Abstract
Purpose
The purpose of this study was to evaluate the levels of neutrophil gelatinase-associated lipocalin (NGAL) in the aqueous humor in eyes with idiopathic acute anterior uveitis (AAU).
Methods
A comparative control study. Aqueous humor was collected from 20 eyes of 20 patients with idiopathic AAU. The control group included 20 aqueous samples from 20 patients about to undergo cataract surgery and without any other ocular or systemic diseases. The level of NGAL was determined with a commercially available ELISA kit.
Results
The concentration of NGAL in aqueous humor was markedly higher in patients with idiopathic AAU than in control subjects (Mann–Whitney U test, p<0.001). The level of NGAL was 139,197.38±183,426.36 (mean±SD) pg/ml in eyes with AAU and 3,169.96±1,595.78 pg/ml in the eyes of the control group.
Conclusions
The aqueous humor NGAL level is increased in eyes with idiopathic AAU. These results imply that NGAL is associated with the regulation of inflammation in patients with AAU and could be used as a biomarker of ocular inflammation and immunomodulatory treatment response.
Introduction
The term “uveitis” is used to describe a group of diseases characterized by inflammation of intraocular structures. In the United States, uveitis is reportedly responsible for an estimated 30 000 new cases of legal blindness annually and causes 2.8% to 10% of cases of blindness [1,2], with a prevalence reported as high as 115,3 cases per 100,000 [2]. The non-infectious uveitis can be associated with autoimmune systemic diseases such as Behçet’s disease, sarcoidosis, spondyloarthropathies, and Vogt-Koyanagi-Harada syndrome, or unclassified uveitis are labeled idiopathic. Although posterior uveitis (affecting the posterior segment) describes a range of different clinical entities, all forms are similar immunohistologically, characterized by an infiltration of mainly lymphocytes T CD4+ (CD4+ T) cells [3].
Idiopathic acute anterior uveitis (AAU), in which there is often a severe inflammatory response in the anterior chamber, or front of the eye, is the most common type that occurs in the general population. The disease’s severity and course vary between individuals, and some patients have ocular complications that can threaten sight. Many different cytokines have been identified in the inflamed eye, including tumor necrosis factor (TNF)-α [4,5].
Neutrophil gelatinase-B associated lipocalin (Lcn2/NGAL or NGAL) is a 21-kD protein of the lipocalin superfamily. NGAL is siderophore-binding antimicrobial protein that is upregulated in epithelial tissues during inflammation and seems to play an important role in this process [6]. This protein is upregulated in several pathological conditions, including cancers [7], inflammation bowel disease [8], nephritis [9], acute kidney injury (AKI) [9], heart failure [10], autoimmune myocarditis [11], polyps [12], preeclampsia [13], arthritis [14], and pancreatitis [15]. Several studies have shown NGAL to be a useful biomarker for early detection of AKI in post-cardiac surgery, nephritis, and radiocontrast exposure [9].
The objective of this study was to measure the levels of NGAL in the aqueous humor of patients with idiopathic AAU and its possible implication as a regulator of inflammation in the acute phase of the uveitis.
Methods
This comparative control study investigates the levels of NGAL in the aqueous humor of patients with idiopathic AAU. Control samples of aqueous humor were taken of cataract patients without other ocular or systemic disease. The study protocol complied with the Helsinki Declaration and was reviewed and approved by the Ethical Committee of our tertiary care hospital. An informed consent was obtained from each patient.
Aqueous humor samples were collected from 20 eyes of 20 patients with idiopathic AAU in their fist documented episode. A battery of serological and laboratory tests were performed in all patients to rule out any other ocular or systemic disease. Control aqueous humor samples were obtained from 20 eyes of 20 patients undergoing cataract surgery and without any other ocular or systemic confounding disease.
Aqueous samples of patients with AAU were collected under sterile conditions using a 30-gauge needle under the slit lamp and applying povidone-iodine before and after anterior chamber puncture. Topical antibiotic prophylaxis was used for 5 days after the sample was taken. The aqueous humor was collected from the controls with a 30-gauge needle before the start of cataract surgery. Samples were collected in volumes of at least 0.05 ml per patient, kept in sterile tubes, and stored immediately at −80 °C until use. NGAL levels were quantified by enzyme-linked immunosorbent assay (ELISA) of aqueous humor (Searchlight Array; Pierce Biotechnology, Inc., Woburn, MA). The demographic characteristics of patients were studied with the statistical program SPSS (SPSS Inc., Chicago, IL) for Windows. The Mann–Whitney U test for independent samples was applied to compare the levels of NGAL in the study groups, accepting p<0.05 as statistically significant.
Results
We analyzed a total of 20 samples of aqueous humor of 20 patients with idiopathic AAU. The age of this group was 47±3.2 (mean±SD) years and 30% were women. Similarly we studied 20 samples of 20 patients that underwent cataract surgery, which constituted the control group, with an age of 55±2.7 (mean±SD) years, 40% were women.
The observed level of NGAL in eyes with idiopathic AAU was 139,197.38±183,426.36 pg/ml (mean±SD) and 3,169.96±1,595.78 pg/ml in the eyes of the control group (Table 1 and Table 2). The NGAL levels were significantly different between groups (Mann–Whitney U Test, p<0.001) in which AAU were significantly higher than that of the control group (Figure 1).
Table 1. Levels of neutrophil gelatinase-associated lipocalin (NGAL) in aqueous humor of all the patients.
|
Neutrophil gelatinase-associated lipocalin levels in aqueous humor (pg/ml) |
||
|---|---|---|
| Patient | Acute anterior uveitis group | Control group |
| 1 |
12307.5 |
5030.2 |
| 2 |
500000 |
2157 |
| 3 |
124925 |
2565.3 |
| 4 |
433957.2 |
1264.3 |
| 5 |
500000 |
1365.5 |
| 6 |
81156.4 |
891.1 |
| 7 |
35275.6 |
3116.9 |
| 8 |
13484.5 |
7163.9 |
| 9 |
17411.9 |
5115.1 |
| 10 |
14422.5 |
1685 |
| 11 |
6610.3 |
1800 |
| 12 |
12704.3 |
2100 |
| 13 |
11474.8 |
2360 |
| 14 |
21760.2 |
4685 |
| 15 |
77611.4 |
4400 |
| 16 |
500000 |
4215 |
| 17 |
6253.8 |
4050 |
| 18 |
136100 |
3185 |
| 19 |
140294.8 |
3400 |
| 20 |
138197.4 |
2850 |
| 139197.38±183426.36 pg/ml (mean±SD)* | 3169.96±1595.78 pg/ml (mean±SD)* | |
The asterisk indicates a statistically significant difference between groups (Mann–Whitney U test; p<0.001)
Table 2. Results of the levels of neutrophil gelatinase-associated lipocalin (NGAL) in aqueous humor in each group.
| Group | Aqueous humor level of NGAL (pg/ml) mean ± SD |
|---|---|
| AAU (n=20) |
139197.38 ± 183426.36 |
| |
p<0.001* |
| Control (n=20) | 3169.96 ± 1595.78 |
The group of patients with idiopathic acute anterior uveitis (AAU) had higher NGAL levels than the control group. NGAL=Neutrophil gelatinase-associated lipocalin; AAU=Acute Anterior Uveitis. *Compared with Control group (Mann–Whitney U test).
Figure 1.
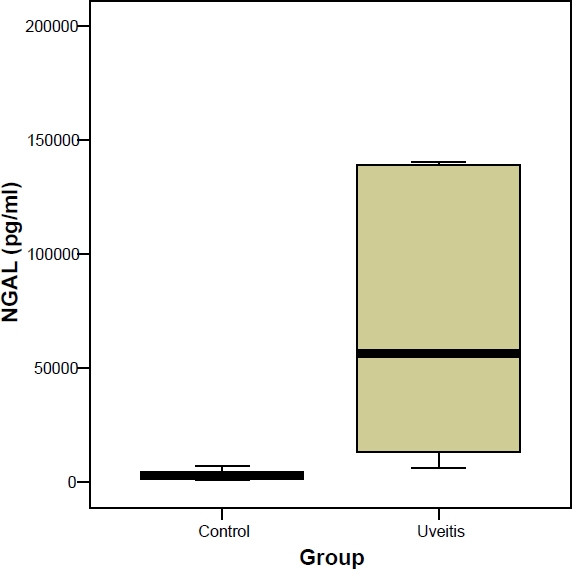
Levels of neutrophil gelatinase-associated lipocalin (NGAL) in aqueous humor of 20 eyes with idiopathic acute anterior uveitis (AAU) and 20 control eyes. NGAL levels were different in both groups; patients with AAU had significantly higher levels than in the control group (Mann–Whitney U test p<0.001).
Discussion
In our study, quantification of NGAL in aqueous humor was conducted in a homogeneous group of patients with idiopathic AAU in which we observed significantly elevated levels of NGAL compared with control subjects. These results imply that NGAL is associated with the regulation of inflammation in patients with AAU. In addition to our direct observation, NGAL is strongly upregulated by interleukin (IL) 1 beta [16] and TNF-α in the presence of IL-17 [17], a pro-inflammatory cytokine produced by the newly discovered subset of CD4+ T helper cells (TH-17), and increased levels of IL-1 has been observed in AAU [4], and this could be one of the reasons of the high levels found in our patients.
Otherwise, NGAL plays a cytoprotective role by transporting iron into cells, promoting differentiation in myocardial [11] and renal cells [18], developing an antioxidant activity and reducing apoptosis. NGAL is a potent inducer of heme oxygenase-1 (HO-1) and superoxide dismutase (1, 2; SOD) [19]. The overexpression of NGAL, significantly reduce cell death by apoptosis-inducing agents related to COX-2 and lipoxygenase inhibitors [19]. Moreover, NGAL is strongly induced by growth factors including insulin-like growth factor (IGF) and TGF-α in primary human keratinocytes, this induction is believed to play a role in wound healing [20]. This suggests that increased levels of NGAL may serve to limit injury in recurrent insults.
Plasma NGAL is a useful early preclinical marker for AKI in a heterogeneous adult intensive care unit population. In addition, it predicts need for renal replacement therapy and correlates with AKI severity [21]. Moreover, the levels of serum NGAL in patients with vasculitis, had a closer correlation with clinical findings (Birmingham Vasculitis Activity Score, BVAS) than erythrocyte sedimentation rate, C-reactive protein, and anti-neutrophil cytoplasmic antibody did [22]. Other studies have confirmed the potential usefulness of NGAL measurement in the evaluation of early responses to therapy or in predicting different clinical outcomes with infliximab in Crohn disease [6] and intravenous immunoglobulin infusion in renal disease [23]. However, the serum levels of NGAL in uveitis have not been determined yet. It would be interesting to analyze the correlation of NGAL serum levels with aqueous humor levels to determine whether NGAL can be used as a biomarker of inflammatory activity. In this context, NGAL could be used to molecularly quantify and monitor the response to an anti-inflammatory treatment, not only in AAU, but also, in inflammatory diseases of different origins.
In summary, the aqueous humor NGAL levels are increased in eyes with idiopathic AAU. We suggest NGAL is involved in the inflammatory response in AAU where it may act as a cytoprotective factor. As was previously reported, NGAL was associated with high levels of gelatinase B in patients with active uveitis, suggesting that neutrophils is a significant source of gelatinase B in these patients. Gelatinase B is a specifically cleaver of type IV collagen of endothelial basement membrane, and may be responsible of the blood-ocular barrier disruption in patients with uveitis [24]. Our results add evidence to the possibility of using selective gelatinase inhibitors as a potential therapy in patients with uveitis. The aqueous humor levels of NGAL may be used as a biomarker of inflammatory ocular activity in patients with idiopathic AAU.
References
- 1.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80:844–8. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Muhaya M, Calder V, Towler HM, Shaer B, McLauchlan M, Lightman S. Characterization of T cells and cytokines in the aqueous humour (AH) in patients with Fuch’s heterochromic cyclitis (FHC) and idiopathic anterior uveitis. Clin Exp Immunol. 1998;111:123–8. doi: 10.1046/j.1365-2249.1998.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ongkosuwito JV, Feron EJ, Van Doornik CE, Van der Lelij A, Hoyng CB, La Heij EC, Kijlstra A. Analysis of immunoregulatory cytokines in ocular fluid samples from patients with uveitis. Invest Ophthalmol Vis Sci. 1998;39:2659–65. [PubMed] [Google Scholar]
- 5.Santos Lacomba M, Marcos Martín C, Gallardo Galera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R, Omar M. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33:251–5. doi: 10.1159/000055677. [DOI] [PubMed] [Google Scholar]
- 6.Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89–94. doi: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolignano D, Donato V, Lacquaniti A, Fazio MR, Bono C, Coppolino G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288:10–6. doi: 10.1016/j.canlet.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Bolignano D, Della Torre A, Lacquaniti A, Costantino G, Fries W, Buemi M. Neutrophil gelatinase-associated lipocalin levels in patients with Crohn disease undergoing treatment with infliximab. J Investig Med. 2010;58:569–71. doi: 10.231/JIM.0b013e3181ccc20c. [DOI] [PubMed] [Google Scholar]
- 9.Devarajan P. NGAL in acute kidney injury: from serendipity to utility. Am J Kidney Dis. 2008;52:395–9. doi: 10.1053/j.ajkd.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail. 2010;16:49–54. doi: 10.1016/j.cardfail.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding L, Hanawa H, Ota Y, Hasegawa G, Hao K, Asami F, Watanabe R, Yoshida T, Toba K, Yoshida K, Ogura M, Kodama M, Aizawa Y. Lipocalin-2/neutrophil gelatinase-B associated lipocalin is strongly induced in hearts of rats with autoimmune myocarditis and in human myocarditis. Circ J. 2010;74:523–30. doi: 10.1253/circj.cj-09-0485. [DOI] [PubMed] [Google Scholar]
- 12.Woo HJ, Min JK, Bai CH, Song SY, Kang HJ, Lee HM, Kim YD. Expression of neutrophil gelatinase-associated lipocalin in nasal polyps. Arch Otolaryngol Head Neck Surg. 2008;134:1182–6. doi: 10.1001/archotol.134.11.1182. [DOI] [PubMed] [Google Scholar]
- 13.D'Anna R, Baviera G, Giordano D, Todarello G, Russo S, Recupero S, Bolignano D, Corrado F. Neutrophil gelatinase-associated lipocalin serum evaluation through normal pregnancy and in pregnancies complicated by preeclampsia. Acta Obstet Gynecol Scand. 2010;89:275–8. doi: 10.3109/00016340903443676. [DOI] [PubMed] [Google Scholar]
- 14.Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56:3326–35. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty S, Kaur S, Muddana V, Sharma N, Wittel UA, Papachristou GI, Whitcomb D, Brand RE, Batra SK. Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowland JB, Muta T, Borregaard N.IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol 20061765559–66. 16622025 [DOI] [PubMed] [Google Scholar]
- 17.Karlsen JR, Borregaard N, Cowland JB. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with IL-17 and TNF-{alpha} is controlled by I{kappa}B-{zeta} but neither by C/EBP-{beta} nor by C/EBP-{delta}. J Biol Chem. 2010;285:14088–100. doi: 10.1074/jbc.M109.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahmani P, Halabian R, Rouhbakhsh M, Roushandeh AM, Masroori N, Ebrahimi M, Samadikuchaksaraei A, Shokrgozar MA, Roudkenar MH. Neutrophil Gelatinase-Associated Lipocalin induces the expression of heme oxygenase-1 and superoxide dismutase (1, 2). Cell Stress Chaperones. 2010;15:395–403. doi: 10.1007/s12192-009-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sørensen OE, Cowland JB, Theilgaard-Mönch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170:5583–9. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 21.Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, Piccinni P, Ronco C. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36:444–51. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Wang F, Zhao MH. Circulating neutrophil gelatinase-associated lipocalin: a useful biomarker for assessing disease activity of ANCA-associated vasculitis. Rheumatology (Oxford) 2009;48:355–8. doi: 10.1093/rheumatology/ken500. [DOI] [PubMed] [Google Scholar]
- 23.Bolignano D, Coppolino G, Aloisi C, Romeo A, Nicocia G, Buemi M. Effect of a single intravenous immunoglobulin infusion on neutrophil gelatinase-associated lipocalin levels in proteinuric patients with normal renal function. J Investig Med. 2008;56:997–1003. doi: 10.2310/JIM.0b013e31818e7e95. [DOI] [PubMed] [Google Scholar]
- 24.Abu El-Asrar AM, Struyf S, Descamps FJ, Al-Obeidan SA, Proost P, Van Damme J, Opdenakker G, Geboes K. Chemokines and gelatinases in the aqueous humor of patients with active uveitis. Am J Ophthalmol. 2004;138:401–11. doi: 10.1016/j.ajo.2004.04.046. [DOI] [PubMed] [Google Scholar]


