eppig JJ, et al. Oocyte control of granulosa cell development: how and why. Human Reproduction. 1997;12:127–132. [PubMed] [Google Scholar]
- 17.Richards JS, et al. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 18.Diaz FJ, Sugiura K, Eppig JJ. Regulation of Pcsk6 Expression During the Preantral to Antral Follicle Transition in Mice: Opposing Roles of FSH and Oocytes. Biol Reprod. 2007 doi: 10.1095/biolreprod.107.063537. [DOI] [PubMed] [Google Scholar]
- 19.Sugiura K, et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 20.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 21.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol. 2007;305:300–311. doi: 10.1016/j.ydbio.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salustri A, et al. Hyaluronic acid synthesis by mural granulosa cells and cumulus cells in vitro is selectively stimulated by a factor produced by oocytes and by transforming growth factor-beta. Journal of Biological Chemistry. 1990;265:19517–19523. [PubMed] [Google Scholar]
- 23.Elvin JA, et al. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Molecular Endocrinology. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 24.Matzuk MM. Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol. 2000;163:61–66. doi: 10.1016/s0303-7207(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 25.Yan C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 26.Elvin JA, Yan C, Matzuk MM. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci U S A. 2000;97:10288–10293. doi: 10.1073/pnas.180295197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varani S, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Molecular Endocrinology. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 28.Fulop C, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- 29.Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- 30.Lofrano-Porto A, et al. Luteinizing hormone beta mutation and hypogonadism in men and women. N Engl J Med. 2007;357:897–904. doi: 10.1056/NEJMoa071999. [DOI] [PubMed] [Google Scholar]
- 31.Qin Y, et al. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet. 2007;81:576–581. doi: 10.1086/519496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledig S, et al. BMP15 mutations in XX gonadal dysgenesis and premature ovarian failure. Am J Obstet Gynecol. 2007 doi: 10.1016/j.ajog.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Suzumori N, Pangas SA, Rajkovic A. Candidate genes for premature ovarian failure. Curr Med Chem. 2007;14:353–357. doi: 10.2174/092986707779941087. [DOI] [PubMed] [Google Scholar]
- 34.Kovanci E, et al. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril. 2007;87:143–146. doi: 10.1016/j.fertnstert.2006.05.079. [DOI] [PubMed] [Google Scholar]
- 35.Chand AL, et al. Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. Fertil Steril. 2006;86:1009–1012. doi: 10.1016/j.fertnstert.2006.02.107. [DOI] [PubMed] [Google Scholar]
- 36.Laissue P, et al. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol. 2006;154:739–744. doi: 10.1530/eje.1.02135. [DOI] [PubMed] [Google Scholar]
- 37.Dixit H, et al. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. 2006;119:408–415. doi: 10.1007/s00439-006-0150-0. [DOI] [PubMed] [Google Scholar]
- 38.Dixit H, et al. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause. 2005;12:749–754. doi: 10.1097/01.gme.0000184424.96437.7a. [DOI] [PubMed] [Google Scholar]
- 39.Di Pasquale E, et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. 2006;91:1976–1979. doi: 10.1210/jc.2005-2650. [DOI] [PubMed] [Google Scholar]
- 40.Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 2004;75:106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 42.Lee KY, et al. Mouse models of implantation. Trends Endocrinol Metab. 2007;18:234–239. doi: 10.1016/j.tem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Lee KY, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paria B, et al. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 45.Dinulescu DM, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 46.Giudice LC. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics. 2004;4:299–312. doi: 10.2165/00129785-200404050-00003. [DOI] [PubMed] [Google Scholar]
- 47.Giudice LC. Elucidating endometrial function in the post-genomic era. Hum Reprod Update. 2003;9:223–235. doi: 10.1093/humupd/dmg019. [DOI] [PubMed] [Google Scholar]
- 48.Giudice LC. Genomics' role in understanding the pathogenesis of endometriosis. Semin Reprod Med. 2003;21:119–124. doi: 10.1055/s-2003-41318. [DOI] [PubMed] [Google Scholar]
- 49.Kao LC, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 50.Burney RO, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 51.Zhao ZZ, et al. KRAS variation and risk of endometriosis. Mol Hum Reprod. 2006;12:671–676. doi: 10.1093/molehr/gal078. [DOI] [PubMed] [Google Scholar]
- 52.Treloar SA, et al. Variants in EMX2 and PTEN do not contribute to risk of endometriosis. Mol Hum Reprod. 2007;13:587–594. doi: 10.1093/molehr/gam023. [DOI] [PubMed] [Google Scholar]