Abstract
Background
Imatinib is a highly effective treatment for chronic myeloid leukemia (CML) that was approved by the Food and Drug Administration in 2001, and thereafter rapidly became front line therapy. This study characterized the prevailing chronic myeloid leukemia therapies in the United States and assessed the impact of imatinib on chronic myeloid leukemia survival and mortality rates in the general population.
Methods
Investigators with the National Cancer Institute’s Patterns of Care study reviewed medical records and queried physicians regarding therapy for 423 chronic myeloid leukemia patients diagnosed in 2003 who were randomly selected from cancer registries in the Surveillance, Epidemiology, and End Results (SEER) Program. Characteristics associated with the receipt of imatinib were documented, as were survival differences between those who received imatinib and those who did not. Population-based data were used to assess chronic myeloid leukemia survival and mortality rates in time periods before and after the introduction of imatinib.
Results
76% of patients in the Patterns of Care study received imatinib. Imatinib use was inversely associated with age: 90%, 75%, and 46% for patients ages 20–59, 60–79, and ≥80, respectively. Elderly patients who received imatinib survived significantly longer than those who did not. After adjusting for age, imatinib use did not vary significantly by race/ethnicity, socioeconomic status, urban/rural residence, presence of co-morbid conditions, or insurance status. Overall, chronic myeloid leukemia survival in the SEER population improved and mortality in the United States declined dramatically during the period when imatinib became widely available; these improvements diminished with increasing age.
Conclusion
Age disparities in treatment with imatinib likely contributed to worse survival for many elderly chronic myeloid leukemia patients.
Keywords: Chronic Myeloid Leukemia, Imatinib, Socioeconomic Status, Age Discrimination
INTRODUCTION
Chronic myelogenous leukemia (CML) can occur at any age, but is most commonly diagnosed in older adults1. The American Cancer Society estimates that approximately 4,830 new cases of the disease will be diagnosed in the United States in 2008, and that 450 individuals will die from the disease2. Approximately 95% of chronic myeloid leukemia cases harbor the aberrant Philadelphia Chromosome, which results from the translocation of the ABL proto-oncogene on chromosome 9 to the breakpoint cluster region (BCR) at the distal region of chromosome 22. The resulting BCR-ABL fusion protein is a key factor in the development and maintenance of chronic myeloid leukemia 3,4.
The initial morbidity of chronic myeloid leukemia results from leukocytosis, and earlier chemotherapeutic agents such as hydroxyurea and bulsulfan targeted the reduction of leukocytes5,6. The introduction of interferon alpha for the treatment of chronic myeloid leukemia resulted in the first major increases in survival in this disease, but this therapy was not curative, and had significant morbidity5,6. The first curative therapy for chronic myeloid leukemia was allogeneic bone marrow transplantation, which resulted in long-term disease-free survivors, but with substantial early mortality5,6. Imatinib (trade name, Gleevec, Novartis, Basel, Switzerland) is a tyrosine kinase inhibitor that binds to the BCR-ABL protein and effectively inhibits the signaling cascade that leads to chronic myeloid leukemia 7,8. Clinical trials established the efficacy of imatinib in altering the biology of chronic myeloid leukemia, resulting in significantly increased survival, with few side effects9–12. The United States Food and Drug Administration (FDA) licensed imatinib for use in treating chronic myeloid leukemia patients on May 10, 200113,14. Since the introduction of imatinib, there has been an improvement in survival in younger but not elderly chronic myeloid leukemia patients on a population basis15,16.
We used three large data sets to characterize the prevailing chronic myeloid leukemia therapies in the United States and to assess the impact of the introduction of imatinib on chronic myeloid leukemia survival and mortality rates. We found that elderly chronic myeloid leukemia patients were not treated with imatinib at the same rate as younger patients, and that this age discrimination resulted in a survival disparity.
METHODS
This investigation was based on data from (1) the NCI’s Patterns of Care study, (2) population-based cancer surveillance data routinely collected by the SEER Program, and (3) population-based mortality rates derived from National Center for Health Statistics (NCHS) records. The Human Research Review Committee at the University of New Mexico determined that this analysis was not subject to federal regulations for review as human research. The SEER Program is a collection of population-based registries that assemble surveillance data on all incident cancer cases that occur in defined geographic regions of the United States17.
The present analyses utilized data from an NCI Patterns of Care study that focused on individuals diagnosed with chronic myeloid leukemia during calendar year 2003. Diagnosis was based on cytogenetic or molecular evidence of the presence of BCR-ABL. The period of this Patterns of Care study started 19 months after the FDA approved imatinib for chronic myeloid leukemia therapy, but preceded general availability of other ABL kinase inhibitors. Patients were ineligible if they were under the age of 20 years at diagnosis, if the sole documentation of chronic myeloid leukemia diagnosis was a death certificate or autopsy report, or if they were diagnosed simultaneously with a second cancer. A total of 423 eligible patients were identified from the following participating SEER Program registries: the metropolitan areas of San Francisco, San Jose/Monterey, Atlanta, Detroit, Los Angeles County, Seattle, the remainder of the state of California, and the states of Connecticut, Iowa, New Mexico, Utah, New Jersey, and Louisiana. Vital status was documented for all chronic myeloid leukemia patients included in this study through December 31, 2005.
SEER Program registries routinely determine the county and census tract of residence at time of diagnosis for all registered cancer cases. Surrogate measures of income and educational level assigned to individual cancer patients based on aggregate data from their respective census tracts of residence, as documented in the 2000 decennial census18. For the purposes of this analysis, income was defined as the median household income and educational level was defined as the percentage of individuals age 25 or older with a high school diploma for individuals living in that same census tract. Designation of rural/urban residence was assigned based on county of residence at diagnosis, according to a classification system developed by the United States Department of Agriculture (USDA)19.
Logistic regression was used to assess the association between selected patient characteristics and receipt of imatinib. Individuals for whom imatinib status could not be determined (n=20) were excluded. Survival following chronic myeloid leukemia diagnosis was compared between those Patterns of Care subjects who received imatinib and those who did not. Cox proportional hazards model was used to generate survival curves for each of three age-groups (20–59, 60–79, and ≥80 years) and for conducting multivariate survival analyses20, 21. Data from the Patterns of Care study were weighted to reflect the population from which the sample was drawn; sample weights, calculated as the inverse of the sampling proportion for each sampling stratum (defined by race/ethnicity and gender), were used to obtain estimates that are representative of all eligible chronic myeloid leukemia patients in the study areas. SUDAAN software, Version 9.0.3 (Research Triangle Institute, Research Triangle Park, NC) was used for all weighted analyses; all statistical tests were two-sided.
Population-based survival rates were calculated using data from nine SEER Program registries included in the SEER limited use dataset22. This analysis was based on all incident cases of chronic myeloid leukemia that were diagnosed during the time period 1990–2004, and that met the following criteria: (1) diagnosis of chronic myeloid leukemia was the first and/or only cancer diagnosis that occurred during the study period; (2) chronic myeloid leukemia diagnosis was confirmed; and (3) cases were 20 years of age and older at the time of this chronic myeloid leukemia diagnosis. Cases included in the SEER Program dataset utilized for this study were followed from date of diagnosis until December 31, 2005. Thus, cases diagnosed in the earlier time periods had been followed for much longer time than were cases diagnosed in more recent years. Accordingly, follow-up time was restricted to 36 months for each group in order to standardize the period of follow-up for cases diagnosed during the three time periods of interest. Survival curves were calculated according to the Kaplan and Meier method20 and multivariate survival analyses were conducted using the Cox proportional hazards model21. All population-based survival analyses were conducted with standard modules from the Statistical Analysis System, Version 9.1.3 (SAS Institute, Cary, NC).
Age-specific chronic myeloid leukemia mortality rates were calculated from death certificate data from the NCHS for each year during the time period 1975–200423; population estimates derived from United States Census Bureau data served as denominators for the mortality rates24. Age-groups for mortality rates were defined as follows: 20–59 years; 60–79 years; and ≥80 years. All rates were calculated with the NCI’s SEER*Stat software25. Temporal changes in age-specific mortality rates were assessed with the joinpoint regression techniques26 using standard statistical software developed by NCI27.
RESULTS
Characteristics of 423 chronic myeloid leukemia cases that were included in the Patterns of Care study are available from the corresponding author upon request. There was a slight male predominance of the disease and a majority of the study sample was non-Hispanic whites. Characteristics of the Patterns of Care study sample were generally consistent with those of all chronic myeloid leukemia patients within the entire SEER Program.
Dissemination of Therapy
Imatinib therapy was documented in 76.1% of the Patterns of Care study subjects and was, by far, the most commonly used therapeutic agent. Hydroxyurea was administered to 40.3% of subjects, most of whom also received imatinib. Stem cell transplantation was performed in 5.2% of subjects: 4.2% of study subjects received allogeneic transplants; 0.4% received autologous transplants; and 0.6% received transplants of unknown type.
The percentage of chronic myeloid leukemia cases treated with imatinib decreased with age in a statistically significant manner: 89.7% of 20–59 year-old patients received imatinib, 75.0% of 60–79 year olds received imatinib, and 46.0% of those 80 years of age and older received imatinib (odds ratio of receiving imatinib per age group- 1.0, 0.4, 0.1 respectively). Imatinib utilization also did not vary significantly by sex, race/ethnicity, socioeconomic status, urban/rural residence, number of co-morbidities, or medical insurance status (specific data available upon request).
Treatment and Survival in the Patterns of Care Study
Failure to receive imatinib and increasing age at diagnosis were both significantly associated with poor survival among Patterns of Care study subjects (Table 1). The hazard ratios for age at diagnosis and imatinib use were slightly diminished when both of these variables were considered together in a multivariable model; nonetheless, both variables remained significant predictors of survival. Socioeconomic status (i.e., income and education level), sex, rural versus urban location, hospital type, and insurance coverage were not statistically related to survival.
Table 1.
Univariate and multivariate analysis of survival in patients with chronic myeloid leukemia in the Patterns of Care study using Cox proportional hazards. Use of imatinib and age were the major determinants of survival.
| Characteristic | Strata | Univariate Analysisa | Multivariate Analysisb | ||
|---|---|---|---|---|---|
| Hazard Ratio | 95 percent Confidence Interval | Hazard Ratio | 95 percent Confidence Interval | ||
| Imatininb | Yes | 1.0 | Reference | 1.0 | Reference |
| No | 4.1c | 2.7, 6.2 | 2.7c | 1.7, 4.2 | |
| Sex | Male | 1.0 | Reference | 1.0 | Reference |
| Female | 0.8 | 0.5, 1.2 | 0.7 | 0.5, 1.1 | |
| Ancestry | Non Hispanic | 1.0 | Reference | 1.0 | Reference |
| Black | 1.0 | 0.6, 2.0 | 1.4 | 0.8, 2.7 | |
| Hispanic White | 0.6 | 0.3, 1.3 | 1.0 | 0.5, 2.1 | |
| Other | 0.9 | 0.4, 2.2 | 1.3 | 0.5, 3.1 | |
| Marital Status | Never Married | 1.0 | Reference | 1.0 | Reference |
| Married | 0.9 | 0.5, 1.6 | 0.6 | 0.3, 1.0 | |
| Separated/Divorced | 0.7 | 0.3, 1.6 | 0.5 | 0.2, 1.2 | |
| Widowed | 1.9 | 0.9, 3.9 | 0.5 | 0.2, 1.1 | |
| Unknown | 0.7 | 0.3, 1.9 | 0.4 | 0.1, 1.1 | |
| Age at Diagnosis (Years) | 20–59 | 1.00 | Reference | 1.00 | Reference |
| 60–79 | 2.6c | 1.5, 4.3 | 2.3c | 1.3, 3.8 | |
| 80+ | 7.8c | 4.5, 13.5 | 5.3c | 3.0, 9.5 | |
| Income (Quartiles) | First (Low) | 1.0 | Reference | 1.0 | Reference |
| Second | 0.9 | 0.5, 1.6 | 1.0 | 0.5, 1.7 | |
| Third | 0.8 | 0.5, 1.5 | 0.8 | 0.4, 1.4 | |
| Fourth (High) | 0.7 | 0.4, 1.3 | 0.7 | 0.4, 1.3 | |
| Education (Quartiles) | First (Low) | 1.0 | Reference | 1.0 | Reference |
| Second | 1.1 | 0.6, 2.0 | 0.9 | 0.5, 1.6 | |
| Third | 1.2 | 0.7, 2.2 | 1.1 | 0.6, 1.9 | |
| Fourth (High) | 0.9 | 0.5, 1.6 | 0.8 | 0.4, 1.4 | |
| Residence | Urban | 1.0 | Reference | 1.0 | Reference |
| Rural | 2.0 | 0.6, 6.1 | 2.4 | 0.8, 6.8 | |
| Insurance | Yes | 1.0 | Reference | 1.0 | Reference |
| No | 0.1 | 0.0, 1.1 | 0.3 | 0.0, 2.0 | |
| Unknown | 0.4 | 0.1, 1.3 | 0.5 | 0.2, 1.4 | |
| One or more co morbidities | No | 1.0 | Reference | 1.0 | Reference |
| Yes | 1.1 | 0.6, 2.1 | 1.3 | 0.7, 2.6 | |
Calculated by univariate Cox proportional hazards model
Calculated by multivariate Cox proportional hazards model, adjusting for receipt of imatinib and age at diagnosis
Hazards ratio significantly different from unity (i.e., 1.00), p<0.005
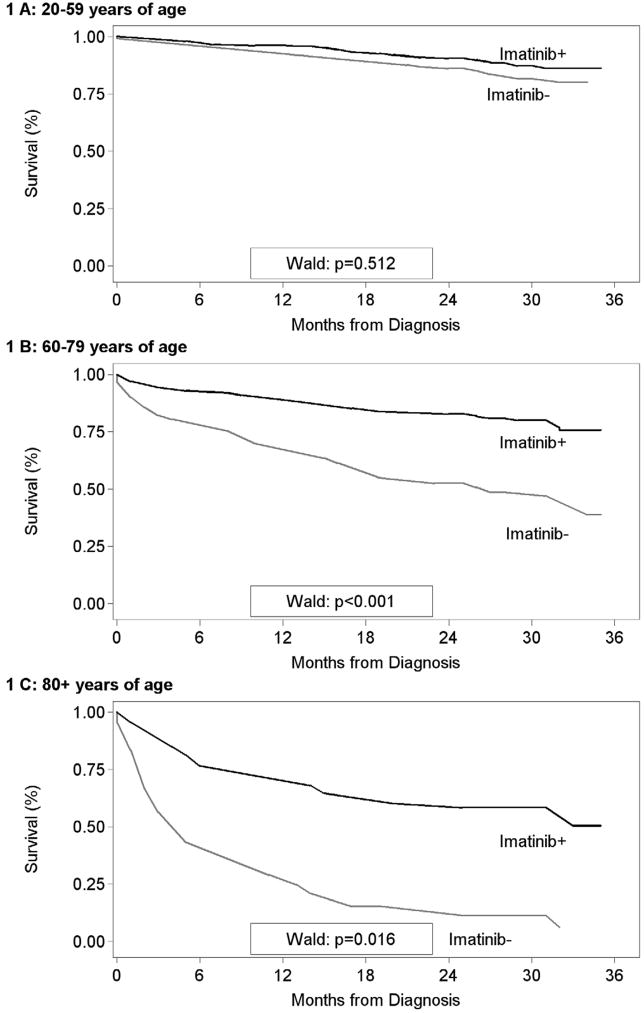
Figure 1 graphically displays the differences in survival between those Patterns of Care subjects who received imatinib and those who did not, by age at diagnosis. Receipt of imatinib did not significantly influence survival among Patterns of Care subjects under the age of 60 years as a group, though it should be noted that a majority of subjects in this age group received imatinib. There was a substantial and statistically significant survival advantage for Patterns of Care subjects greater than 60 years of age at diagnosis who received imatinib compared to those who did not (Figure 1).
Figure 1.
Survival (months) among Patterns of Care Study subjects by age at diagnosis and imatinib utilization status. A- 20–59 years of age. B- 60–79 years of age. C- 80 and over years of age. These data indicate that elderly patients that did not receive imatinib fared more poorly compared to those that did. There was no significant difference in socioeconomic status or co-morbidities between those that received imatinib and those that did not.
SEER-wide Chronic Myeloid Leukemia Survival
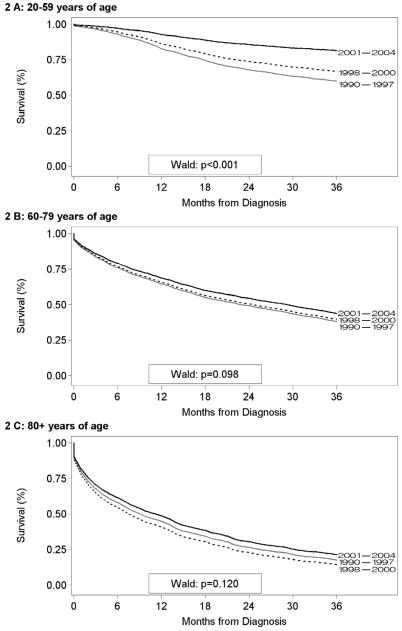
Population-based data from the SEER Program were used to assess whether survival patterns observed in Patterns of Care study subjects were mirrored in the general population. A total of 6,418 incident cases of chronic myeloid leukemia were diagnosed among residents of the specified SEER Program areas from 1990–2004. A total of 1,351 (21.1%) of these cases did not meet eligibility criteria, leaving 5,067 subjects available for this analysis. We found that chronic myeloid leukemia survival generally declined with age (Figure 2). Within each age group, however, chronic myeloid leukemia survival was highest among those who were diagnosed from 2001–2004, the period immediately following FDA approval of imatinib therapy for chronic myeloid leukemia. However, the elderly did not benefit nearly as much from the improvement in chronic myeloid leukemia survival after 2001. The differences among survival curves for the periods 1990–1997, 1998–2000, and 2001–2004 become less distinct with increasing age (Figure 2).
Figure 2.
Survival (months) for chronic myeloid leukemia cases in nine core Surveillance, Epidemiology, and End Results (SEER) Program participants by age at diagnosis and time period of diagnosis. A- 20–59 years of age. B- 60–79 years of age. C- 80 and over years of age. These data indicate that elderly patients with CML had a worse survival on a population basis.
Chronic Myeloid Leukemia Mortality Rates in the United States
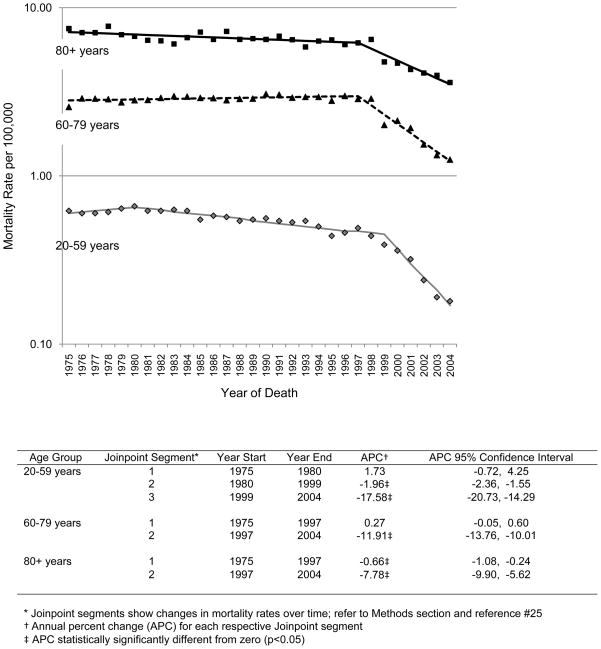
We also queried NCHS records to determine if changes in chronic myeloid leukemia survival were reflected in mortality rates for the general population. Dramatic declines in age-specific mortality rates were observed in a time frame that corresponded to the introduction of imatinib (Figure 3). By joinpoint regression, the greatest Annual Percent Change (APC) in mortality rates occurred after calendar year 2000 for 20–59 year olds (APC = −17.58; 95% confidence interval (CI) −20.73, −14.29), after 1997 for 60–79 year olds (APC=−11.91; 95% CI −13.76, −10.01), and after 1997 for those 80 years of age and older (APC=−7.78; 95% CI −9.90, −5.62). Also, the magnitude of the APC was greatest in the youngest group and decreased with age.
Figure 3.
Joinpoint analysis of chronic myeloid leukemia (CML) patients by age at year of death. These data show that there has been an overall improvement in survival of chronic myeloid leukemia patients in the imatinib era, but that this improvement extends less well to elderly patients.
DISCUSSION
By the time Patterns of Care study subjects were diagnosed in 2003, imatinib was standard first-line therapy for chronic myeloid leukemia, supplanting the existing standards of care9–12, 28. Results from the analysis of three data bases included in this report indicate that increasing age was a barrier to receipt of imatinib therapy for chronic myeloid leukemia. Nearly 90% of Patterns of Care study subjects less than 60 years of age received imatinib. Although there was no significant difference in survival in these patients, it may be that the Patterns of Care study lacked the power to detect the difference with such a large percentage of patients on imatinib. Less than half of chronic myeloid leukemia patients 80 years of age and older received the drug. These results are disturbing because of the profound survival advantage accrued by the elderly Patterns of Care study subjects who received imatinib. Perhaps because of this, reductions in chronic myeloid leukemia mortality rates in the United States in recent years were most pronounced at younger ages and were least evident among the elderly.
Results from previous studies have shown that age can be a barrier to receipt of optimal treatment due, in part, to the presence of co-morbid conditions29–33, increased toxicity of some therapies in the elderly29–34, lack of data on the elderly from clinical trials35–38, social marginalization30,31, and patient and physician preferences31,32,39–41. It is unlikely that co-morbid conditions influenced receipt of imatinib in the Patterns of Care study. Co-morbid conditions were documented here in similar proportions of those subjects who did and did not receive imatinib; this pattern persisted after adjustment for age at diagnosis.
It is possible that some prescribing physicians avoided imatinib therapy in elderly chronic myeloid leukemia patients out of concern for toxicity. Little toxicity was reported in clinical trials that established the efficacy of imatinib therapy for chronic myeloid leukemia 9–12. It is possible that patients and physicians were unaware of such findings since results from clinical trials were often not reported by age. One study that specifically demonstrated the benefits of imatinib without significant toxicity in older chronic myeloid leukemia patients was published in 200328. It is unlikely that those results were widely disseminated by the time most Patterns of Care study subjects were accrued. For these reasons, it is plausible that anxiety for, or lack of knowledge of, toxicity of imatinib therapy in the elderly could have contributed to the age disparity in treatment. Certainly, drug interactions with imatinib could be worse in the elderly, and this could also have been a limiting factor in physician decisions.
Imatinib use did not vary substantially by race/ethnicity, socioeconomic status, urban/rural residence, or insurance status; these findings persisted after adjustment for age at diagnosis. These findings suggest progress in the dissemination of novel cancer therapy since factors such as lower socioeconomic status, rural residency, and lack of insurance had previously influenced receipt of state-of-the-art cancer treatment30, 31. Previous studies have shown that elderly patients generally do not perceive their age or current health as a barrier to treatment39,40,42 or as a limiting factor for their enrollment in clinical trials43. Physician preferences for treatment may be influenced if elderly patients are perceived as less able to tolerate therapy or as having a short life expectancy, or if there is a dearth of clinical trial data specific to elderly patients39–41,44.
Several limitations should be considered when interpreting results from this investigation. The study was based on a population-based sample that was generally representative of chronic myeloid leukemia cases diagnosed in participating SEER Program registries. Nonetheless, the sample size of 423 resulted in some analyses that were based on relatively few observations. For example, results regarding race/ethnicity and urban/rural residence should be interpreted with caution since the study sample included relatively few members of minority populations and rural residents. Indicators of socioeconomic status (i.e., income and education) were derived from aggregate measures that represented the census tract of residence for each Patterns of Care study subject. Nonetheless, previous studies have shown that such aggregate data can serve as reasonable surrogate measures of education and income18. Finally, existing co-morbid conditions were documented from medical record review and physician statements, and were not categorized by severity. Indeed, ethical considerations may be important in the physician decision process for imatinib use in elderly with severe co-morbid conditions, such as dementia.
The high efficacy of imatinib resulted in dramatic increases in survival and diminished chronic myeloid leukemia mortality rates in the general population of the United States15–17. However, results from this study also show that the percentage of chronic myeloid leukemia patients treated with imatinib declined with age. These findings indicate that age disparities in receipt of imatinib resulted in worse survival for many elderly chronic myeloid leukemia patients who might have benefited from this new therapy. Age disparities in treatment of cancer patients have been well-documented in the medical literature29–33 and a number of positive steps have been taken to address these concerns. For example, the FDA issued guidelines for the study of drugs likely to be used in the elderly45, the National Cancer Care Network has issued guidelines for care of elderly cancer patients46,47, and clinical trial groups have developed tools for uniformly evaluating elderly cancer patients48. The present study serves as a reminder that these steps are not sufficient, and additional work is needed to ensure that novel therapy is available to all.
Patient advocates have challenged the status quo of drug development and delivery49, and there is undoubtedly a role for such advocacy in the dissemination of new cancer therapies. However, physicians must also bear responsibility for the dissemination of novel cancer therapy to elderly patients. Specifically, several further measures are recommended: 1) Mandatory inclusion of elderly patients in federally-funded clinical trials, 2) Requiring separate reporting of study results for elderly patients, and 3) Implementing standards that require pharmaceutical firms to educate oncologists regarding the safety and efficacy of novel therapy in the elderly. In summary, age is a major health disparity when it comes to the introduction of novel cancer therapy, and this disparity results in mortality that could be prevented.
Acknowledgments
Dr. Wiggins acknowledges the support of NCI Contracts NO1-PC-35138, N01-PC-35133, N01-PC-35135, N01-PC-35141, N01-PC-35136, N01-PC-35137, N01-PC-35139, N01-PC-35142, N01-PC-35143, N01-PC-35145, N01-PC-54402, N01-PC-54404, and N01-PC-54405, and the UNM Cancer Center, a recipient of NCI Cancer Center Support Grant P30-CA118100. Dr Willman was supported by LLS SCOR 7388-06 and the NCI 5UO1 CA88361, and Dr. Hromas was supported by LLS 7388-06, NIH CA139429, NIH CA100862, NIH CA140442, and NIH HL075783. We thank Ms. Lorna Marchand (New Mexico Tumor Registry) for editorial assistance.
Funding sources: The National Cancer Institute of the National Institutes of Health and the Leukemia and Lymphoma Society supported this study.
Footnotes
Disclaimers: The content of this article does not necessarily reflect the views or policies of the National Cancer Institute or of the University of New Mexico, nor does the mention of trade names, commercial products, or organizations imply endorsement by the National Cancer Institute or by the University of New Mexico.
All authors had access to the data and assisted in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 01.Linet MS, Devesa SS, Morgan GJ. The Leukemias. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. Chapter 44. Oxford University Press; 2006. pp. 841–871. [Google Scholar]
- 02.American Cancer Society. Cancer Facts & Figures 2008. American Cancer Society; 2008. p. 4. [Google Scholar]
- 03.Savona M, Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer. 2008;8:341–350. doi: 10.1038/nrc2368. [DOI] [PubMed] [Google Scholar]
- 04.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 05.Carella AM, Frassoni F, Melo J, et al. New insights in biology and current therapeutic options for patients with chronic myelogenous leukemia. Haematologica. 1997;82:478–495. [PubMed] [Google Scholar]
- 06.Kantarjian HM, Giles FJ, O’Brien S, et al. Therapeutic choices in younger patients with chronic myelogenous leukemia. Cancer. 2000;89:1647–1658. doi: 10.1002/1097-0142(20001015)89:8<1647::aid-cncr1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 07.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of BCR-ABL positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 08.Deiniger MW, Goldman JM, Lydon N, et al. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997;90:3691–3698. [PubMed] [Google Scholar]
- 09.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 12.Druker BJ, Guilhot F, O’Brien SG, et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration, Center for Drug Evaluation and Research. Approval Letter for Application Number NDA 21-335. 2001 May 10; Available from http://www.fda.gov/cder/foi/nda/2001/21-335_Gleevec_Approv.pdf.
- 14.Cohen MH, Johnson JR, Pazdur R. Approval summary: imatinib mesylate capsules for treatment of adult patients with newly diagnosed philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase. Clin Cancer Res. 2003;9:1972–1979. [PubMed] [Google Scholar]
- 15.Brenner H, Gondos A, Pulte D. Recent trends in long-term survival of patients with chronic myelocytic leukemia: disclosing the impact of advances in therapy on the population level. Haematologica. 2008;93:1544–1549. doi: 10.3324/haematol.13045. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen LC, Nielsen OJ, Johansen C. Trends in adult leukemia incidence and survival in Denmark, 1943–2003. Cancer Causes Control. 2009;20:1671–1680. doi: 10.1007/s10552-009-9417-9. [DOI] [PubMed] [Google Scholar]
- 17.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 18.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annual Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 19.Butler MA, Beale CL. Staff Report No. 9425. Agriculture and Rural Economic Division, Economic Research Service, U.S. Department of Agriculture; 1994. Rural-urban continuum codes for metro and non-metro counties, 1993. [Google Scholar]
- 20.Elandt-Johnson RC, Johnson NL. Survival models and data analysis. New York: John Wiley & Sons, Inc; 1980. [Google Scholar]
- 21.Cox DR, Oaks D. Analysis of survival data. London: Chapman and Hall; 1984. [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Incidence - SEER 17 Registries Limited-Use + Hurricane Katrina Impacted Louisiana Cases, November 2007 Submission (1973–2005 varying); Linked To County Attributes - Total U.S., 1969–2005 Counties. Available from: www.seer.cancer.gov. [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Mortality - All Causes of Death, Aggregated With State, Total U.S. (1969–2004) released April 2007. Underlying mortality data provided by National Center for Health Statistics ( www.cdc.gov/nchs). Available from URL: www.seer.cancer.gov. [Google Scholar]
- 23.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute. Population estimates used in SEER*Stat software. Available from URL: http://seer.cancer.gov/popdata/methods.html.
- 24.Surveillance Research Program. SEER*Stat Software, Version 6.4.4. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; 2008. Available from URL: http://www.seer.cancer.gov/seerstat. [Google Scholar]
- 25.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. (correction: 20 (2001), p. 655) [DOI] [PubMed] [Google Scholar]
- 26.Statistical Research and Applications Branch, National Cancer Institute. Joinpoint Regression Program, Version 3.2.0. Jan, 2008. [Google Scholar]
- 27.Cortes J, Talpaz M, O’Brien S, et al. Effects of age on prognosis with imatinib mesylate therapy for patients with Philadelphia chromosome-positive chronic myelogenous leukemia. Cancer. 2003;98:1105–1113. doi: 10.1002/cncr.11629. [DOI] [PubMed] [Google Scholar]
- 28.Crivellari D, Bonetti M, Castiglione-Gertsch M, et al. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: the International Breast Cancer Study Group Trial VII. J Clin Oncol. 2000;18:1412–1422. doi: 10.1200/JCO.2000.18.7.1412. [DOI] [PubMed] [Google Scholar]
- 29.Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev. 2005;31:380–402. doi: 10.1016/j.ctrv.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 31.Silliman RA, Guadagnoli E, Weitberg AB, et al. Age as a predictor of diagnostic and initial treatment intensity in newly diagnosed breast cancer patients. J Gerontol. 1989;44:M46–50. doi: 10.1093/geronj/44.2.m46. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Cantor A, Meyer J, et al. Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer. 2003;97:1107–1114. doi: 10.1002/cncr.11110. [DOI] [PubMed] [Google Scholar]
- 33.Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol. 2002;3:289–297. doi: 10.1016/s1470-2045(02)00730-1. [DOI] [PubMed] [Google Scholar]
- 34.Hutchins LF, Unger JM, Crowley JJ, et al. Under-representation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 35.Yee KW, Keating A. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–1623. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 36.Kimmick GG, Peterson BL, Kornblith AB, et al. Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol. 2005;23:2201–2207. doi: 10.1200/JCO.2005.01.222. [DOI] [PubMed] [Google Scholar]
- 37.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 38.Kearney N, Miller M, Paul J, et al. Oncology healthcare professionals’ attitudes toward elderly people. Ann Oncol. 2000;11:599–601. doi: 10.1023/a:1008327129699. [DOI] [PubMed] [Google Scholar]
- 39.Fentiman IS, Tirelli U, Monfardini S, et al. Cancer in the elderly: why so badly treated? Lancet. 1990;335:1020–1022. doi: 10.1016/0140-6736(90)91075-l. [DOI] [PubMed] [Google Scholar]
- 40.Maly RC, Leake B, Silliman RA. Breast cancer treatment in older women: impact of the patient-physician interaction. J Am Geriatr Soc. 2004;52:1138–1145. doi: 10.1111/j.1532-5415.2004.52312.x. [DOI] [PubMed] [Google Scholar]
- 41.Moore DH, Kauderer JT, Bell J, et al. An assessment of age and other factors influencing protocol versus alternative treatments for patients with epithelial ovarian cancer referred to member institutions: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;94:368–374. doi: 10.1016/j.ygyno.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 42.Townsley CA, Chan KK, Pond GR, et al. Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC Cancer. 2006;6:34. doi: 10.1186/1471-2407-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keime-Guibert F, Chinot O, Taillandier L, et al. Association of French-Speaking Neuro-Oncologists. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 44.Food and Drug Administration. Guideline for the Study of Drugs Likely to Be Used in the Elderly. Fed Register. 1989 November;59(1994):39398–39400. [Google Scholar]
- 45.Balducci L, Cohen HJ, Engstrom PF, et al. Senior Adult Oncology Clinical Practice Guidelines in Oncology. J Natl Comp Canc Netw. 2005;3:572–590. doi: 10.6004/jnccn.2005.0032. [DOI] [PubMed] [Google Scholar]
- 46.Carlson RW, Moench S, Hurria A, et al. NCCN Task Force Report: Breast Cancer in the Older Woman. J Natl Comp Canc Netw. 2008;6(Suppl 4):S1–S25. [PubMed] [Google Scholar]
- 47.Repetto L, Fratino L, Audisio RA, et al. Comprehensive Geriatric Assessment Adds Information to Eastern Oncology Group Performance Status in elderly cancer patients: An Italian Group for Geriatric Oncology study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 48.Epstein S. The construction of lay expertise: AIDS activism and the forging of credibility in the reform of clinical trials. Science, Technology, and Human Values. 1995;20:408–437. doi: 10.1177/016224399502000402. [DOI] [PubMed] [Google Scholar]
- 49.Lerner B. Breast cancer activism: Past lessons, future directions. Nat Rev Cancer. 2002;2:225–230. doi: 10.1038/nrc744. [DOI] [PubMed] [Google Scholar]