Abstract
Brain plasticity and adult neurogenesis may play a role in many ecologically-important processes including mate recognition, song learning and production, and spatial memory processing. In a number of species, both physical and social environments appear to influence attributes (e.g., volume, neuron number, and neurogenesis) of particular brain regions. The hippocampus in particular is well known to be especially sensitive to such changes. Although social grouping in many taxa includes the formation of male and female pairs, most studies of the relationship between social environment and the hippocampus have typically considered only solitary animals and those living in same-sex groups. Thus, the aim of the present study was to compare the volume of the hippocampal formation, the total number of hippocampal neurons, and the number of immature neurons in the hippocampus (as determined by doublecortin expression) in mountain chickadees (Poecile gambeli) housed in groups of males and females, male-female pairs, same-sex pairs of either males or females, and as solitary individuals. The different groups were visually and physically, but not acoustically, isolated from each other. We found no significant differences between any of our groups in hippocampal volume, the total number of hippocampal neurons, or the number of immature neurons. Our results thus provided no support to the hypothesis that social group composition and/or size have an effect on hippocampal morphology and neurogenesis.
Keywords: social grouping, hippocampus, doublecortin, Poecile gambeli
INTRODUCTION
It is now generally accepted that the brains of adult animals are more malleable than originally thought, and that changes in brain morphology, including the production of new neurons, occur in adults in all major vertebrate taxa (Eriksson et al., 1998; Garcia-Verdugo et al., 2002; Nottebohm, 2002; Kempermann et al., 2004). Moreover, plasticity in various brain regions appears to play a role in processes including spatial memory (e.g., Kempermann, 2002; Nottebohm, 2002), mate selection and/or recognition (Smith et al., 2001; Mak et al., 2007), and seasonal song learning (Nottebohm, 2005).
Within the brain, the hippocampus appears to be especially sensitive to variation in the environment (McEwen, 1999), which may affect volume, the number of neurons, and neurogenesis. For example, adult mice housed in enriched environments (i.e., those with greater physical complexity) have higher levels of neurogenesis (see van Praag et al., 2000 for a review) and larger hippocampi with more neurons than those housed in standard cages (Kempermann et al., 1997). The effects of environmental enrichment may be partly due to increased physical activity, which has been shown to increase neurogenesis in the hippocampus (van Praag et al., 1999a,b). Some of the effects of environmental enrichment on the hippocampal architecture may also be due to hippocampal-dependent experiences related to space use or spatial memory (Gould et al., 1999; van Praag et al., 1999; LaDage et al., 2010). Additionally, reductions in hippocampal attributes and neurogenesis have been linked to cognitive impairment in older individuals (e.g., Drapeu et al., 2003; McEwen, 2007) and to depression and other psychiatric conditions (e.g., Jacobs et al., 2000; Sapolsky, 2004; Malberg and Schechter, 2005), meaning that understanding the factors governing neural plasticity in adult animals is a crucial mental-health issue.
Like the physical environment, some aspects of the social environment may also influence brain attributes, although not much is known about the relationship between social group structure and the hippocampus in particular. It has been reported that both socially-isolated animals and subordinate animals have lower levels of neurogenesis and/or cell proliferation rates than dominants (Fowler et al., 2002; Kozorovitskiy and Gould, 2004; Pravosudov and Omanska, 2005; Sørensen et al., 2007). However, most studies of the relationship between social environment and hippocampal attributes have either compared solitary animals to animals of the same sex housed in unisex groups (e.g., Stranahan et al., 2006; Ibi et al., 2008; Leasure and Decker, 2009) or used either only males or only females to create strong dominance hierarchies (e.g., Pravosudov et al., 2003; Kozorovitskiy and Gould, 2004; Sørensen et al., 2007; Veenema et al., 2007). In addition, the formation of male-female relationships appears to promote neurogenesis in the hippocampus and in other regions of the brain such as the subventricular zone (Smith et al., 2001; Fowler et al., 2002; Baudoin et al., 2005; Hawken et al., 2009). Individuals that are prevented from maintaining species-typical social relationships may experience social stress due to atypical group composition, which may negatively affect hippocampal structure and neurogenesis (e.g., Lu et al., 2003). Furthermore, Hawken et al. (2009) suggests that these changes may be related to the ability to recognize or discriminate between sexual partners. The effects of long-term male-female pair bonds on the brain, however, have been virtually ignored.
It is possible that the effects of the social environment may depend on group size rather than group composition alone. Larger groups are by definition associated with increased social complexity, which has been linked to enhancements in brain attributes (Barnea et al., 2006; Emery et al., 2007). The need to remember more individuals, for example, may result in increased neuron survival (Lipkind et al., 2002). It is also possible that living in isolation or in smaller groups may be associated with increased social stress in species that typically occur in large groups (Takeda et al., 2003; Kikusui et al., 2006).
The aim of the present study was to investigate how social group composition and/or size affects hippocampal formation (referred to as the hippocampus from now on) volume, the number of hippocampal neurons, and the number of immature neurons in the hippocampus in mountain chickadees (Poecile gambeli). Chickadees are socially monogamous birds that maintain pair bonds throughout the year (i.e., during both the breeding and non-breeding seasons; e.g., Ekman, 1989). During the winter, chickadees form large social groups consisting of multiple male-female pairs that tend to maintain visual contact and the potential for physical interaction (Ekman, 1989; Lemmon et al., 1997).
We compared the hippocampal attributes of birds living in aviary groups consisting of two males and two females, birds living in single male-female pairs, birds living in single unisex (male-male and female-female) pairs, and solitary birds. We expected that birds living in atypical social groupings (solitary, same-sex pairs) should have smaller hippocampi and fewer hippocampal neurons, and should exhibit fewer immature neurons in the hippocampus than birds housed in male-female groups. It is also possible that differences in hippocampal attributes may be driven by group size. In this case, when chickadees are maintained under short-day (winter) photoperiod we would expect to see differences between aviary birds and paired birds and between paired birds and solitary birds.
METHODS
Subjects and Initial Housing
Subjects were 94 juvenile mountain chickadees (52 males and 42 females), captured on Sept. 11–12, 2007 (24 males and 24 females) and Sept. 8–9, 2008 (28 males and 18 females) around Sagehen Creek, Tahoe National Forest, CA (near Truckee, CA). Birds for this study were captured at a network of 40 feeders situated at spatially distinct locations spread over a distance of approximately 11 km over two forest roads. When birds were captured, they were individually marked with a unique combination of two colored leg bands on the left leg, and wing chord length was measured to the nearest 0.5 mm.
Birds were transferred to the laboratory at the University of Nevada, Reno, and were housed individually in wire-mesh cages (60 × 42 × 60 cm) which were visually and physically, though not acoustically, isolated by solid metal partitions between cages. Birds were initially housed on 11.5 h : 12.5 h light/dark cycle, which was gradually changed to 9.5 h : 14.5 h light/dark. The photoperiod was initially changed to 11 h : 13 h light/dark on 8 October in 2007 and 2008, further changed to 10 h : 14 h light/dark on 25 October 2007 and 28 October 2008 , and set to its final ratio (9.5 h : 14.5 h light/dark) on 23 November 2007 and 1 December 2008 to resemble natural photoperiod. Rooms were maintained at a constant 20°C temperature, and a mixture of pine nuts, shelled and unshelled sunflower seeds, crushed peanuts, Roudybush bird pellets (Roudybush Inc., Woodland, CA) and water was available ad libitum. Each bird was also given 6–10 mealworms daily.
Genetic Sexing
After 7 days in captivity, we collected 1 capillary tube (~75 μl) of blood from the brachial vein of each bird for genetic sex determination. DNA was extracted from samples using a Qiagen DNEasy kit (Qiagen Inc.,Valencia, CA). Sex was determined by amplifying a portion of the sex-linked CHD genes (CHD-W in females only and CHD-Z in both sexes) in a polymerase chain reaction using microsatellite primers P2 and P8 (Griffiths et al., 1998). Sex was confirmed by visual examination of the gonads after birds were sacrificed at the end of the study. Results of the visual and genetic sexing matched 100%.
Social Group Treatments
On 19 October 2007 and 20 October 2008 (approximately six weeks after initial capture), all birds captured during that year were, within sexes, randomly assigned to one of five treatment groups: aviary, male-female pair, male-male pair, female-female pair, or solitary. Birds in the “aviary” group were housed two males and two females (four birds) per aviary (122 × 70 × 70 cm). Aviaries were equipped with natural fir branches for perching, four water dishes, and eight feeders. A large water dish in the bottom of each aviary was provided for bathing. Aviaries were placed two to a room, with a cloth covering on one wall of the aviary preventing visual but not acoustic contact with birds in the other aviary. We formed 8 aviary groups (4 aviaries in 2007 and 4 aviaries in 2008, containing a total of 16 males and 16 females).
Members of pairs shared a single 120 × 42 × 60 cm cage equipped with a dish for bathing, a water bottle, two feeders, and two perches). Pair cages were visually and physically, but not acoustically, isolated from cages holding other pairs and solitary birds. We formed 8 male-female pairs (four in 2007 and four in 2008; a total of 16 birds), 8 male-male pairs (four in 2007 and four in 2008; a total of 16 birds), and 7 female-female pairs (four in 2007 and three in 2008; a total of 14 birds). The remaining 16 birds (12 males and 4 females) were assigned to the ‘solitary’ group (8 males in 2008; 4 males and 4 females in 2007). Solitary birds were housed in the individual cages that were half the size of pairs’ cages (60 × 42 × 60 cm). All cages (single, double and aviary) provided approximately the same space volume per bird. Within aviary and same-sex male groups, we determined dominance status of all males following our previously published methods (Pravosudov and Omanska 2005). In male-female pairs, the male is always dominant; however, we did not determine dominance in female-female pairs because females rarely express aggression towards each other.
Nissl Staining and Immunohistochemistry
Between 30 January and 1 February 2008, and between 31 January and 1 February 2009 (when birds were approximately 7–8 months of age), birds captured the previous fall were perfused, and their brains were removed, sectioned, and processed as described elsewhere (Pravosudov and Omanska, 2005; LaDage et al., 2009). Briefly, birds were anesthetized with a lethal overdose of Nembutal (0.07 ml of 50 mg/ml sodium pentobarbitol), then transcardially perfused with 0.1 M phosphate buffered saline (PBS) for 10 min followed by 4% paraformaldehyde in 0.1 M PBS for another 15–20 min. Brains were extracted and post-fixed in 4% paraformaldehyde solution for 24 h, then cryoprotected in 15% sucrose followed by 30% sucrose. After cryoprotection, brains were flash-frozen on dry ice and stored at −80°C until sectioning.
Brains were sectioned every 40 μm in the coronal plane using a cryostat (Leica CM 3050S, Bannockburn, IL) at −20°C, and sections were collected in four series. Sections from the first series were Nissl-stained using thionin, and sections from the second series were processed for doublecortin (DCX), an endogenous marker of immature neuronal phenotype (i.e., neurogenesis; Brown et al., 2003; Rao and Shetty, 2004; Couillard-Després et al., 2005; Hairston et al., 2005; Balthazart et al., 2008; LaDage et al., 2010). In passerine birds, doublecortin expression occurs for at least 25–30 days after a neuron is born and ceases when neurons begin expressing a mature phenotype (Balthazart et al., 2008). Therefore, our analysis of neurogenesis only concerns the population of immature neurons less than 30 days of age. Because social groups were formed and maintained for almost three months, the use of doublecortin effectively rules out any possibility that our results were affected by group formation, but rather reflects the effect of group maintenance. We have previously used doublecortin as a marker of neurogenesis in mountain chickadees and have shown that ecologically-relevant spatial memory use increases the number of cells expressing doublecortin specifically in the hippocampus in captive mountain chickadees (LaDage et al., 2010).
To visualize doublecortin, sections were washed in Tris-buffered saline (TBS), incubated in 30% hydrogen peroxide plus TBS (1:50) at room temperature for 30 min, washed in TBS, incubated in blocking buffer (normal horse serum (1:33.3), TX-100 (1:39.8) and TBS) at room temperature for 30 min, and then incubated in anti-doublecortin antibody plus blocking buffer (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, SC-8066) overnight (~18 h) at 4oC. The following day, sections were washed in TBS, incubated in biotinylated horse anti-goat antibody in blocking buffer (1:200; Vector Laboratories, Burlingame, CA, BA-9500) at room temperature for 2 h, washed in TBS, incubated in ABC Elite kit (Vector Laboratories, Burlingame, CA, PK-6100) at room temperature for 1 h, washed in TBS, reacted with DAB+nickel kit (Vector Laboratories, Burlingame, CA, SK-4100) at room temperature for 2 min, washed in TBS, and mounted on slides. Slides were dried, lightly Nissl-stained and coverslipped. DCX-expressing neurons are readily identified in stained sections, as they stain quite darkly relative to surrounding Nissl-stained cells (Fig. 1). Omitting primary antibody resulted in no staining in control tissues (LaDage et al., 2010).
Figure 1.
Representative example of doublecortin labeling in the hippocampus of captive chickadees.
The boundaries of the hippocampus were determined by the change in density of Nissl-stained cells at the boundary, as in Krebs et al. (1989) and our previous studies (Pravosudov and Clayton, 2002; Pravosudov et al., 2002; Pravosudov and Omanska, 2005; LaDage et al., 2009; Roth and Pravosudov, 2009). The hippocampus is also bounded by the midline and ventrally by the septum (Krebs et al. 1989).
All slides were scored blind to treatment and measurements were made using standard stereological methods (StereoInvestigator, MicroBrightfield, Inc.; Leica M4000B microscope). In Nissl-stained sections from the first series, we measured the hippocampal volume, the telencephalon volume (telencephalon volume minus hippocampal volume), and neuron numbers in the hippocampus. In sections from the second series, which had been processed for doublecortin, we measured the number of hippocampal neurons expressing doublecortin. Volumes of the telencephalon and the hippocampus were measured in their entirety and estimated using the Cavalieri procedure (Gundersen and Jensen, 1987). Following our previous work (Pravosudov et al., 2002; Pravosudov and Omanska, 2005; LaDage et al., 2009), the hippocampal volume was measured with a 200 μm grid and the telencephalon volume was measured with a 1200 μm grid. The total number of hippocampal neurons were estimated using Nissl-stained slides, an optical fractionator procedure at 1000 × (West et al., 1991), and StereoInvestigator software. The optimal grid size (250 μm), counting frame (30 × 30 μm), dissector height (5 μm), and frequency of section sampling (12) have previously been established for mountain chickadees (Pravosudov et al., 2002; Pravosudov and Omanska, 2005). All estimations used actual thickness of mounted sections as required by the optical fractionator procedure (West et al., 1991). Counts of neurons expressing doublecortin were also performed using the same procedure, except with a counting frame size of 70 × 70 μm, as established by LaDage et al. (2010). The left and right hemispheres were both measured for the hippocampus and telencephalon volumes, hippocampal neuron numbers, and the number of hippocampal neurons expressing doublecortin, and summed to produce the values given in this paper.
Statistical analyses
Data were analyzed using SAS PROC MIXED (SAS Systems, Cary NC; SAS Institute, 2008). All analyses were conducted using an unbalanced repeated-measures design with “cage” as the repeated factor (i.e., data from all birds that were housed in the same cage or aviary were treated as repeated measurements), as birds housed in the same enclosure could not be considered statistically independent. . Year (2007 or 2008), group (aviary, male-female pair, female-pair, male-male pair, or solitary), and group x year score were included as factors in all models. When telencephalon volume was the dependent variable, wing length was also included as a covariate to account for variation due to body size. In the models for hippocampal volume and number of neurons in the hippocampus, telencephalon volume was included to account for variation due to brain size. The level of significance was set as α = 0.05 for all tests. Reported means are least squares means ± SE.
For all major non-significant results we determined if our tests had the statistical power to detect differences determined from previous studies on chickadees. Previous studies reported significant differences in the hippocampal volume ranging from 26% to 30% (Smulders et al. 1995; LaDage et al. 2009) and so we used 26% for our power analyses. The significant differences detected in the number of hippocampal neurons have been reported to range from 26% to 45% (Smulders et al. 1995, 2000) and so we used 26% for our power analyses. The significant differences detected in hippocampal neurogenesis rates have been reported to range from 40% to 72% using cell division markers (Barnea and Nottebohm 1994; Pravosudov and Omanska 2005) and from 16% to 63% using doublecortin (LaDage et al. 2010). We therefore used 16% for our power analyses for the results with doublecortin. We used the program G*Power (Faul et al. 2007) to conduct post-hoc power analyses for a repeated-measures ANOVA (between factors), using the mean of the brain measures for solitary birds as the baseline value. To estimate standard deviation, we used the group with the largest standard deviation for that brain measure.
Ethical note
All procedures were conducted under Animal Use and Care Protocol #A05/06-39, approved by the University of Nevada, Reno Institutional Animal Care and Use Committee and complied with APA ethical standards for the treatment of animals. Birds were collected under the U.S. Federal Fish and Wildlife (MB022532) and California State (802017-05) scientific collecting permits. We regularly observed more than 20 birds at each feeder, and estimate that we had more than 800 chickadees coming to the feeder grid in a given year. Removal of chickadees for this experiment had no detectable effect on the local population (Pravosudov, pers. observ.).
RESULTS
Telencephalon Volume
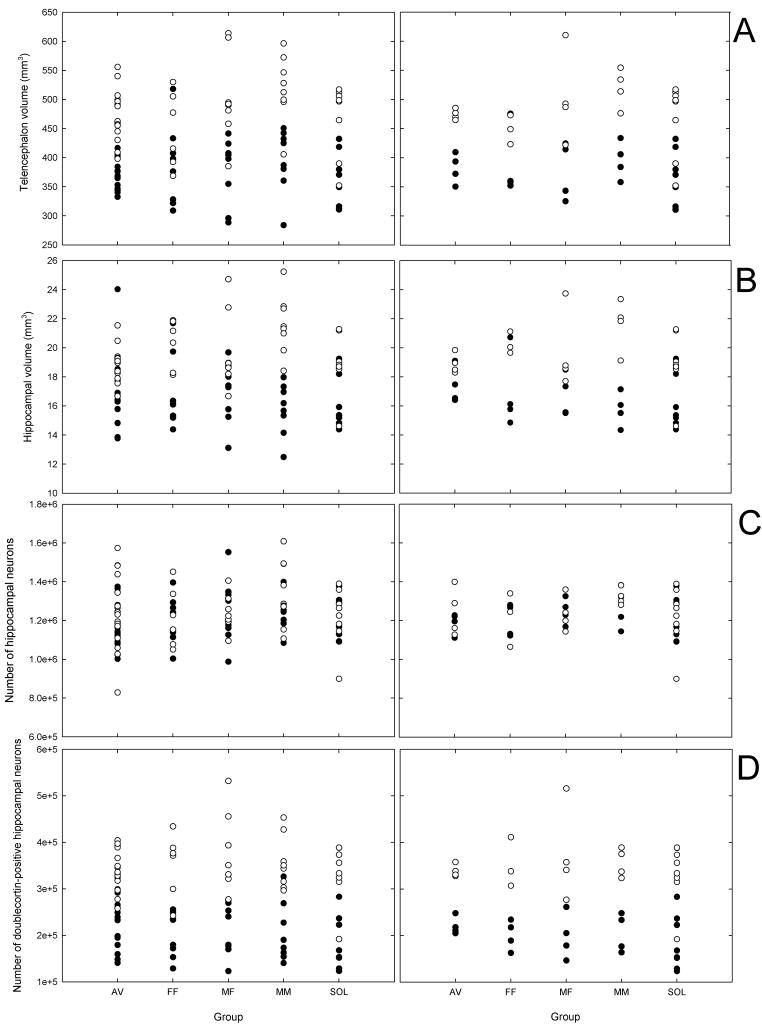
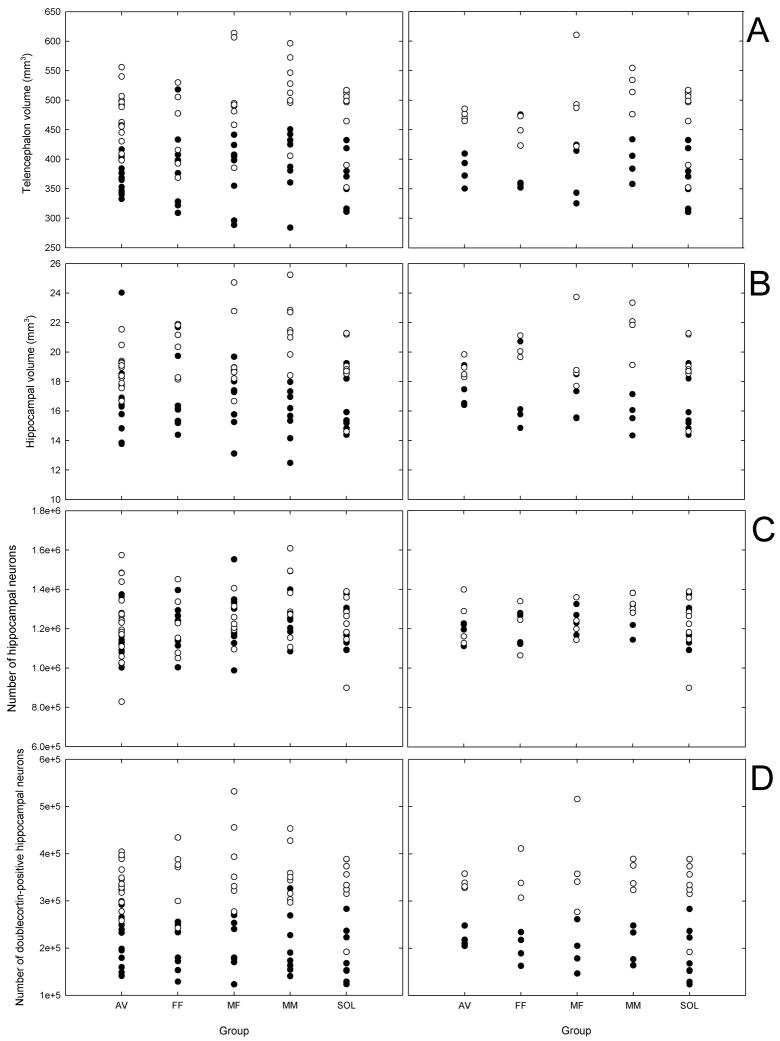
Body size, group and the group by year interaction were non-significant predictors of telencephalon volume (Table 1; Fig. 2a). Statistical power to detect 10% differences in the telencephalon volume among our experimental groups was 0.99. However, there was a significant effect of year on telencephalon volume (Table 1), with birds captured in 2008 having larger telencephalon volume relative to body size (average relative telencephalon volume: 482.15 ± 8.59 mm3) than those captured in 2007 (380.46 ± 8.31 mm3).
Table 1.
Effect of grouping on the telencephalon volume, the HF volume, the total number of HF neurons and the total number of DCX-positive neurons in the HF of mountain chickadees. Significant effects are in boldface type.
| Telencephalon volume | HF volume | Total number of neurons in the HF | Number of DCX-positive neurons in the HF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | F | df | p | F | df | p | |
| Year | 54.47 | 1,37 | <0.001 | 2.46 | 1,37 | 0.13 | 1.82 | 1,37 | 0.18 | 105.85 | 1,37 | <0.001 |
| Group | 1.43 | 4,37 | 0.24 | 0.74 | 4,37 | 0.57 | 0.36 | 4,37 | 0.84 | 0.43 | 4,37 | 0.78 |
| Year*Group | 0.62 | 4,37 | 0.65 | 3.94 | 4,37 | 0.009 | 0.31 | 4,37 | 0.87 | 0.46 | 4,37 | 0.76 |
| Telencephalon volume | n/a | n/a | n/a | 60.86 | 1,46 | <0.001 | 7.97 | 1,46 | 0.007 | n/a | n/a | n/a |
| Wing length | 0.38 | 1,46 | 0.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Figure 2.
Measurements of hippocampal attributes and telencephalon in aviary birds (AV), male-female (M–F) pairs, female-female (F-F) pairs, male-male (M-M) pairs, and solitary birds (SOL) plotted for all individuals and for cage averages. Closed circles represent data collected in 2007, and open circles represent data collected in 2008.
(a) Telencephalon (minus the hippocampus) volume (mm3).
(b) Volume of the hippocampus (mm3).
(c) Total number of neurons in the hippocampus.
(d) Total number of doublecortin neurons (i.e., immature neurons) in the hippocampus.
Hippocampal Formation Volume
Hippocampal volume was significantly and positively related to telencephalon volume, but there were no significant group or year effects (Table 1; Fig 2b; statistical power to detect significant differences among the groups was 1.0). There was a significant group × year interaction (Table 1), which is likely to have been a spurious effect of the fact that males randomly assigned to male-male pairs had, on average, the smallest hippocampus in 2007 and the largest hippocampus in 2008.
Number of Neurons
There were no significant differences among groups in the total number of hippocampal neurons relative to telencephalon volume (Fig. 2c; statistical power to detect significant differences among the groups was 1.0). The only significant effect in the model was that of telencephalon volume (Table 1).
Number of Neurons Expressing Doublecortin
Year of capture was the only significant effect on the number of hippocampal neurons expressing doublecortin with more doublecortin-labeled cells during the second year (Table 1; Fig. 2d). The effect of group and the group × year interaction were non-significant (Table 1; statistical power to detect significant differences among the groups was 0.99).
The Effects of Dominance and Sex
Our main results were based on a repeated-measures analysis, which averages individuals within groups. However, it is possible that the actual composition of the groups (e.g., dominance status) may be affected by grouping differently. Thus, it may mask the group effect. To determine if dominance or sex had an effect on hippocampal attributes or hippocampal neurogenesis, we ran several additional repeated-measures analyses. The first analysis used sex and dominance status of individuals within groups as factors. The second analysis used only a single dominant or subordinate individual from each group as independent units. In addition, we also ran a mixed general linear model with social composition, sex, and year as fixed factors and cage as a random intercept and random coefficient simultaneously. All of these analyses yielded a non-significant effect of group on telencephalon volume, hippocampal volume, the total number of hippocampal neurons, and the number of doublecortin-labeled neurons (all p’s > 0.20). We also performed the above analyses without using telencephalon volume or body size as covariates (for the hippocampal attributes and telencephalon volume, respectively). These analyses also showed no significant effect of grouping (all p’s > 0.26).
DISCUSSION
Our results provided no support for the hypothesis that social group composition and/or group size have an effect on hippocampal morphology and neurogenesis, as we found no significant differences in hippocampal attributes among our experimental groups maintained in visual and physical isolation.
Although we did not expect to see significant variation in brain attributes between years, we found a strong effect of year on both telencephalon volume and the number of doublecortin-expressing neurons in the hippocampus. We cannot pinpoint the source of this variation as these birds came from a wild population. Unknown differences in the physical and social environment prior to capture could have contributed to the variation between years. For example, differences in food abundance during early development have been shown to affect hippocampal attributes (e.g., Pravosudov et al., 2005). It is also possible that slight variation in histological procedures between the years resulted in these differences.
There are several potential reasons that may explain the negative results of our study. It is theoretically possible that we did not have a sufficient sample size to detect biologically meaningful differences. We do not think that this explanation is likely because our sample size (94 birds in 47 independent cages) was one of the largest of any other published study of hippocampal attributes. Further, we have previously shown significant effects of other manipulations (e.g., dominance status, memory use) on cell proliferation and neurogenesis in the hippocampus of captive mountain chickadees using much smaller sample sizes (Pravosudov and Omanska, 2005; LaDage et al., 2010). Furthermore, we had sufficient statistical power (> 0.9) for all of our tests, which suggests that our sample size was sufficient to detect statistical differences among the groups.
Yet another possible explanation for our negative findings may be related to the fact that our groups were visually and physically, but not acoustically, isolated from one another. Exposure to the vocalizations of familiar conspecifics has been shown to attenuate the physiological stress response associated with housing animals in social isolation, a process termed “vocal buffering” (Rukstalis and French, 2005). Chickadees live in male-female pairs within non-breeding social groups and they maintain frequent visual contact and the potential for physical interaction (Ekman 1989; V. Pravosudov, pers. observations), even if in some cases they rely on acoustical contact alone. It is possible that having acoustic interactions alone without the ability for visual or physical interaction may have been even more stressful than total isolation. Therefore, we think that the physical and visual isolation in our study provides highly unnatural (and thus stressful) social conditions, even if the birds are able to hear one another. Nonetheless, additional acoustic isolation might have provided an additional stressor, which was not present in our study; therefore, our study is limited to the effects provided only by visual and physical isolation.
Chickadees form a dominance hierarchy in their social groups (Ekman 1989) and thus averaging individuals within the groups could mask potential differences between dominant and subordinate individuals and the effects of different grouping on brain attributes (Pravosudov and Omanska 2005). However, both including dominance status as a factor and analyzing only dominant or subordinate individuals from each group yielded no significant effects of group on any of the measured brain parameters. Therefore, we are reasonably confident that our negative results were not produced by the differences between dominants and subordinates. Interestingly, our results did not support our previous study showing that subordinates have lower hippocampal cell proliferation rates (Pravosudov and Omanska 2005). However, there were a number of differences between these studies that may have contributed to this inconsistency. First, Pravosudov and Omanska (2005) specifically measured cell proliferation rates using BrdU labeling and so they measured only production of all new cells, not just new neurons. Our current study measured a population of new neurons generated over several weeks. Second, this study was not designed to detect differences between dominants and subordinates and the variance between groups and a relatively small sample size of groups with male-male and female-female pairings may have prevented us from detecting potential differences. More studies will be needed to test specifically whether dominance status has a direct effect on neuron production and survival.
It is also possible that our use of doublecortin prevented us from discovering differences in long-term neuronal survival. Studies have shown that BrdU and doublecortin estimates are parallel when measured in response to treatments known to increase neurogenesis (Nacher et al., 2002; Couillard-Després et al., 2005), and thus, doublecortin has been used as an alternative to BrdU to investigate neurogenesis (e.g., Brown et al., 2003; Balthazart et al., 2008; LaDage et al., 2010). Doublecortin labels only immature neurons about 20–30 days old (Balthazart et al., 2008), and thus our results can only be extended to the population of surviving immature neurons that were produced within 30 days of sacrifice. We have already shown that doublecortin is effective in detecting significant effects of environmental manipulations other than social grouping on the number of immature neurons in the HF of captive mountain chickadees (LaDage et al., 2010). However, we cannot measure neuronal survival beyond 30 days using doublecortin, and thus it remains possible that social group composition and/or size may still have a longer-term effect on neuronal survival.
Many previous studies have documented an effect of the social environment on neurogenesis in the brain (Tramontin et al., 1999; Fowler et al., 2002; Lipkind et al., 2002; Mak et al., 2007; Sørensen et al., 2007; Hawken et al., 2009), but only a few of those detected such differences in the hippocampus (Kozorovitskiy and Gould, 2004; Pravosudov and Omanska, 2005). Interestingly, Fowler et al. (2002) failed to detect a significant effect of social isolation on neurogenesis in the hippocampus of prairie voles (Microtus ochrogaster), but reported such effects on other brain areas. It is also possible that the effects of social environment on hippocampal neurogenesis are time specific and may not be long-lived. In zebra finches, for example, the effect of social separation on hippocampal neurogenesis was only significant at 40, but not at 60 or 150 days following social separation (Barnea et al. 2006).
Most other studies have also focused on neurogenesis in brain regions other than the hippocampus such as the subventricular zone, amygdala, and forebrain, and nuclei in the song system (HVC and RA) in songbirds (Tramontin et al., 1999; Fowler et al., 2002; Lipkind et al., 2002; Mak et al., 2007; Sørensen et al., 2007; Hawken et al., 2009). Therefore, it remains possible that the social group composition/size may not influence the hippocampus while providing strong effects on other brain areas. Measuring neurogenesis in other brain regions would be interesting, but it was beyond the scope of our study.
In summary, the results of the present study suggest that social group composition and group size do not seem to influence the volume of the hippocampus, the number of hippocampal neurons, or the number of immature neurons in the hippocampus of mountain chickadees. These findings suggest that the effects of social grouping may be unrelated to the hippocampus and may be specific to other brain regions involved in mediating social interactions. Nonetheless, it remains uncertain whether longer-term neuronal survival in the hippocampus may still be affected by the social environment. Future studies would be needed to address this question.
Acknowledgments
This study was supported by grants from the National Institutes of Health (MH079892 and MH076797) and the National Science Foundation (IOB-0615021) to Vladimir Pravosudov. We thank Geniveve Hanson, Leia Chancellor, Kathleen Cornfield, and Ashley Rolfe for help in bird maintenance and in running the experiment.
References
- Balthazart J, Boseret G, Konkle ATM, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci. 2008;27:801–817. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci USA. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Mishal A, Nottebohm F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: an anatomical representation of time? Behav Brain Res. 2006;167:63–74. doi: 10.1016/j.bbr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Baudoin C, Busquet N, Dobson FS, Gheusi G, Feron C, Durand J-L, Heth G, Patris B, Todrank J. Male-female associations and female olfactory neurogenesis with pair bonding in Mus spicilegus. Biol J Linn Soc. 2005;84:323–334. [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Couillard-Després S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn H-G, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Drapeu E, Mayo W, Arousseu C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Nat Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman J. Ecology of non-breeding social systems of Parus. Wilson Bull. 1989;101:263–288. [Google Scholar]
- Emery NJ, Seed AM, von Bayern AMP, Clayton NS. Cognitive adaptations of social bonding in birds. Phil Trans R Soc B. 2007;362:489–505. doi: 10.1098/rstb.2006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordberg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Faul F, Erfelder E, Buchner A, Lang A-G. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;41:1149–1160. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fowler C, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Mol Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its predictions. J Microsc-Oxford. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Little MTM, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hawken PAR, de St Jorre JJ, Rodger J, Esmaili T, Blache D, Martin GB. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod. 2009;80:1146–1151. doi: 10.1095/biolreprod.108.075341. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T, Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;3:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psych. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Phil Trans R Soc B. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. Hippocampal specialization of food-storing birds. Proc Nat Acad Sci USA. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDage LD, Roth TC, Fox RA, Pravosudov VV. Effects of captivity and memory-based experiences on the hippocampus in mountain chickadees. Behav Neurosci. 2009;123:284–291. doi: 10.1037/a0014817. [DOI] [PubMed] [Google Scholar]
- LaDage LD, Roth TC, Fox RA, Pravosudov VV. Ecologically-relevant spatial memory use modulates hippocampal neurogenesis. Proc R Soc B. 2010 doi: 10.1098/rspb.2009.1769. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Lemmon D, Withiam ML, Barkan CPL. Mate protection and winter pair-bonds in black-capped chickadees. Condor. 1997;99:424–433. [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav Brain Res. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- Lu L, Bao B, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Ann Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Schechter LE. Increasing hippocampal neurogenesis: a novel mechanism for antidepressant drugs. Curr Pharm Design. 2005;11:145–155. doi: 10.2174/1381612053382223. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2002;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Why are some neurons replaced in adult brain? J Neurosci. 2002;22:624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F. The neural basis of birdsong. PLOS Biol. 2005;3:e164. doi: 10.1371/journal.pbio.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov VV, Clayton NS. A test of the adaptive specialization hypothesis: population differences in caching, memory and the hippocampus in black-capped chickadees (Poecile atricapilla) Behav Neurosci. 2002;116:515–522. [PubMed] [Google Scholar]
- Pravosudov VV, Omanska A. Dominance-related changes in spatial memory are associated with changes in hippocampal cell proliferation rates in mountain chickadees. J Neurobiol. 2005;62:31–41. doi: 10.1002/neu.20065. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Lavenex P, Clayton NS. Changes in spatial memory mediated by experimental variation in food supply do not affect hippocampal anatomy in mountain chickadees (Poecile gambeli) J Neurobiol. 2002;51:142–148. doi: 10.1002/neu.10045. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Mendoza SP, Clayton NS. The relationship between dominance, corticosterone, memory, and food caching in mountain chickadees (Poecile gambeli) Horm Behav. 2003;44:93–102. doi: 10.1016/s0018-506x(03)00119-3. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Lavenex P, Omanska A. Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behav Neurosci. 2005;119:1368–1374. doi: 10.1037/0735-7044.119.5.1368. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyze the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Roth TC, Pravosudov VV. Hippocampal volume and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc R Soc B. 2009;276:401–405. doi: 10.1098/rspb.2008.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm Behav. 2005;47:1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Why Zebras Don’t Get Ulcers. New York: Henry Holt; 2004. p. 564. [Google Scholar]
- SAS Institute, Inc. SAS 9.2 User’s Guide. Cary, NC: SAS Institute, Inc; 2008. [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm Behav. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Sasson AD, DeVoogd TJ. Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee. J Neurobiol. 1995;27:15–25. doi: 10.1002/neu.480270103. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Shiflett MW, Sperling AJ, DeVoogd TJ. Seasonal changes in neuron numbers in the hippocampal formation of a food-hoarding bird: the black-capped chickadee. J Neurobiol. 2000;44:414–422. doi: 10.1002/1097-4695(20000915)44:4<414::aid-neu4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Sørensen C, Øverli Ø, Summers CH, Nilsson G. Social regulation of neurogenesis in teleosts. Brain Behav Evol. 2007;70:239–246. doi: 10.1159/000105487. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khali D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K-I, Sato S, Sugawara K. Familiarity and group size affect emotional stress in Japanese black heifers. Appl Anim Behav Sci. 2003;82:1–11. [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Nat Acad Sci USA. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Veenema AH, de Kloet ER, de Wilde MC, Roelofs AJ, Kawata M, Buwalda B, Neumann ID, Koolhaas JM, Lucassen PJ. Differential effects of stress on adult hippocampal cell proliferation in low and high aggressive mice. J Neuroendocrinol. 2007;19:489–498. doi: 10.1111/j.1365-2826.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]